Abstract
Objective
To examine the effect of weight loss on sleep duration, sleep quality, and mood in 390 obese men and women who received one of three behavioral weight loss intervention in the Practice-based Opportunities for Weight Reduction trial at the University of Pennsylvania (POWER-UP).
Methods
Sleep duration and quality were assessed at baseline and months 6 and 24 by the Pittsburgh Sleep Quality Index (PSQI) questionnaire and mood by the Patient Health Questionnaire-8 (PHQ-8). Changes in sleep and mood were examined according to treatment group and based on participants’ having lost ≥5% of initial weight vs <5%.
Results
There were few significant differences between treatment groups in changes in sleep or mood. At month 6, however, mean (±SD) min of sleep increased significantly more in participants who lost ≥5% vs <5% (21.6±7.2 vs 1.2±6.0 min, p=0.0031). PSQI total scores similarly improved (declined) more in those who lost ≥5% vs <5% (−1.2±0.2 vs −0.4±0.2, p < 0.001), as did PHQ scores (−2.5±0.4 vs −0.1±0.3, p <0.0001). At month 24, only the differences in mood remained statistically significant (p < 0.05).
Conclusion
Losing ≥ 5% of initial weight was associated with short-term improvements in sleep duration and sleep quality, as well as favorable short- and long-term changes in mood.
Keywords: Behavioral weight loss, obesity, sleep quality, sleep duration, mood
INTRODUCTION
Weight loss of 5–10% of initial weight is associated with improvements in risk factors for cardiovascular disease (CVD).1,2 The Look AHEAD trial, for example, found that weight loss of this magnitude, achieved with a behavioral intervention, was associated with improvements in glycemic control, blood pressure, triglyceride levels, and HDL cholesterol.1 It was also associated with reductions in symptoms of depression and in the risk of future depression (as assessed over 8 years of follow up).3 Findings of reduced symptoms of depression confirm results of earlier behavioral weight loss studies,4–7 and are important because depression is an independent risk factor for CVD morbidity and mortality.8–11
Weight loss may also be associated with improvements in sleep duration and quality, as shown by studies by Verhoef et al12 and Chaput et al,13 respectively, in patients who lost approximately 5–10% of initial weight with behavioral interventions. Furthermore, studies have shown that reduced sleep, defined as an average of less than 7 hours per night, is associated with an increased risk of both obesity and CVD.14–18 Gangwicsh et al15 for example, examined the National Health Examination Survey (NHANES) and found that individuals (32–49 yr of age) who reported sleep duration of less than 7 hours per night had a higher average body mass index (BMI) and were more likely to be obese than were individuals who reported ≥7 hours of sleep. Grander et al16 found that short sleep duration was associated with increased risk of hypertension, diabetes, and hyperlipidemia.
Given that the aforementioned studies which examined weight loss and changes in mood involved intensive behavioral intervention, it is unclear whether a less intensive behavioral weight loss program, delivered in primary care practice, would have a similarly favorable effect on sleep and mood. We examined this issue using data from the Practice-based Opportunities for Weight Reduction trial at the University of Pennsylvania (POWER-UP), which provided moderate intensity (i.e., monthly) brief counseling visits to obese patients in primary care practice.19 Two-year changes in weight in this randomized trial have been reported previously and ranged from 1.7 to 4.6 kg across three different interventions.19 The present study evaluated changes for the three groups in sleep duration and sleep quality, as measured by the Pittsburgh Sleep Quality Index (PSQI),20,21 as well as changes in mood, as assessed by the Patient Health Questionnaire-8 (PHQ-8).22 We also examined changes in sleep and mood based on achievement of a 5% or greater reduction in initial weight, regardless of participants’ original group assignment. In addition, we assessed categorical changes in both sleep (i.e., a change from <7 hours to 7–8 hours per night) and depression (i.e., a change from mild or greater symptoms of depression to no/minimal symptoms) to determine the percentage of participants who experienced clinically meaningful changes in these conditions with a loss ≥5% of initial weight.
