Abstract
Objective
This study investigated the effect of type 2 diabetes duration on glucose regulation 24 months post-bariatric surgery.
Methods
Twenty-seven adults with short (<5 years) and long-duration (≥10 years) type 2 diabetes received a mixed-meal tolerance test at baseline and 24 months (m) post-surgery. Body weight, insulin sensitivity, 1st and 2nd phase meal-stimulated insulin secretion, disposition index (i.e. DI or pancreatic β-cell function), and incretin responses were examined.
Results
Adults with short-duration type 2 diabetes had better HbA1c, greater insulin secretory capacity, and greater DI compared with adults with long-duration type 2 diabetes, despite similar weight loss and incretin responses. Diabetes duration correlated with smaller improvements in HbA1c and DI, but not weight loss.
Conclusions
Enhanced β-cell function characterizes the effect of bariatric surgery in adults with diabetes for <5 years, independent of weight loss or incretins. Additional therapy post-surgery may be required to improve glycemia for people with long-standing type 2 diabetes.
Keywords: Bariatric surgery, type 2 diabetes, beta cells, glycemic control
INTRODUCTION
Not all individuals with type 2 diabetes meet remission criteria following bariatric surgery (1) and diabetes duration has emerged as an important clinical factor in predicting disease resolution. In fact, recent work suggests that individuals with short- vs. long-duration diabetes improve glycemic control up to 3 months post-op through improvements in insulin sensitivity (2). However, differences in pre-existing HbA1c and adiposity between groups (2) limit our understanding of the interaction between diabetes duration per se and bariatric surgery. High fasting C-peptide levels pre-surgery have also been reported to predict diabetes remission 1-5 years post-surgery (3), and we recently demonstrated that bariatric surgery lowered blood glucose by predominantly augmenting pancreatic function via gastrointestinal hormone-related mechanisms (4, 5). Given that individuals with long-duration diabetes typically exhibit severe β-cell dysfunction, we hypothesized that bariatric surgery would promote greater glycemic benefit 24 months post-surgery in individuals with short- vs. long-duration diabetes matched on glycemic control and BMI, and that this would relate to accentuated β-cell function.
RESEARCH DESIGN & METHODS
This prospective study was performed as part of the STAMPEDE trial, of which the methods have been reported and approved by our Institutional Review Board (1). Metabolic studies on diabetes duration were performed before and 24 months following bariatric surgery (i.e. Roux-en Y Gastric Bypass (RYGB) or Sleeve Gastrectomy (SG)). Our sample consisted of 27 adults (Table 1) with short (n=14; <5y; 2y [2, 3], 46% SG) and long-duration (n=13; ≥10y, 11y [10, 13] 53% SG) diabetes. Type 2 diabetes duration was identified using electronic medical records and confirmed by a health history questionnaire. Diabetes remission was defined as HbA1c < 6.5% and fasting glucose levels < 126 mg/dl. DiaRem scores were also calculated to characterize factors involved in diabetes remission (6). Subjects abstained from diabetes medication 24-hours prior to testing. After an overnight fast, subjects were given a mixed meal tolerance test (MMTT: Boost; 350 kcal) and blood samples were obtained for HbA1C, glucose, insulin, and C-peptide at 0, 30, 60, 90 and 120 min of the MMTT to determine glycemic control, insulin sensitivity (IS) and β-cell function. GIP (gastric inhibitory polypeptide) and GLP-1 (glucagon-like polypeptide-1) were also measured at 0 and 60 minutes to determine incremental incretin responses. IS was estimated using the Matsuda Index (7) and insulin secretion rates (ISR) during the MMTT were calculated using C-peptide deconvolution techniques (4). Meal-stimulated insulin secretion (MSIS) was calculated as incremental area under the curve (iAUC) of C-peptide ISR divided by glucose during the first 30 and last 60 minutes of the MMTT (i.e. C-pep0-30/Glc0-30 and C-pep60-120/Glc60-120). Pancreatic β-cell function was estimated using the disposition index (DI; MSIS x IS). Plasma insulin was assayed by a radioimmunoassay (Linco Research), C-peptide by a chemiliuminescence immunoassay (Linco Research), and total GIP and GLP-1 were measured using an ELISA (ALPCO Diagnostics). Data were analyzed using R (Vienna, Austria). Group differences were evaluated using Wilcoxon rank sum tests, while categorical baseline factors were tested with Fisher's exact test. Spearman's correlation was used to examine associations. Significance was accepted as p≤0.05.
