Abstract
Streptococcus agalactiae (Group B Streptococcus, GBS) is an encapsulated, Gram-positive bacterium that is a leading cause of neonatal pneumonia, sepsis and meningitis, and an emerging aquaculture pathogen. The zebrafish (Danio rerio) is a genetically tractable model vertebrate that has been used to analyze the pathogenesis of both aquatic and human bacterial pathogens. We have developed a larval zebrafish model of GBS infection to study bacterial and host factors that contribute to disease progression. GBS infection resulted in dose dependent larval death, and GBS serotype III, ST-17 strain was observed as the most virulent. Virulence was dependent on the presence of the GBS capsule, surface anchored lipoteichoic acid (LTA) and toxin production, as infection with GBS mutants lacking these factors resulted in little to no mortality. Additionally, interleukin-1β il1b and CXCL-8 (cxcl8a) were significantly induced following GBS infection compared to controls. We also visualized GBS outside the brain vasculature, suggesting GBS penetration into the brain during the course of infection. Our data demonstrate that zebrafish larvae are a valuable model organism to study GBS pathogenesis.
1.1 Introduction
Streptococcus agalactiae, also known as Group B Streptococcus (GBS), is a leading cause of severe, invasive bacterial infection in human newborns. Neonatal infection can present as early-onset or late-onset disease. In early-onset cases, bacteria are transferred from the mother to the infant in utero, following ascending infection of the placental membranes, or during passage through the birth canal, by aspiration of infected vaginal fluids [1]. Despite routine screening and administration of antibiotic prophylaxis in pregnant women to reduce GBS vaginal colonization and prevent transmission, infection rates remain high [2]. Late-onset GBS disease can occur in infants up to several months old, and is distinguished by bloodstream infection with a high rate of progression to meningitis [3]. Infants that survive GBS meningitis can suffer long-term neurological sequelae, such as seizures, blindness, hearing loss and cognitive impairment. To cause meningitis, GBS must interact with, and penetrate the blood-brain barrier (BBB) [4]. The BBB is primarily comprised of a single layer of specialized brain microvascular endothelial cells (BMEC) and serves as a critical barrier to separate the surrounding tissues from the circulating blood and protect the central nervous system (CNS) against invading microorganisms [4]. Previously we, and others, have described a number of bacterial factors that promote GBS virulence and BBB penetration including the polysaccharide capsule [5], pili [6], HvgA [7], serine rich repeat (Srr) proteins [8, 9], lipoteichoic acid (LTA) [10], β-hemolysin/cytolysin (β-h/c) [11], and fibronectin binding proteins (SfbA) [12]. Further, disease progression is exacerbated by neutrophils that are recruited to the site of infection and contribute to BBB dysfunction and the development of meningitis by further destruction of the BBB and greater cytokine production (as reviewed in [4]).
Although studies using tissue culture and small animal challenge models have enhanced our understanding of the molecular pathogenesis of GBS disease [6, 7, 9–13], limitations exist in live imaging and tracking of disease progression in real time. Over the last decade, zebrafish (Danio rerio) have emerged as a valuable tool for modeling a number of human diseases [14, 15]. Zebrafish are easy to genetically manipulate, lay large clutches, and are transparent for the first week of life. Additionally, the zebrafish and mammalian innate immune systems are striking similar [16–18]. GBS infection in adult zebrafish has been described, and infected adult fish exhibit mortality, cerebral edema, and bacteria in the brain [19]. However, to date GBS infection of zebrafish larvae has not been described. Here we examine GBS infection in zebrafish larvae at three days post fertilization (dpf). We show that zebrafish larvae are susceptible to GBS, and that bacteria are able to cross the BBB. Furthermore, bacterial mutants that are deficient in virulence factors are attenuated in the larval model. These findings propose a novel system for examining GBS virulence in zebrafish larvae, which allow for the visualization of infection in vivo.