MATERIALS AND METHODS
Participants
Participants were 390 obese men and women who were recruited from six primary care practices in the University of Pennsylvania Health System, described previously.19 Inclusion criteria included age ≥21 years, body mass index (BMI) of 30–50 kg/m2, and at least two components of the metabolic syndrome. Exclusion criteria included comorbid illnesses that could be complicated by weight loss, severe psychiatric illness, high blood pressure (defined ≥160/100 mm Hg), the presence of recent cardiovascular disease, use of medications known to affect body weight, substance abuse, weight loss ≥5% in the prior 6 months, and participation in a weight loss program or prior or planned bariatric surgery.19
Interventions
Participants were randomly assigned to one of three interventions: 1) Usual Care; 2) Brief Lifestyle Counseling; or 3) Enhanced Brief Lifestyle Counseling. As described previously,19 participants in all three groups were prescribed the same diet and physical activity goals but received different amounts of behavioral support to reach these targets. Individuals who weighed <113.6 kg were prescribed a balanced diet of 1200–1500 kcal/day, with 1500–1800 kcal/day for those who weighed ≥113.6 kg. The diet recommended 15–20% kcal from protein, 20–35% from fat, and the remainder from carbohydrate. All participants were instructed to gradually increase their physical activity to 180 min per week and were given a pedometer, a calorie counting book,23 and handouts from Aim for a Healthy Weight, published by the National Heart, Lung, and Blood Institute (NHLBI).24
During the 2-year intervention, participants assigned to Usual Care had brief (10–15 min) quarterly visits with their primary care provider (PCP), at which they reviewed their health status, as well as handouts from Aim for a Healthy Weight. PCPs did not provide specific suggestions for behavior change (e.g., keeping food or physical activity records). Participants in the Brief Lifestyle Counseling group (Brief LC) had the same quarterly PCP visits, plus monthly10–15 min meetings with an auxiliary health care provider, typically a medical assistant (MA), who delivered behavioral counseling following an adapted version of the Diabetes Prevention Program (DPP).2,19 Participants were instructed to keep daily food and activity records, which they reviewed with MAs at monthly visits. Individuals in Enhanced Brief Lifestyle Counseling (Enhanced Brief LC) received the same intervention as those in Brief LC but, in consultation with their PCPs, added either meal replacements or a weight loss medication (orlistat or sibutramine) to their regimen in order to increase weight loss. (After sibutramine was removed from the market in October 2010 because of its association with cardiovascular disease, participants on this medication chose either orlistat or meal replacement.25 This switch was also offered when the Food and Drug Administration first issued an alert regarding sibutramine’s safety in November 2009.) Participants in Enhanced Brief LC were allowed to change their choice of enhancements (i.e., meal replacements or medication) but were not allowed to use two enhancements at the same time. Individuals who chose meal replacements replaced two meals and one snack daily with liquid shakes or meal bars (provided by SlimFast). All enhancements were provided free of charge.
Outcomes and Measurements
Sleep duration and quality were assessed by the PSQI questionnaire, which was administered at baseline and months 6 and 24. The PSQI contains 19 questions which evaluate (for the prior month) sleep duration and quality, sleep latency, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. The sum of the component scores yields a global maximum score of 21. A total PSQI score greater than 5 indicates poor sleep.20 We used self-reported hours of actual sleep (rather than time in bed) to assess sleep duration; we did not count nap time, and we defined optimal sleep as 7–8 hours nightly.26–28 Mood was measured by the PHQ-8, on the same schedule as sleep. This questionnaire assesses depression according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV),22,29 but excludes the question about suicidal ideation contained in the PHQ-9.30 Participants indicate the number of days in the preceding 2 weeks that they experienced various symptoms of depression. Total scores range from 0–24; scores of 0–4 indicate no to minimal symptoms of depression; those 5–9 suggest mild depression; 10–14 moderate; and scores ≥ 15 are indicative of moderately severe to severe depression.22
Weight was measured on a calibrated scale (Tanita BWB-800) and height using a wall mounted stadiometer. Demographic data including gender, age, race/ethnicity, and educational level were collected by a self-report questionnaire at baseline.
