Abstract
It was recently demonstrated that herpes simplex virus (HSV) successfully infects Chinese hamster ovary (CHO) cells expressing glycoprotein D (gD) receptors and HeLa cells by an endocytic mechanism (A. V. Nicola, A. M. McEvoy, and S. E. Straus, J. Virol. 77:5324-5332, 2003). Here we define cellular and viral requirements of this pathway. Uptake of intact, enveloped HSV from the cell surface into endocytic vesicles was rapid (t1/2 of 8 to 9 min) and independent of the known cell surface gD receptors. Following uptake from the surface, recovery of intracellular, infectious virions increased steadily up to 20 min postinfection (p.i.), which corresponds to accumulation of enveloped virus in intracellular compartments. There was a sharp decline in recovery by 30 min p.i., suggesting loss of the virus envelope as a result of capsid penetration from endocytic organelles into the cytosol. In the absence of gD receptors, endocytosed virions did not successfully penetrate into the cytosol but were instead transported to lysosomes for degradation. Inhibitors of phosphatidylinositol (PI) 3-kinase, such as wortmannin, blocked transport of incoming HSV to the nuclear periphery and virus-induced gene expression but had no effect on virus binding or uptake. This suggests a role for PI 3-kinase activity in trafficking of HSV through the cytosol. Viruses that lack viral glycoproteins gB, gD, or gH-gL were defective in transport to the nucleus and had reduced infectivity. Thus, similar to entry via direct penetration at the cell surface, HSV entry into cells by wortmannin-sensitive endocytosis is efficient, involves rapid cellular uptake of viral particles, and requires gB, gD, and gH-gL.
Entry of herpes simplex virus (HSV) is a complex process comprised of several events that can be studied individually. These include binding of the virus to the cell surface, penetration of a cell membrane via fusion of the viral envelope with either the plasma membrane or an intracellular membrane, transport of the virion or virion components from the cell periphery to the nuclear periphery, binding of the genome-containing capsid to the nuclear pore complex, and injection of the viral DNA, culminating in viral gene expression.
In cell types such as Vero, HSV penetrates the plasma membrane and delivers its capsid directly into the cytosol. Microtubules then propel the naked capsids to the nuclear region (15, 45, 48). However, in other common continuous cell lines, endocytosis of virions is required to initiate successful entry of HSV (33). Two such cell types are Chinese hamster ovary (CHO) cells engineered to express entry receptors, such as HveA (HVEM), nectin-1, or nectin-2, that bind the HSV glycoprotein D (gD), and HeLa cells. In these cells, entry, as measured by virus-induced gene expression, is inhibited by agents that block either uptake from the cell surface or endosomal acidification (33).
In HeLa and CHO-nectin-1 cells, HSV uptake by endocytosis can be defined as delivery of intact, enveloped particles from the cell surface to intracellular vesicles. This is followed by penetration from an endocytic compartment, in which the capsid is deposited into the cytosol as a result of its separation from the viral envelope.
Not all cases in which HSV has been detected in vesicles at early times postinfection (p.i.) indicate the onset of productive virus infection. For example, particles in vesicles do not initiate replication when either the cells overexpress gD (6) or when an entry-defective mutant strain is used (51). Moreover, in Vero cells, endocytosed HSV does not seem to result in productive infection (15, 24, 45). Penetration of HSV at the plasma membrane of Vero cells is rapid, efficient, and complete by ∼30 min (15, 21, 24, 45). In Vero cells, unenveloped, cytosolic capsids appear within minutes of infection (15, 33, 45), whereas degraded, enveloped virions in vesicles appear at 1 h p.i. and later (45). Because enveloped HSV is detected in endocytic compartments of Vero cells after successful penetration has already taken place, it is unlikely that endocytosed virions have an important role for entry into these cells. For these reasons, uptake by endocytosis as a means of infection by HSV has not been widely studied.