Table 1.
Comparison between short- and long-duration diabetes subjects following bariatric surgery at baseline and 24 months.
| Short-Duration | Long-Duration | P-value | ||||
|---|---|---|---|---|---|---|
| Pre | Δ | Pre | Δ | Pre vs. Pre | Δ vs. Δ | |
| n (M,F) | 14 (11F, 3M) | - | 13 (7F, 6M) | - | 0.24 | - |
| n (RYGB, SG) | 14 (7, 7) | - | 13 (5, 8) | - | 0.70 | - |
| Age (years) | 50.4 [38.2, 55.5] | - | 52.0 [42.5, 59.1] | - | 0.39 | - |
| DiaRem | 10.5 [9.25, 17] | −8 [−12.75, −6] | 18 [10, 19] | −2 [−10, 0] | 0.39 | 0.12 |
| Medication Sum | 4 [2, 4.7] | −2.5 [−4, −2] | 5 [4, 5] | −3 [−4, −2] | 0.02 | 0.69 |
| Weight (kg) | 99.3 [93.8, 111.5] | −22.6 [−29.0, −17.8] | 105.8 [91.2, 112.5] | −19.7 [−23.5, −16.8] | 0.83 | 0.17 |
| BMI (kg/m2) | 36.76 [34.77, 37.99] | −8.2 [−10.1, −6.8] | 35.3 [34.1, 37.8] | −6.81 [−8.6, −5.5] | 0.83 | 0.12 |
| HbA1c (%) | 9.3 [8.9, 10.5] | −3.0 [−4.9, −2.1] | 8.9 [8.6, 10.5] | −1.6 [−3.1, −0.8] | 0.61 | 0.04 |
| HbA1c (mmol/mol) | 74 [70, 91] | −33.6 [−54.2, −23.7] | 78 [74.2, 84.2] | −18 [−34, −9] | 0.61 | 0.04 |
| FPG (mg/dl) | 204.5 [144, 238] | −95.5 [−141.7, −60.7] | 171 [112, 209] | −72 [−104, −8] | 0.45 | 0.42 |
| FPI (μU/ml) | 26.35 [15.1, 40.8] | −14.7 [−30.0, −7.1] | 11.5 [5.7, 22.8] | −1 [−5.7, 3.4] | 0.009 | 0.003 |
| FPCpep (ng/ml) | 3.8 [2.4, 4.6] | −1.8 [−2.5, −0.5] | 1.4 [0.5, 2.6] | −0.2 [−0.8, 0.3] | 0.003 | 0.01 |
| PG AUC0-30 | 7.1 [4.3, 12.5] | 12 [3.5, 23.6] | 6.5 [3.5, 8.2] | 20.2 [13.5, 27] | 0.42 | 0.22 |
| PI AUC0-30 | 3.2 [1.2, 5.9] | 24.0 [18.7, 31.9] | 2.0 [0.7, 2.5] | 9.7 [6.2, 17.9] | 0.28 | 0.01 |
| ISR/PG (iAUC0-30) | 0 [−0.02, 0.02] | 0.04 [0, 0.07] | 0.01 [0, 0.02] | 0 [0, 0.02] | 0.49 | 0.15 |
| PG AUC60-120 | 90.2 [57.5, 99.6] | −52.3 [−67.8, −40.0] | 80.2 [75.7, 82.2] | −12.2 [−42.2, 21] | 0.45 | 0.003 |
| PI iAUC60-120 | 20.2 [5.3, 34.3] | 12.9 [−13.4, 18.9] | 8.9 [7.7, 17.7] | 6.2 [−0.9, 20.2] | 0.28 | 0.87 |
| ISR/PG ( iAUC60-120) | 0.01 [0, 0.02] | 0.07 [0.04, 0.1] | 0.01 [0, 0.01] | 0.01 [0, 0.03] | 0.65 | <0.001 |
| Insulin Sensitivity | 1.3 [1.1,2.7] | 2.3[1.6,3.2] | 3.9 [2.7,6.2] | 0.89 [0, 3.0] | 0.008 | 0.17 |
| DI (1st phase) | 0.07 [−0.2, 0.2] | 0.9 [0.7, 2.2] | 0.2 [−0.04, 0.7] | 0.2 [−0.3, 0.7] | 0.30 | 0.04 |
| DI (2nd phase) | 0.1 [0.01, 0.3] | 2.8 [1.8, 4.5] | 0.1 [−0.04, 0.6] | 0.4 [0.1, 1.3] | 0.62 | 0.01 |
| fGLP-1 | 1.1 [0.8, 2] | 0 [−0.3, 1.0] | 1.2 [0.6, 2.3] | 0 [−0.2, 0.2] | >0.99 | >0.99 |
| ΔGLP-1 (60 min) | 3.0 [2.5, 3.8] | 4.6 [3.1, 9.6] | 1.9 [0.9, 3.1] | 8.5 [5.9, 30.3] | 0.09 | 0.21 |
| fGIP | 5 [1.8, 8.7] | −1.2 [−4.2, 0.5] | 3.7 [2, 4.9] | −0.2 [−2.1, 1.9] | 0.92 | 0.26 |