Statistical Analysis
Changes in weight, sleep, and mood were examined using mixed effect general linear regression models, fit with the between-subject factor of treatment group and the within-subject factor of time (changes from baseline to months 6 and 24). All mixed models were fit using an unstructured variance-covariance matrix and controlling for a priori chosen covariates: gender, ethnicity, and baseline age. Pairwise comparisons were used to determine differences between treatment groups at months 6 and 24. The p value was set at ≤ 0.05 for all comparisons, except for body weight, the study’s primary outcome (which was set at ≤ 0.025 for month 24).19 (This study was powered to detect differences in change in body weight and not those in sleep and mood.) The mixed effect general linear model was also used to assess the relationship between the achievement of <5% vs ≥5% reduction in initial weight and changes in sleep and mood, while adjusting for the above mentioned covariates. All analyses were performed using SAS version 9.3 (SAS Institute Inc. 2011. Cary, NC).
RESULTS
Baseline Characteristics
Table 1 shows that participants (N=390) had a mean (±SD) age of 51.5±11.5 yr, weight of 107.7±18.3 kg, and BMI of 38.5±4.7 kg/m2. Participants were predominantly non-Hispanic, white women. The three groups did not differ significantly at baseline on these or other characteristics shown in Table 1. Participant retention at month 24 averaged 86%, with no significant difference among groups.20
Table 1.
Baseline characteristics by intervention group in the POWER-UP trial (n=390).
| Variable | Usual Care (n=130) | Brief LC (n=131) | Enhanced Brief LC (n=129) | All (n=390) |
|---|---|---|---|---|
| Gender (%) | ||||
| Male | 32 (24.6) | 21(16.0) | 26 (20.2) | 79 (20.3) |
| Female | 98 (75.4) | 110 (84.0) | 103(79.8) | 311 (79.7) |
| Race or Ethnicity group (%) | ||||
| White | 81 (62.3) | 75 (57.3) | 74 (57.4) | 230 (59.0) |
| African American | 46 (35.4) | 52 (39.7) | 52 (40.3) | 150 (38.5) |
| Asian | 2 (1.5) | 0 | 2 (1.6) | 4 (1.0) |
| More than one race | 1 (0.8) | 4 (3.1) | 1 (0.8) | 6 (1.5) |
| Self-reported Hispanic | ||||
| Yes | 6 (4.6) | 6 (4.6) | 6 (4.7) | 18 (4.6) |
| No | 124 (95.4) | 125 (95.4) | 123 (95.3) | 372 (95.4) |
| Age, yr | 51.7 ± 12.1 | 52.0 ± 12.2 | 51.0 ± 10.1 | 51.5 ± 11.5 |
| Weight, kg | 111.2 ± 20.0 | 106.3 ± 17.3 | 105.4 ± 17.2 | 107.7± 18.3 |
| BMI, kg/m2 | 39.0 ± 4.8 | 38.5 ± 4.6 | 37.8 ± 4.7 | 38.5 ± 4.7 |
| Waist circumference, cm | 119.8 ± 13.9 | 117.1 ± 11.9 | 115.9 ± 11.7 | 117.6 ± 12.6 |
| Education (%) | ||||
| Less than high school | 10 (7.7) | 5 (3.8) | 6 (4.7) | 21 (5.4) |
| High school | 25 (19.2) | 27 (20.6) | 26 (20.2) | 78 (20.0) |
| Some college | 43 (33.1) | 50 (38.2) | 48 (37.2) | 141 (36.2) |
| College or more | 52 (40) | 49 (37.4) | 49 (38.0) | 150 (38.5) |
| Sleep Duration (hr) | 6.5 ± 1.2 | 6.3 ± 1.3 | 6.5 ± 1.4 | 6.4 ± 1.3 |
| PSQI score | 8.3 ± 3.2 | 8.4 ± 3.6 | 8.2 ± 3.6 | 8.3 ± 3.4 |
| PHQ-8 | 5.3 ± 4.2 | 5.3 ± 4.2 | 5.4 ± 4.2 | 5.3 ± 4.2 |
Values shown are number (%) or mean ± SD; BMI= body mass index; PSQI= Pittsburgh Sleep Quality Index; PHQ-8= Patient Health Questionnaire 8.