The goal of the present work is to characterize molecular and cellular details of the endocytic entry pathway that leads to productive infection by HSV. The presence of a gD receptor is necessary for the successful infection of CHO cells by endocytosis (16, 28, 33). The precise function of the interaction of gD with its receptor in the HSV entry process remains unclear. It has been proposed that binding of virions to a gD receptor initiates membrane fusion, which results in penetration of the capsid into the cytosol. The fact that cell fusion induced by the expression of HSV glycoproteins gB and gD and the gH-gL heterodimer requires the presence of a gD receptor has been used to support this notion (48). In the present study, we address the step(s) in the endocytic entry pathway at which this virus-receptor interaction is required: uptake by endocytosis, penetration from an intracellular compartment, or both.
As with many viruses (43, 44), HSV, once taken up by endocytosis, likely exploits cellular retrograde transport mechanisms to deliver its genetic material to the site of replication. Trafficking of endocytic vesicles is a highly regulated process involving the coordinated action of cytoskeletal elements and regulatory molecules, such as kinases and GTPases (11). Phosphatidylinositol (PI) 3-kinase is involved in many cellular processes, including internalization, cell cycle progression, cytoskeletal rearrangements, and membrane trafficking (49). PI 3-kinase is also implicated in virus entry pathways. For example, the endocytic entry of adenovirus (25), Semliki Forest virus (27), and human herpesvirus 8 (HHV-8) (30) has been proposed to require PI 3-kinase activity, based in part on studies that used PI 3-kinase inhibitors such as wortmannin and LY294002.
Penetration of HSV through the Vero plasma membrane requires viral envelope glycoproteins gB and gD and the gH-gL heterodimer. Virus mutants devoid of these glycoproteins fail to penetrate the cell surface (5, 14, 26, 39). Furthermore, antibodies specific for gB, gD, and gH can neutralize entry into and infection of Vero cells (10, 15, 17, 20, 31, 34, 37). An additional purpose of the present study, then, was to examine viral glycoprotein determinants of endocytic entry of HSV.
The series of studies reported here delineate cellular and viral requirements of HSV entry via endocytosis. We demonstrate that (i) endocytic uptake of enveloped HSV from the cell surface is rapid and independent of the known gD receptors; (ii) efficient entry via endocytosis requires cellular PI 3-kinase activity; and (iii) the viral glycoproteins gB, gD, and gH-gL are required for endocytic entry of HSV. Although entry of HSV by endocytosis is comprised of processes that are molecularly and spatially distinct from those of penetration at the plasma membrane, both pathways are rapid and efficient and require a similar set of envelope glycoproteins.
MATERIALS AND METHODS
Cells and viruses.
CHO IEβ8 cells (provided by P. Spear, Northwestern University) are CHO-K1 cells stably transformed with the Escherichia coli lacZ gene under the control of the HSV ICP4 promoter (28). CHO IEβ8 cells that were further stably transformed to express nectin-1 (16) (provided by G. Cohen and R. Eisenberg, University of Pennsylvania) were propagated in Ham's F12 medium (Life Technologies, Grand Island, N.Y.) supplemented with 10% fetal calf serum (FCS; Life Technologies), 150 μg of puromycin (Sigma, St. Louis, Mo.)/ml, and 250 μg of Geneticin (Life Technologies)/ml. Cells were subcultured in nonselective medium prior to use in all experiments. HeLa and Vero cells (both from American Type Culture Collection, Rockville, Md.) were propagated in Dulbecco's modified Eagle's medium (Life Technologies) supplemented with 10% FCS.
Wild-type HSV-1 strain KOS and its derivative KOS 7134, which has the lacZ gene in place of the immediate-early ICP0 gene (4), were obtained from P. Schaffer (Harvard University). HSV-1 KOS K26GFP contains green fluorescent protein (GFP) fused to the N terminus of the VP26 capsid protein (13) (provided by P. Desai, Johns Hopkins University).