2.1 Materials and Methods
Bacterial strains
The Streptococcus agalactiae (GBS) highly virulent clinical isolate COH1 (serotype III, sequence type (ST)-17) [20], NCTC10/84 (V) [21], and A909 (Ia) [22] were used. GBS mutants on the COH1 background are deficient in anchored LTA, COH1Δiag [10], capsule, HY106 [23], and β-h/c production, COH1ΔcylE [11]. Generation of GFP expressing GBS, COH1-GFP, was described previously [12]. GBS was grown in Todd Hewitt Broth to mid-log phase OD600=0.4 and harvested by centrifugation. Pellets were washed, suspended in PBS, and diluted to desired concentration for injection into zebrafish larvae.
Zebrafish Stocks and Larvae
AB*, Tg(lysC:DsRed2)nz50, and Tg(flk1:ras-cherry)s896 zebrafish were mated, staged, and raised as previously described [24, 25]. All fish were raised and maintained under the guidelines of the University of California San Diego Institutional Animal Care and Use Committee guidelines.
Infection experiments
Groups of 11–15 zebrafish were used per condition. 3 dpf larvae were anesthetized with tricane (MS-222; Sigma) and put on a 2% agar plate with E3 (5.0 mM NaCl, 0.17mM KCl, 0.33 CaCl, 0.33 MgSO4) for stabilization. 1 nl of diluted GBS was injected into the area surrounding the heart with a femtojet microinjector (Eppendorf). 3% phenol red (Sigma) was added to the GBS dilution to visualize the injection. Fish were examined every 6 hours presence of a heart beat, or sacrificed at 48 h for cytokine analysis.
Transcript analysis
At time of death, zebrafish larvae were homogenized in the first buffer RA1 + BME from the Machery Nagel RNA isolation kit. RNA isolation (Machery Nagel), cDNA synthesis was performed using the qScript kit from Quantas Biosciences and quantative PCR were performed using Quantas PerfeCTa SYBR Green FastMix run on the Bio-Rad CFX96 according to manufacturer’s instructions. Primer sequences for IL-1β (il1b), CXCL-8 (cxcl8a) [24], and elongation factor 1 alpha (ef1α [25] are as follows: il1b, F-5’AGAATGAAGCACATCAAACC3’, R-5’AATCCACCACGTTCACTTC3’; cxcl8a, F-5’GTCGCTGCATTGAAACAGAA3’, R-5’CTTAACCCATGGAGCAGAGG3’; ef1α, F-5’GAGAAGTTCGAGAAGGAAGC3’, R-5’CGTAGTATTTGCTGGTCTCG3’. Ef1a was used as a housekeeping gene. Gene expression in infected and mock-infected fish was normalized to uninfected control animals.
Visualization of GBS zebrafish larvae
Tg(flk1:ras-cherry)s896 or Tg(lysC:DsRed2)nz50 zebrafish were infected with GFP expressing GBS. At various time points fish were anesthetized as described above and mounted in 1.5% agarose. Fish were then imaged with a confocal microscope under 100X (Leica SPX using Leica LAS AF software).
Statistical analysis
GraphPad Prism version 5.0a was used for statistical analysis. Students t test was used to analyze significance. Statistical significance was accepted at p < 0.05.