Weight Loss
Mean (±SE) weight losses at month 6 in the Usual Care, Brief LC and Enhanced Brief LC groups were 2.0±0.5, 3.5±0.5 and 6.6±0.5 kg, respectively, as reported previously.19 Participants in both lifestyle groups lost significantly more weight than those in Usual Care (see Table 2). At month 24, participants in Usual Care, Brief LC and Enhanced Brief LC lost 1.7±0.7, 2.9±0.7 and 4.6±0.7 kg respectively. Enhanced Brief LC lost significantly more than Usual Care, with no other differences between groups. The percentages of participants in the three groups who lost ≥5% of initial weight at month 24 were 21.3, 26.0, and 34.9%, respectively, with significant (p=0.02) differences only between Enhanced Brief LC and Usual Care.
Table 2.
Changes in weight, sleep, and mood for the randomized groups.
| Variable | Usual Care | Brief LC | Enhanced Brief LC | P value month 6 | P value month 24 | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Brief LC vs. Usual Care | Enhanced Brief LC vs. Usual Care | Enhanced Brief LC vs. Brief LC | Brief LC vs. Usual Care | Enhanced Brief LC vs. Usual Care | Enhanced Brief LC vs. Brief LC | ||||
| Weight, kg | 0.0333 | <0.0001 | <0.0001 | 0.222 | 0.003 | 0.076 | |||
| Baseline | 111.2 ± 20.0 | 106.3 ± 17.3 | 105.4 ± 17.2 | ||||||
| Δmonth 6 | −2.0 ± 0.5 | −3.5 ± 0.5 | −6.6 ± 0.5 | ||||||
| Δmonth 24 | −1.7 ± 0.7 | −2.9 ± 0.7 | −4.6 ± 0.7 | ||||||
| Sleep Duration (minutes) | 0.562 | 0.562 | 0.994 | 0.174 | 0.045 | 0.529 | |||
| Baseline | 390 ± 72 | 378 ± 78 | 390 ± 84 | ||||||
| Δmonth 6 | 12.6 ± 8.4 | 6.6 ±8.4 | 6.6 ±8.4 | ||||||
| Δmonth 24 | −9.0 ± 9.0 | 6.6 ± 9.0 | 13.8 ± 9.0 | ||||||
| PSQI score (Sleep Quality) | 0.616 | 0.558 | 0.939 | 0.454 | 0.899 | 0.523 | |||
| Baseline | 8.3 ± 3.2 | 8.4 ± 3.6 | 8.2 ± 3.6 | ||||||
| Δ month 6 | −0.8 ± 0.3 | −0.6 ± 0.3 | −0.6 ± 0.3 | ||||||
| Δ month 24 | −1.0 ± 0.3 | −0.7 ± 0.3 | −0.9 ± 0.3 | ||||||
| PHQ-8 | 0.509 | 0.099 | 0.325 | 0.215 | 0.833 | 0.295 | |||
| Baseline | 5.3 ± 4.2 | 5.3 ± 4.2 | 5.4 ± 4.2 | ||||||
| Δmonth 6 | −0.5 ± 0.4 | −0.9 ± 0.4 | −1.5 ± 0.4 | ||||||
| Δmonth 24 | −1.2 ± 0.5 | −0.3 ± 0.5 | −1.0 ± 0.5 | ||||||
Values shown are mean ± SE; PSQI=Pittsburgh Sleep quality Index; PHQ-8=Patient Health Questionnaire-8; All change scores are based on the difference from baseline values
Changes in Sleep Duration and Sleep Quality
Table 2 shows that at month 6 participants in all three groups reported increases in their nightly minutes of sleep, ranging from 6.6 to 12.6 min/night. However, there were no significant differences between groups and no statistically significant effect of time. At month 24, self-reported sleep duration decreased by 9.0±9.0 min in Usual Care participants, while increasing by 13.8±9.9 min in the Enhanced Brief LC group, resulting in a significant difference between groups (see Table 2). Differences between the other groups were not statistically significant.