All HSV mutants studied here are in the KOS strain background. KO82 lacks gB coding sequences and is propagated on gB-expressing Vero cells designated D6 (5) (both provided by S. Person, Johns Hopkins University). Viral mutant gCΔ2-3 (provided by C. Brandt, University of Wisconsin) lacks gC coding sequences and is grown on Vero cells. KOSgDβ lacks gD coding sequences and is propagated on gD-expressing Vero cells designated 15D1 (12) (both provided by P. Spear). KOS gLβ lacks gL coding sequences and is propagated on gL-expressing Vero cells designated VL60 (39) (both provided by D. Johnson, Oregon Health Sciences University). The number of DNA-containing particles in each virus preparation was quantitated by real-time PCR.
Protease protection assay for uptake of HSV particles.
Radiolabeled HSV was prepared by infecting Vero cells grown to 90% confluence in a 175-cm2 flask with HSV-1 KOS at a multiplicity of infection (MOI) of 2. At 6 h p.i., inoculum was removed and medium containing 0.75 mCi of [35S]methionine plus [35S]cysteine (ICN Biomedical) was added. After detection of significant cytopathic effect but prior to complete detachment of the cell monolayer, the virus-containing medium was collected and spun at 1,600 × g to remove cell debris.
Radiolabeled HSV (specific activity of 1 cpm/PFU) was bound to CHO or CHO-nectin-1 cells at 4°C for 2 h. The cells were washed four times with phosphate-buffered saline (PBS) to remove unbound virus. There was no discernible difference in the amount of virus bound to CHO or CHO-nectin-1 cells (typically 10,000 to 20,000 cpm). Culture temperatures were raised to 37°C for various lengths of time. Cells were washed twice and then incubated with 2 mg of proteinase K (Invitrogen, Carlsbad, Calif.)/ml or were mock treated for 1 h on ice. Proteinase K treatment removed ∼90% of radioactivity bound to control cells that were kept at 4°C without temperature shift. Proteolysis was halted by adding 62.5 mM phenylmethylsulfonyl fluoride and 1.5% bovine serum albumin (BSA; Sigma). The cells were collected and the wells were washed with PBS supplemented with 0.2% BSA. The cells and wash buffer were spun at 200 rpm for 5 min. Cells were washed two additional times, and then the pellets were solubilized. Radioactivity was measured in a liquid scintillation counter. For a given time point, the counts per minute (cpm) that remained cell associated following protease treatment relative to the total cpm bound in the corresponding untreated sample represents HSV that had been endocytosed. About 50 to 60% of total virus bound to CHO and CHO-nectin-1 cells was ultimately taken up by 30 to 120 min.
Transmission electron microscopy.
HSV-1 (KOS) was added to cells (MOI of 50) for 2 h at 4°C to allow binding to occur. Cells were washed three times with PBS and then were incubated at 37°C for 3 min or 1 h. Infected cells were fixed in 2% glutaraldehyde, embedded, thin sectioned, and stained as previously described (33). Samples were examined and photographed with a Hitachi H7000 electron microscope (National Cancer Institute, Frederick, Md.).
Uptake of infectious HSV by endocytosis.
HSV-1 KOS was bound to CHO-nectin-1 cells on coverslips for 2 h at 4°C (MOI of 0.5). Cells were washed twice with PBS and were then incubated at 37°C, and extracellular virus was inactivated by treatment for 1 min with sodium citrate buffer (pH 3.0) at the indicated times. At 8 h p.i., cells were washed twice with PBS and fixed in methanol. Coverslips were air dried and blocked for 1 h with PBS containing 1% BSA. Cells were then incubated with anti-HSV polyclonal antibody HR50 (Fitzgerald Industries, Concord, Mass.) for 1 h. Following addition of Alexa Fluor 488-labeled goat anti-rabbit secondary antibody (Molecular Probes, Eugene, Oreg.), 400 to 500 cells per sample were evaluated by fluorescence microscopy. Nuclei were stained with 25 ng of 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI; Roche Diagnostics, Indianapolis, Ind.)/ml to determine the total cell number, and Alexa Fluor 488-positive cells were quantitated. Maximum infectivity was set to 100%.