3.1 Results and Discussion
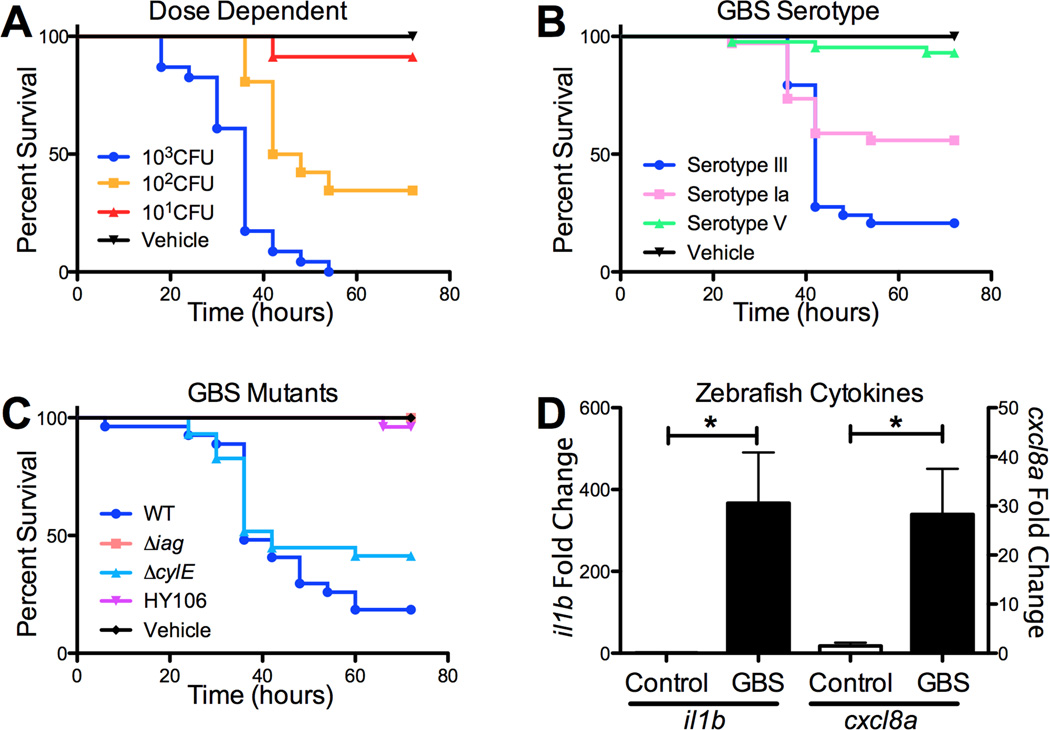
To determine if GBS causes mortality in zebrafish larvae, WT hypervirulent serotype III, sequence type (ST)-17 GBS (strain COH1) was injected into zebrafish larvae at 3 dpf. Zebrafish larvae were injected with 101, 102, or 103 CFU GBS, and control fish were injected with PBS alone. Mortality of the larvae was dose-dependent. An MOI of 102 CFU represented an LD50, and an MOI of 103 CFU resulted in 100% larval death by 36 hours (Fig. 1A). For all future experiments a dose of 102 CFU was used. To determine if infection with other clinically relevant GBS strains would also result in larval death, we infected larvae with 102 CFU serotype V (NCTC 10/84) and serotype Ia (A909). Both GBS serotypes resulted in increased mortality compared to PBS-injected larvae. However, at the same dose, the hypervirulent serotype III strain resulted in increased mortality compared to serotypes V and Ia (Fig. 1B). Next, we sought to determine whether characterized GBS virulence determinants, which contribute to disease pathogenesis in murine models of infection, also promote virulence in the zebrafish larval model. We examined GBS mutants in the serotype III background (COH1), which were deficient in capsule (HY106) [5], the β-h/c toxin (ΔcylE) [11], and properly anchored LTA (Δiag) [10]. We observed that all of these mutants were attenuated in zebrafish larvae (Fig. 1C). Infection with GBS mutants lacking capsule and anchored LTA resulted in very little to no mortality, respectively. Infection with the ΔcylE mutant resulted in 43% mortality by 72 hpi compared to 76% mortality induced by the WT strain. These results are consistent with previous studies in mice and adult zebrafish demonstrating the capsule is a virulence factor required for GBS survival in the bloodstream [5, 19]. Further, the iagA gene has been demonstrated to be critical for anchoring LTA on the GBS surface and promoting GBS invasion of the BBB endothelium [10]. During experimental meningitis in the mouse model, we have previously observed that GBS induces pro-inflammatory signaling molecules, including those responsible for neutrophil recruitment and activation [11, 13]. Therefore we sought to examine whether GBS induces in a similar upregulation of pro-infammatory cytokines and chemokines in zebrafish larvae. Following GBS infection, we extracted RNA from larvae and analyzed transcript levels of interleukin-1β and CXCL8 by real time qPCR at 48 h post infection (hpi). IL-1β and CXCL-8 transcript were significantly induced compared to PBS-infected controls (Fig. 1D). These results suggest that zebrafish larvae respond similarly to mice during disease progression in response to GBS infection. Future studies to examine cytokine protein levels during infection will be important once reagents become available for zebrafish.
Figure 1. GBS infection in zebrafish larvae.