At month 6, PSQI scores declined in all three groups (ranging from −0.6 to −0.8), indicating improved sleep quality. There were no significant differences between groups, but sleep quality did improve over time across the three groups (p=0.0005). A similar pattern of findings was observed at month 24, with a significant (p<0.0001) effect of time but no significant differences between groups.
5% weight loss
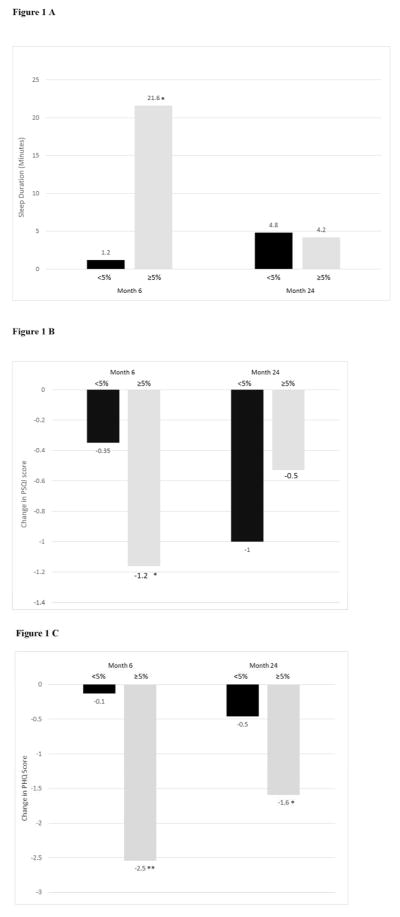
Participants were divided into two groups based on their 6-month weight loss, regardless of their original group assignment. As shown in Figure 1A, those who lost ≥5% of initial weight reported a 21.6±7.2 min/night increase in sleep duration at month 6, compared with a significantly (p=0.0049) smaller 1.2±6 min/night increase in participants who had lost <5% of weight at this time. Of the 224 participants who reported suboptimal sleep duration at baseline (< 7 hours or >8 hours) 30.7% of those who lost ≥5% (at month 6) achieved optimal sleep duration (7–8 hours), compared with only 19.5% of those who lost <5% (p=0.061). Changes in sleep quality followed the same pattern at month 6, as shown in Figure 1B. Reductions (i.e., improvements) on the PSQI were significantly greater in participants who lost ≥5%, compared with <5% of initial weight (−1.2±0.2 vs −0.4±0.2, p=0.0012).
Figure 1.
Figure 1 A: Changes in sleep duration (minutes) at months 6 and 24 based on losing <5% of initial weight (Ns = 202 and 178, respectively) vs ≥5% (Ns = 113 and 87, respectively). *Significant difference between groups, p <0.05.
Figure 1 B: Changes in sleep quality (measured by the PSQI) at months 6 and 24 based on losing <5% of initial weight (Ns =200 and 179, respectively) vs ≥5% (Ns = 116 and 87, respectively). *Significant difference between groups, p<0.05.
Figure 1 C: Changes in mood (measured by the PHQ-8) at months 6 and 24 based on losing <5% of initial weight (Ns = 209 and 187, respectively) vs ≥5% (Ns =115 and 93, respectively). *Significant p<0.05; ** Significant difference between groups, p<0.0001.