Intracellular trafficking of enveloped, infectious HSV.
HSV-1 strain KOS was added to confluent cell monolayers for 2 h on ice at an MOI of 50. Cells were washed with PBS and then were incubated at 37°C for the indicated times. Extracellular virus was inactivated by adding Ham's F12 medium buffered to pH 3.0 for 1 min at 37°C (33). Monolayers were immediately put on ice and washed with cold PBS. One milliliter of Ham's F12 medium with 20 mM HEPES and 1% FCS was added, and cells were lysed by two cycles of freezing and thawing. The titers of lysates were determined on Vero cells. Each value shown is the mean of triplicate determinations.
β-Galactosidase reporter assay.
Confluent cell monolayers grown in 96-well dishes were infected with HSV-1 (strain KOS for CHO-nectin-1 cells or strain 7134 for Vero or HeLa cells) at an MOI of 1. Cells were treated with a range of concentrations of wortmannin or calphostin C (Sigma) or were mock treated at 37°C for 30 min prior to addition of virus. Infected cells were incubated at 37°C in the presence or absence of inhibitor for 6 h. Cells were lysed in 0.5% Nonidet P-40, chlorophenol red-β-d-galactopyranoside (Roche Diagnostics) was added, and β-galactosidase activity was determined by absorbance at 560 nm using a microtiter plate reader (Dynatech, Chantilly, Va.). Mean results were calculated for four replicate samples. The β-galactosidase activity from infected but mock-treated cells was defined as 100%.
Binding of radiolabeled virus to cells.
HSV labeled with [35S]cysteine and [35S]methionine was added to CHO-nectin-1 cells in HAM's F12 medium buffered with 20 mM HEPES for 2 h at 4°C in the presence of 200 nM wortmannin or 100 μg of heparin (Sigma)/ml. Cells were washed three times with PBS, and then 0.5% Nonidet P-40 lysates were prepared. Radioactivity was measured in a liquid scintillation counter. Cell-associated cpm represent bound virus.
Effect of inhibitors on uptake of infectious HSV.
HSV-1 KOS was bound to CHO-nectin-1, HeLa, or Vero cells on coverslips for 2 h at 4°C (MOI of 0.5). Cells were washed with cold PBS and incubated in medium containing 200 nM wortmannin or glucose-free medium supplemented with a solution of 2% BSA, 0.05% sodium azide, and 0.3% 2-deoxy-d-glucose for 15 min at 4°C. Cultures were shifted to 37°C in the presence of inhibitors for 1 h. Media containing inhibitors were removed and extracellular virus was inactivated by treatment with sodium citrate buffer (pH 3.0). Cells were washed with PBS and incubated for 7 h at 37°C in normal culture medium. Cells were fixed in methanol, and infected cells were detected by fluorescence microscopy as described above for uptake of infectious HSV by endocytosis.
Fluorescence microscopy of HSV transport to the nucleus.
Cells were grown on glass coverslips overnight. Cultures were then chilled on ice, and either HSV-1 (KOS), glycoprotein mutant derivatives, or K26GFP was added at an MOI of 20 for 2 h on ice. Cells were washed with PBS, after which cultures were warmed to 37°C for 2.5 h in medium containing cycloheximide. Cells were washed with PBS and then fixed in methanol. Alternatively, cells infected with HSV K26GFP were fixed with 3% paraformaldehyde and permeabilized with 0.2% Triton X-100. Cells on coverslips were blocked for 1 h with PBS containing 1% BSA. Nuclei were counterstained with DAPI. Virions were visualized by staining with a 2-μg/ml concentration of a mouse monoclonal antibody against HSV-1 VP5 (ABI Technologies, Rockville, Md.) followed by an Alexa Fluor 488-labeled goat-anti-mouse secondary antibody. Coverslips were washed with PBS, mounted with Fluoromount G (Electron Microscopy Sciences, Fort Washington, Pa.), and viewed with a Zeiss Axioplan 2 imaging microscope. Digital images were captured with a Zeiss AxioCam using Openlab 3.1 (Improvision, Lexington, Mass.) and were processed with Adobe Photoshop 6.0.