Data are reported as median [25%, 75%]. BMI = body mass index. FPG = fasting plasma glucose. FPI = fasting plasma insulin. FPCpep = fasting plasma C-peptide. iAUC = incremental area under the curve. ISR = meal-stimulated insulin secretion rate (iAUC C-peptide divided by iAUC Glucose). RYGB = Roux-en-Y Gastric Bypass. SG = Sleeve Gastrectomy. Bold type denotes statistical significance.
RESULTS
Before surgery, BMI, HbA1c, DiaRem, MSIS and DI, as well as incretin levels, were comparable between patients with short and long-duration diabetes (all p>0.05, Table 1). Subjects with long-duration diabetes, compared to those with short-duration diabetes, had similar use of incretin mimetics (92.3 vs. 57.1%; p=0.07), metformin (92.9 vs. 100%; p=0.99), and insulin (53.8 vs. 28.5%; p=0.25). However, long-duration subjects with diabetes required more overall oral medications when compared to short-duration diabetes (Table 1), and this was specifically reflected by the difference in thiazolidinediones usage (100 vs. 50%; p=0.006). Lastly, patients with long-duration diabetes had lower fasting C-peptide levels (p=0.003, Table 1) compared to short-duration subjects.
At 24 m post-surgery, improvements in BMI, insulin sensitivity, and incretin responses were similar between groups (all p>0.05, Table 1). However, short-duration subjects had significantly better HbA1c, higher 2nd phase MSIS, and greater 1st and 2nd DI responses compared with long-duration subjects (all p<0.05, Table 1 and Figure 1). In fact, 42.9% of short-duration diabetics were in remission compared to only 7.7% in the long duration diabetes group. Moreover, short-duration diabetes subjects had lower total medication intake than subjects with long duration diabetes (Table 1), which was primarly the result of decreased metformin use (21.4 vs. 84.6%, p=0.002). Use of other medications was not statistically different at 24 m, including thiazolidinediones (0 vs. 7.7%, p=0.48), incretin mimetics (21.4 vs. 23.1, p=0.99), insulin use (0 vs. 23.1, p=0.09) or secretagogues (0 v. 23.1%, p=0.09) in patients with short- vs. long-duration diabetes. Baseline diabetes duration correlated with smaller improvements in HbA1c (r=0.39, p<0.04) as well as 1st (r=-0.50, p=0.002) and 2nd (r=-0.40, p=0.03) phase DI (Figure 2). Pre-operative 1st phase MSIS and DI were inversely linked to changes in 1st phase MSIS and DI, respectively (r=-0.79, p<0.001 and r=-0.58, p=0.002). However, pre-operative 2nd phase MSIS, or DI were not statistically associated with changes in 2nd phase MSIS or DI (r=-0.18, p=0.37 and r=-0.30, p=0.13).