When the same analyses were repeated based upon participants’ losing ≥5% vs <5% of weight at month 24, no significance differences were observed between groups in changes in sleep duration (Figure 1A). At this time, of the 198 participants who reported suboptimal sleep duration at baseline (and completed the 24-month assessment), only 11.3% who lost ≥5% at month 24 achieved optimal sleep duration, compared with 22.8% who lost <5% (p=0.057). Additional analyses showed that participants who lost ≥5% of initial weight at month 24 reported smaller reductions (i.e., improvements) on the PSQI than those who lost <5% (−0.53±0.31 vs −1.0±0.23, p=0.14) (see Figure 1B).
Changes in Mood
Table 2 shows that mean PHQ scores declined (improved) in all three groups at month 6, ranging from −0.5 to −1.5 points. Changes among groups did not differ significantly, but there was a significant (p = 0.0008) reduction in scores over time across the three groups. A similar pattern of findings was observed at month 24, with no significance between groups but a significant (p=0.016) effect of time.
5% weight loss
Participants again were grouped based upon losing ≥5% and <5% of their initial weight at month 6, regardless of their initial group assignment. Figure 1C shows that PHQ scores decreased by −2.5±0.4 in participants who lost ≥5% of weight and by a significantly (p <0.0001) smaller −0.1±0.3 in those who lost <5%. At month 24, mean PHQ scores decreased by −1.6±0.5 in participants who lost ≥5% of weight and by a significantly (p=0.047) smaller −0.5±0.4 in those who lost <5%.
Additional analyses examined categorical changes in mood (i.e., from one category of severity to another) in the 190 individuals who at baseline reported mild or greater symptoms of depression. At month 6, 84.2% of participants who lost ≥5% of initial weight improved (declined) by one or more categories of severity (e.g., from mild to no/minimal symptoms of depression) compared with 66.4% of participants who lost <5% of weight (p=0.014). (Mean changes in PHQ scores for these two groups were −4.7±4.4 and −1.0±4.4, respectively [p<0.0001], from baseline values of 8.6±3.7 and 8.5±3.3, respectively.) At month 24, 82.2% of participants who lost ≥5% of initial weight improved by one or more categories of severity, compared with 68.6% of participants who lost <5% of initial weight. The difference between groups was no longer statistically significant (p=0.083). (Mean changes in PHQ scores for these two groups were −3.5±3.8 and −1.7±4.5, respectively; p=0.03.)
Of the 171 participants who scored 0–4 (i.e., no/minimal depression) on the PHQ-8 at baseline, 27.9% of those who lost ≥5% of initial weight increased by one category of depression severity or more (e.g., from no/minimal depression to mild or greater), as compared with a significantly (p=0.042) greater 43.6% among those who lost <5% of weight. The benefit of weight loss in preventing symptoms of depression remained at month 24, when corresponding values were 24.5% and 44.3%, respectively (p=0.018).
DISCUSSION
This 2-year randomized controlled trial found that three different behavioral weight loss interventions, delivered in primary care practice by practice staff, helped approximately one fifth to one third of participants in the three groups lose 5% or more of initial weight. Losses of this magnitude are considered clinically meaningful because of their reduction of traditional CVD risk factors, including triglyceride levels, blood pressure, and blood glucose.1,2 The present study found that a loss ≥5% also was associated with improvements in two behavioral risk factors for CVD – short sleep duration and depression.
At month 6, participants who lost ≥5% of initial weight reported a 21.6±7.2 min/night increase in sleep duration, which was significantly greater than the 1.2 ± 6 min/night increase in participants who lost <5% of weight. In addition, more participants in the former group than latter (30.7 vs 19.5%; p<0.061) achieved optimal sleep duration (i.e., 7–8 hours nightly). Improvements in PSQI scores followed the same pattern at month 6 in participants who lost ≥5% vs. <5% of initial weight. However, when these same analyses were repeated at month 24, no significance differences were observed between the two groups on either of these outcomes, with changes tending to be more favorable in participants who lost <5% of initial weight.