Dose-dependent survival of zebrafish larvae injected with GBS Serotype III (A). Survival of zebrafish larvae (n=24) in response to clinical isolates of GBS representing common serotypes (B). Survival of zebrafish larvae (n=26) in response to attenuated GBS mutants (C). Induction of il1b and cxcl8a transcript following GBS infection of zebrafish larve, n=6. Experiments were performed at least three times and combined experiments are shown. * p < 0.05.
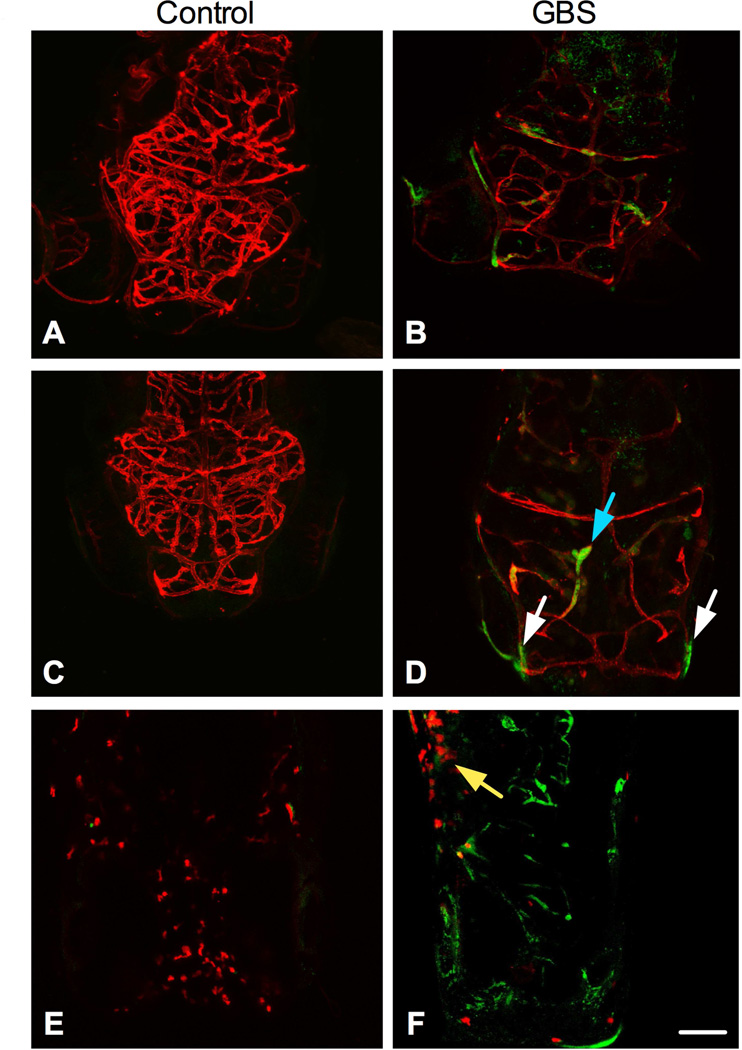
3.2
To further examine if GBS is able to cross the BBB of zebrafish larvae, we infected Tg(flk1:ras-cherry)s896 transgenic zebrafish with COH1-GFP. Larvae were examined 24 and 48 hpi. At 24 and 48hrs post infection we observed that GBS colocalize with the brain vasculature (yellow), and also observed GBS independent of the brain vasculature (green) (Fig. 2A-D). Interestingly, uninfected (saline treated) larvae appear to have increased vasculature compared to infected larvae. Further analysis is needed to determine if infection results in a developmental defect, apoptosis or decreased blood flow that could account for the observed decreased vascular density. During bacterial meningitis, neutrophils are known to migrate to the brain and contribute to disease pathogenesis. To visualize neutrophils we infected Tg(lysC:DsRed2)nz50 transgenic fish with GBS in order to examine neutrophil migration during the course of infection. We observed neutrophils at the site of bacterial injection (Fig. 2E-F), however at the early time points before larval death we did not observe neutrophil migration to the brain. While neutrophils and other leukocytes may contribute to BBB disruption during disease progression, GBS penetration of the BBB prior to the inflammatory response, represents a key first step to the pathogenesis of meningitis. We propose that this model will be more useful to assess bacterial factors responsible for the initial BBB penetration. In summary, we describe a new in vivo model to examine GBS virulence using zebrafish larvae. Our results recapitulate findings of GBS infection in mice and adult zebrafish [19]. The ability to image and monitor the infection process in vivo makes the zebrafish larvae a powerful model for studying host-GBS interactions.