Figure 1.
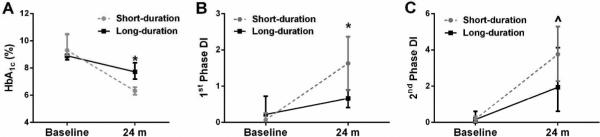
Effect of bariatric surgery on HbA1c and DI in subjects with short vs. long-duration type 2 diabetes. Change in HbA1c (A), 1st phase DI (B), and 2nd phase DI (C) from baseline to 24 months post-surgery. Compared with baseline, *p=0.04 and ^p=0.01. DI = disposition index (meal-stimulated insulin secretion rate [iAUC C-peptide/iAUC Glucose] multiplied by insulin sensitivity).
Figure 2.
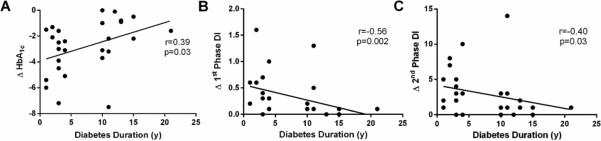
Correlations between diabetes duration and 1st phase DI (A), 2nd phase DI (B), and HbA1c (C). DI = disposition index (meal-stimulated insulin secretion rate [iAUC C-peptide/iAUC Glucose] multiplied by insulin sensitivity). HbA1c reflects absolute difference between Post-Pre.
DISCUSSION
Consistent with prior work following lifestyle intervention in hyperglycemic adults (8, 9), our novel findings suggest that bariatric surgery promotes better glycemic control in individuals with short- vs. long-duration diabetes 2 years after bariatric surgery. Unlike prior short-term data following bariatric surgery (2), our data indicate that β-cell function, not insulin sensitivity, is the key determinant of post-operative glycemic control. There are several reasons for this apparent difference between studies. Weight loss has been directly linked to both improved insulin sensitivity and secretion through anti-inflammatory mediated mechanisms following bariatric surgery (10). However, both groups in the current study lost similar amounts of weight, suggesting that caloric restriction per se and/or inflammatory changes are unlikely to explain the 2 year differences in glycemic benefit between individuals with short- vs. long-duration diabetes (11). Moreover, the intravenous glucose tolerance test was previously used to determine insulin sensitivity and β-cell function independent of incretin effects (2). However, a strength of the current study is that using the MMTT increases the clinical application and physiologic insight of our findings. In this context, Dutia and colleagues recently observed that incretins are a major determinant of pancreatic function following bariatric surgery (5, 12, 13). In fact, GLP-1 is a leading candidate for not only increasing β-cell mass in rodents, but also augmenting β-cell function in humans following bariatric surgery (14, 15). Despite significant parallel increases in meal-stimulated GLP-1 and GIP with enhanced DI at 24 m post-surgery in short-duration diabetes, adults with long-duration diabetes had similar rises in circulating incretins. Although blood incretins do not necessarily reflect GLP-1 or GIP action and total circulating levels were determined, our data are in line with recent work by Jimenez and colleagues suggesting that subjects with long-duration diabetes are either less responsive to these endogenous incretins and/or due to a pre-operative inability to secrete insulin (16). In either case, the blunted rise in insulin secretion in those with long duration diabetes suggests that using gut-mediated treatments for pancreatic function remains a feasible approach for glycemic control. It is also worth noting that pre-operative use of GLP-1 agonists may be effective at enhancing diabetes remission rates following gastric bypass (17). While there were no differences in baseline incretin mimetics in our study, suggesting that pre-operative GLP-1 treatment was unlikely to have impacted glycemic responses in long-duration diabetes following our intervention, our study was not powered to test such a hypothesis. Further work is required pre- and post-operation to clarify the optimal therapy behind bariatric surgery-induced diabetes remission.