The present findings are similar to those reported by Vorhoef et al12 whose participants lost 10% of initial weight in 2 months (on a very low energy diet) and reported a 24 minute increase in sleep duration. Chaput et al13 reported similar improvements in sleep quality with a 5% reduction in initial weight. Similar to the present study, however, the improvements in sleep duration and quality in these studies were not maintained at follow-up.
We observed only one significant difference in sleep duration or quality between the three randomized treatment groups in the POWER-UP trial, potentially because the differences in weight loss between the Usual Care group and two lifestyle interventions were not sufficiently large (ranging from 1.5 to 4.6 kg at month 6 and from 1.7 to 2.9 kg at month 24). Rubin et al31 similarly observed no significant differences in sleep quality between the control and two intervention groups in the 2-year Hopkins POWER trial, which used similar methods and outcomes as our POWER-UP study. These investigators, however, did not include analyses that examined the percentage of participants who achieved optimal sleep duration or the effects on outcomes of losing ≥5% vs <5%. Additional studies of the relationship between weight loss and sleep quality are needed in which participants achieve a larger range of weight losses, as compared with those obtained in the two POWER studies.19,31 In addition, study is needed of factors associated with the deterioration in improved sleep quality, observed in participants in this study who lost ≥5% of initial weight at month 6.
In contrast to sleep, the improvements in mood observed with weight loss were better maintained over the full 2 years of the study. Participants who lost ≥5% of initial weight, compared with those who lost less, had larger mean reductions on the PHQ-8 at both months 6 and 24. In addition, more than 80% of participants who began the study with mild or greater symptoms of depression, and proceeded to lose ≥5% of initial weight, potentially achieved a clinically meaningful improvement in mood, as indicated by a decline in depression severity of one category or more (e.g., from mild to no depression or moderate to mild depression). A significantly smaller portion of participants who lost <5%, compared with ≥5%, achieved this categorical improvement at 6 months (66.4% vs 84.2%). Other behavioral interventions have reported similar improvements in mood with weight loss,3–7 which probably reflect participants’ enhanced physical function, body image, and self-esteem. (We note that neither the present study, nor the Hopkins’ POWER trial, observed significant differences between intervention and usual care conditions on changes in symptoms of depression, again probably because of the limited differences in weight loss observed between groups.)
In addition to its association with improvements in mood in participants with mild or greater symptoms of depression at baseline, losing ≥5% of initial weight was associated with significantly lower rates of depression onset (i.e., mild or greater symptoms) in participants who reported no mood disturbance at baseline. This finding parallels results of the Look AHEAD study in which an intensive behavioral weight loss program was associated with a 15% reduction in the risk of developing mild or greater symptoms of depression over 8 years of treatment and follow-up.3 The present study would have been improved by the inclusion of a diagnostic interview to assess mood, rather than relying upon a self-report questionnaire.
Strengths of the present study include its large and diverse sample of participants, relative to previous studies of this topic. Our study (along with the Hopkins POWER trial) also had a longer duration of intervention and follow up (24 months) than prior studies and a high rate of retention (86%). This investigation also had limitations, including the lack of an objective measure of sleep duration and the absence of larger mean weight losses, which may be associated with even greater improvements in sleep duration and mood.
In conclusion, the present findings indicate that losing ≥5% of initial weight is associated with short-term improvements in sleep duration and quality, as well as favorable short- and long-term changes in mood in individuals who initially reported mild or greater symptoms of depression. These findings provide additional reasons for primary care practitioners to recommend weight loss to their overweight and obese patients.
What is already known about this subject
Short-term studies have demonstrated increased sleep duration and improved sleep quality and mood in behavioral weight loss interventions.
What this study adds
This 24-month study provides a long-term assessment of changes in sleep duration and mood occurring with weight loss and evaluates the clinical significance of the observed changes.