Figure 2. Visualization of GBS in the vasculature.
Tg(flk1:ras-cherry)s896 fish were injected with GFP-GBS or a vehicle control. At 24 hours (A-B) and 48 hpi (C-D) GBS is present outside the endothelium of the brain. Tg(lysC:DsRed2)nz50 fish were injected with GFP-GBS or vehicle control. At all time points assessed, granulocytes migrated to the site of injection, 24hpi shown (E-F). Scale bar = 100uM. The blue arrow points to GBS localized with the vasculature. White arrows point to GBS that does not co-localize with the vasculature. Yellow arrow points to the injection site. Experiments were repeated at least three times and representative images are shown.
Highlights.
Zebrafish larvae model of Group B Streptococcus infection
Visualization of bacterial penetration of the blood brain barrier
Group B Streptococcus mutants are attenuated in the zebrafish larvae model of infection
Acknowledgements
We are grateful to Samira Dahesh and Victor Nizet for assistance and the use of the confocal microscope. B.J.K acknowledges the San Diego chapter of the ARCS Foundation for support. This work was supported by fellowships from Kyoto/Inamori Foundation, Rees Steely Research Foundation/SDSU Heart Institute, American Heart Association 14PRE18690037 to B.J.K., and an R01NS051247 from the NIH/NINDS to K.S.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maisey HC, Doran KS, Nizet V. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert reviews in molecular medicine. 2008;10:e27. doi: 10.1017/S1462399408000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards MS, Baker CJ. Group B streptococcal infections in elderly adults. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41:839–847. doi: 10.1086/432804. [DOI] [PubMed] [Google Scholar]
- 3.Johri AK, Paoletti LC, Glaser P, Dua M, Sharma PK, Grandi G, et al. Group B Streptococcus: global incidence and vaccine development. Nature reviews Microbiology. 2006;4:932–942. doi: 10.1038/nrmicro1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Sorge NM, Doran KS. Defense at the border: the blood-brain barrier versus bacterial foreigners. Future microbiology. 2012;7:383–394. doi: 10.2217/fmb.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubens CE, Wessels MR, Heggen LM, Kasper DL. Transposon mutagenesis of type III group B Streptococcus: correlation of capsule expression with virulence. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:7208–7212. doi: 10.1073/pnas.84.20.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maisey HC, Hensler M, Nizet V, Doran KS. Group B streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. Journal of bacteriology. 2007;189:1464–1467. doi: 10.1128/JB.01153-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tazi A, Disson O, Bellais S, Bouaboud A, Dmytruk N, Dramsi S, et al. The surface protein HvgA mediates group B streptococcus hypervirulence and meningeal tropism in neonates. The Journal of experimental medicine. 2010;207:2313–2322. doi: 10.1084/jem.20092594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo HS, Mu R, Kim BJ, Doran KS, Sullam PM. Binding of glycoprotein Srr1 of Streptococcus agalactiae to fibrinogen promotes attachment to brain endothelium and the development of meningitis. PLoS pathogens. 2012;8:e1002947. doi: 10.1371/journal.ppat.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Sorge NM, Quach D, Gurney MA, Sullam PM, Nizet V, Doran KS. The group B streptococcal serine-rich repeat 1 glycoprotein mediates penetration of the bloodbrain barrier. The Journal of infectious diseases. 2009;199:1479–1487. doi: 10.1086/598217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doran KS, Engelson EJ, Khosravi A, Maisey HC, Fedtke I, Equils O, et al. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. The Journal of clinical investigation. 2005;115:2499–2507. doi: 10.1172/JCI23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doran KS, Liu GY, Nizet V. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. The Journal of clinical investigation. 2003;112:736–744. doi: 10.1172/JCI17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mu R, Kim BJ, Paco C, Del Rosario Y, Courtney HS, Doran KS. Identification of a Group B Streptococcal Fibronectin Binding Protein, SfbA, That Contributes to Invasion of Brain Endothelium and Development of Meningitis. Infection and immunity. 