The progression from normal glucose tolerance to diabetes is characterized by reductions in β-cell mass that, in turn, lead to impaired β-cell function (18). The exact mechanism(s) for this exhausted β-cell mass is unclear, but glucotoxicity is known to promote apoptosis and cause proliferative defects in β-cells (19). As such, it is reasonable to expect that extended periods of time or ambient hyperglycemia, as reflected by the blunted reduction in HbA1c, induce β-cell apoptosis and dysfunction (19). While fasting C-peptides were lower in subjects with long-duration diabetes both before and after the intervention, baseline DI was similar between short-and long-duration subjects in our study, suggesting that the functionality of pre-existing β-cells may have impacted the responsiveness to bariatric surgery. Indeed, those individuals with lower pre-operative MSIS or β-cell function were observed to have larger increases in insulin secretion following bariatric surgery, which is consistent with pancreatic responses to lifestyle modification in adults with prediabetes (20). Our findings do not exclude the benefit of enhanced insulin sensitivity, as individuals with short-duration diabetes had greater improvements, although not statistically (p=0.17), than long-duration subjects. However, there was no relationship between insulin sensitivity and glycemic control. Whether “resting the β-cell” or accentuating pancreatic endocrine function improves glycemic control in long-duration subjects with lifestyle modification or pharmacology awaits further investigation. Therefore, our data call attention to inadequate β-cell function as a key factor characterizing the attenuated glycemic responses to bariatric surgery in people with long-duration diabetes.
What is already known about this subject?
Bariatric surgery induces type 2 diabetes remission
Not all individuals meet diabetes remission criteria following bariatric surgery
Diabetes duration may be a clinical factor that predicts disease resolution
What this study adds:
Bariatric surgery promotes better glycemic control in individuals with short- vs. long-duration diabetes 2 years after bariatric surgery
Inadequate β-cell function is a key factor characterizing the attenuated glycemic responses to bariatric surgery in people with long-duration diabetes
ACKNOWLEDGMENTS
We thank Sarah Neale from the Cleveland Clinic Preventive Research Lab for performing blood analysis, the nursing staff for technical assistance, and the dedicated research assistants and subjects for their effort. We also thank the Cleveland Clinic Coordinating Center for Clinical Research for providing the database and statistical analysis.
FUNDING
This research was supported by Ethicon endo-surgery EESIIS 19900 (PRS), American Diabetes Association clinical translational award 1-11-26 CT (SRK), NIH RO1-DK089547 (PRS, SRK, JPK), and National Institutes of Health National Center for Research Resources, 1UL1RR024989, Cleveland, OH.
Footnotes
AUTHOR CONTRIBUTIONS
All authors contributed to data collection and organization. VK and SKM wrote the manuscript and all authors provided edits. SKM, JB, and SRK share responsibility for the integrity of analysis. SRK is the guarantor of this work. RW performed C-peptide deconvolution measures of ISR.