Weight losses ≥5 % of initial weight were associated with short-term improvements in sleep duration and quality, as well as short- and long-term improvements in mood that appeared to be clinically meaningful in participants who reported mild or greater symptoms of depression at baseline.
References
- 1.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–6. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubin RR, Wadden TA, Bahnson JL, Bray GA, Coday M, Crow SJ, et al. Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: the Look AHEAD Trial. Diabetes Care. 2014;37:1–10. doi: 10.2337/dc13-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faulconbridge LF, Wadden TA, Rubin RR, Wing RR, Walkup MP, Fabricatore AN, et al. One-year changes in symptoms of depression and weight in overweight/obese individuals with type 2 diabetes in the Look AHEAD study. Obesity (Silver Spring) 2012;20:783–93. doi: 10.1038/oby.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wadden TA, Vogt RA, Andersen RE, Bartlett SJ, Foster GD, Kuehnel RH, et al. Exercise in the treatment of obesity: effects of four interventions on body composition, resting energy expenditure, appetite, and mood. J Consult Clin Psychol. 1997;65:269–77. doi: 10.1037//0022-006x.65.2.269. [DOI] [PubMed] [Google Scholar]
- 6.Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med. 2009;169:163–71. doi: 10.1001/archinternmed.2008.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med. 1994;97:354–62. doi: 10.1016/0002-9343(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 8.Anda R, Williamson D, Jones D, Macera C, Eaker E, Glassman A, et al. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of US adults. Epidemiology. 1993;4:285–94. doi: 10.1097/00001648-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93:1976–80. doi: 10.1161/01.cir.93.11.1976. [DOI] [PubMed] [Google Scholar]
- 10.Ferketich AK, Schwartzbaum JA, Frid DJ, Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. Arch Intern Med. 2000;160:1261–8. doi: 10.1001/archinte.160.9.1261. [DOI] [PubMed] [Google Scholar]
- 11.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–26. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 12.Verhoef SP, Camps SG, Gonnissen HK, Westerterp KR, Westerterp-Plantenga MS. Concomitant changes in sleep duration and body weight and body composition during weight loss and 3-mo weight maintenance. Am J Clin Nutr. 2013;98:25–31. doi: 10.3945/ajcn.112.054650. [DOI] [PubMed] [Google Scholar]
- 13.Chaput JP, Drapeau V, Hetherington M, Lemieux S, Provencher V, Tremblay A. Psychobiological impact of a progressive weight loss program in obese men. Physiol Behav. 2005;86:224–32. doi: 10.1016/j.physbeh.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Clark A, Lange T, Hallqvist J, Jennum P, Rod NH. Sleep impairment and prognosis of acute myocardial infarction: a prospective cohort study. Sleep. 2014;37:851–8. doi: 10.5665/sleep.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 16.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15:42–50. doi: 10.1016/j.sleep.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen LS, Danielsen KV, Sorensen TI. Short sleep duration as a possible cause of obesity: critical analysis of the epidemiological evidence. Obes Rev. 2011;12:78–92. doi: 10.1111/j.1467-789X.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- 18.Sabanayagam C, Shankar A. Sleep duration and cardiovascular disease: results from the National Health Interview Survey. Sleep. 2010;33:1037. doi: 10.1093/sleep/33.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two- year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969–79. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Borushek A. The CalorieKing Calorie, Fat, & Carbohydrate Counter: Family Health. 2013 [Google Scholar]
- 24.National Heart, Lung, and Blood Institute. Aim for a Healthy Weight. National Institutes of Health; Bethesda, MD, USA: 2005. p. 05–5213. [Google Scholar]
- 25.James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Eng J Med. 2010;363:905–17. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 26.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–61. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 27.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–23. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. DSM IV. 4. American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin RR, Peyrot M, Wang NY, Coughlin JW, Jerome GJ, Fitzpatrick SL, et al. Patientreported outcomes in the practice-based opportunities for weight reduction (POWER) trial. Qual Life Res. 2013;22:2389–98. doi: 10.1007/s11136-013-0363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]