2014;82:2276–2286. doi: 10.1128/IAI.01559-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee A, Kim BJ, Carmona EM, Cutting AS, Gurney MA, Carlos C, et al. Bacterial Pili exploit integrin machinery to promote immune activation and efficient blood-brain barrier penetration. Nature communications. 2011;2:462. doi: 10.1038/ncomms1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neely MN, Pfeifer JD, Caparon M. Streptococcus-zebrafish model of bacterial pathogenesis. Infection and immunity. 2002;70:3904–3914. doi: 10.1128/IAI.70.7.3904-3914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Sar AM, Stockhammer OW, van der Laan C, Spaink HP, Bitter W, Meijer AH. MyD88 innate immune function in a zebrafish embryo infection model. Infection and immunity. 2006;74:2436–2441. doi: 10.1128/IAI.74.4.2436-2441.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan C, Kim CH. Zebrafish as a model for infectious disease and immune function. Fish Shellfish Immun. 2008;25:341–350. doi: 10.1016/j.fsi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Traver D, Herbomel P, Patton EE, Murphey RD, Yoder JA, Litman GW, et al. The zebrafish as a model organism to study development of the immune system. Adv Immunol. 2003;81 253-+ [PubMed] [Google Scholar]
- 18.Yoder JA, Nielsen ME, Amemiya CT, Litman GW. Zebrafish as an immunological model system. Microbes Infect. 2002;4:1469–1478. doi: 10.1016/s1286-4579(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 19.Patterson H, Saralahti A, Parikka M, Dramsi S, Trieu-Cuot P, Poyart C, et al. Adult zebrafish model of bacterial meningitis in Streptococcus agalactiae infection. Developmental and comparative immunology. 2012;38:447–455. doi: 10.1016/j.dci.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Wessels MRBV, Kasper DL, Heggen LM, Rubens CE. Type III capsule and virulence of group B streptococci. Washington DC USA: American Society for Microbiology; 1991. [Google Scholar]
- 21.Wilkinson HW. Nontypable group B streptococci isolated from human sources. Journal of clinical microbiology. 1977;6:183–184. doi: 10.1128/jcm.6.2.183-184.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madoff LC, Michel JL, Kasper DL. A monoclonal antibody identifies a protective C-protein alpha-antigen epitope in group B streptococci. Infection and immunity. 1991;59:204–210. doi: 10.1128/iai.59.1.204-210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yim HH, Nittayarin A, Rubens CE. Analysis of the capsule synthesis locus, a virulence factor in group B streptococci. Advances in experimental medicine and biology. 1997;418:995–997. doi: 10.1007/978-1-4899-1825-3_234. [DOI] [PubMed] [Google Scholar]
- 24.de Oliveira S, Reyes-Aldasoro CC, Candel S, Renshaw SA, Mulero V, Calado A. Cxcl8 (IL-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J Immunol. 2013;190:4349–4359. doi: 10.4049/jimmunol.1203266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page DM, Wittamer V, Bertrand JY, Lewis KL, Pratt DN, Delgado N, et al. An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood. 2013;122:e1–e11. doi: 10.1182/blood-2012-12-471029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lembo A, Gurney MA, Burnside K, Banerjee A, de los Reyes M, Connelly JE, et al. Regulation of CovR expression in Group B Streptococcus impacts blood-brain barrier penetration. Molecular microbiology. 2010;77:431–443. doi: 10.1111/j.1365-2958.2010.07215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin S, Kim KS. RhoA and Rac1 contribute to type III group B streptococcal invasion of human brain microvascular endothelial cells. Biochemical and biophysical research communications. 2006;345:538–542. doi: 10.1016/j.bbrc.2006.04.130. [DOI] [PubMed] [Google Scholar]
- 28.Tenenbaum T, Bloier C, Adam R, Reinscheid DJ, Schroten H. Adherence to and invasion of human brain microvascular endothelial cells are promoted by fibrinogen-binding protein FbsA of Streptococcus agalactiae. Infection and immunity. 2005;73:4404–4409. doi: 10.1128/IAI.73.7.4404-4409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]