Conflict of Interest: Authors report none for this analysis
REFERENCES
- 1.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavin TP, Ernst JM, Caudill SE, Dohm GL, Pories WJ, Dar M, et al. Insulin sensitivity is related to glycemic control in type 2 diabetes and diabetes remission after Roux-en Y gastric bypass. Surgery. 2014;155:1036–1043. doi: 10.1016/j.surg.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Aarts EO, Janssen J, Janssen IM, Berends FJ, Telting D, de Boer H. Preoperative fasting plasma C-peptide level may help to predict diabetes outcome after gastric bypass surgery. Obes Surg. 2013;23:867–873. doi: 10.1007/s11695-013-0872-8. [DOI] [PubMed] [Google Scholar]
- 4.Kashyap SR, Bhatt DL, Wolski K, Watanabe RM, Abdul-Ghani M, Abood B, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013;36:2175–2182. doi: 10.2337/dc12-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malin SK, Samat A, Wolski K, Abood B, Pothier CE, Bhatt DL, et al. Improved acylated ghrelin suppression at 2 years in obese patients with type 2 diabetes: effects of bariatric surgery vs standard medical therapy. Int J Obes. 2014;38:364–370. doi: 10.1038/ijo.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Still CD, Wood GC, Benotti P, Petrick AT, Gabrielsen J, Strodel WE, et al. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol. 2014;2:38–45. doi: 10.1016/S2213-8587(13)70070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 8.Solomon TP, Malin SK, Karstoft K, Haus JM, Kirwan JP. The influence of hyperglycemia on the therapeutic effect of exercise on glycemic control in patients with type 2 diabetes mellitus. JAMA Intern Med. 2013;173:1834–1836. doi: 10.1001/jamainternmed.2013.7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malin SK, Haus JM, Solomon TP, Blaszczak A, Kashyap SR, Kirwan JP. Insulin sensitivity and metabolic flexibility following exercise training among different obese insulin-resistant phenotypes. Am J Physiol Endocrinol Metab. 2013;305:E1292–1298. doi: 10.1152/ajpendo.00441.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghow R. Bariatric surgery-mediated weight loss and its metabolic consequences for type-2 diabetes. World J Diabetes. 2013;4:47–50. doi: 10.4239/wjd.v4.i3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, et al. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell Function in type 2 diabetic patients. Diabetes. 2013;62:3027–3032. doi: 10.2337/db12-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holst JJ. Enteroendocrine secretion of gut hormones in diabetes, obesity and after bariatric surgery. Curr Opin Pharmacol. 2013;13:983–988. doi: 10.1016/j.coph.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Dutia R, Brakoniecki K, Bunker P, Paultre F, Homel P, Carpentier AC, et al. Limited recovery of beta-cell function after gastric bypass despite clinical diabetes remission. Diabetes. 2014;63:1214–1223. doi: 10.2337/db13-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habegger KM, Heppner KM, Amburgy SE, Ottaway N, Holland J, Raver C, et al. GLP-1R responsiveness predicts individual gastric bypass efficacy on glucose tolerance in rats. Diabetes. 2014;63:505–513. doi: 10.2337/db13-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YS, Jun HS. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism. 2014;63:9–19. doi: 10.1016/j.metabol.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Jimenez A, Mari A, Casamitjana R, Lacy A, Ferrannini E, Vidal J. GLP-1 and Glucose Tolerance After Sleeve Gastrectomy in Morbidly Obese Subjects With Type 2 Diabetes. Diabetes. 2014;63:3372–3377. doi: 10.2337/db14-0357. [DOI] [PubMed] [Google Scholar]
- 17.Wood GC, Gerhard GS, Benotti P, Petrick AT, Gabrielsen JD, Strodel WE, et al. Preoperative Use of Incretins Is Associated With Increased Diabetes Remission After RYGB Surgery Among Patients Taking Insulin: A Retrospective Cohort Analysis. Ann Surg. 2015;261:125–128. doi: 10.1097/SLA.0000000000000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura T. The role of FOXO1 in beta-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013;9:615–623. doi: 10.1038/nrendo.2013.157. [DOI] [PubMed] [Google Scholar]
- 20.Malin SK, Solomon TP, Blaszczak A, Finnegan S, Filion J, Kirwan JP. Pancreatic beta-cell function increases in a linear dose-response manner following exercise training in adults with prediabetes. Am J Physiol Endocrinol Metab. 2013;305:E1248–1254. doi: 10.1152/ajpendo.00260.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