Immunofluorescence assay for infectivity of glycoprotein-null HSVs.
Cells on coverslips were infected with HSV mutants that lacked gB, gC, gD, or gH-gL in their viral envelopes or with complemented mutant HSVs grown on the appropriate glycoprotein-expressing cell lines. Such complemented HSV mutants are genotypically negative for gB, gD, or gL but contain a full set of glycoproteins in the envelope. At 10 h p.i., infected cells were detected as described above for uptake of infectious virus by endocytosis. A value of 100% represents the level of infection of an equivalent number of particles of complemented virus. Control samples were infected with complemented virus at an MOI of 0.5.
RESULTS
Rapid uptake of HSV by endocytosis.
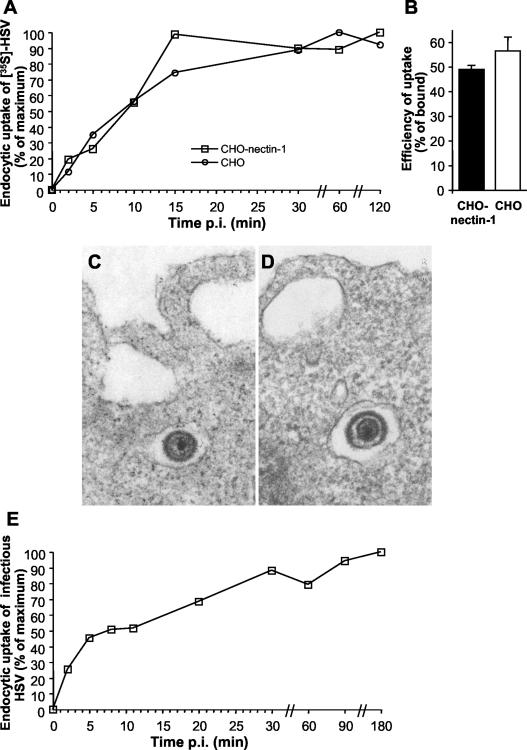
To determine the kinetics of HSV uptake by endocytosis, we used a protease protection assay (18) to measure the uptake of radiolabeled virions by CHO-nectin-1 cells. Upon warming to 37°C and endocytic uptake, 35S-labeled HSV is protected within the cells from Proteinase K treatment. We found that radiolabeled virus was endocytosed rapidly with a half-life (t1/2) of ∼9 min (Fig. 1A). More than 90% of uptake had occurred by 30 min. The rate of endocytic uptake of HSV in CHO-nectin-1 cells is similar to that reported for HSV penetration of the plasma membrane of other cell types, such as Vero cells (21, 24, 45). Thus, uptake of HSV from the cell surface, whether it occurs via endocytosis or by direct penetration, is rapid.
FIG. 1.
Kinetics of HSV uptake by endocytosis and role of gD receptors. (A) Role of nectin-1 in uptake from the cell surface. 35S-labeled virus was added to CHO cells, which lack any known gD receptors, or CHO-nectin-1 cells for 2 h at 4°C. Following shift to 37°C for the time indicated as minutes p.i., nonendocytosed virus was removed by treatment with 2 mg of proteinase K/ml or cells were mock treated to determine the amount of total virus bound. The fraction of total cell-associated cpm that is protected from protease treatment reflects HSV that has been endocytosed. Uptake at time zero was set to 0%, and maximum uptake was set to 100%. Each value is the mean of triplicate determinations. (B) Efficiency of uptake. Uptake of radiolabeled HSV at 30 min p.i. was determined as described for panel A. Efficiency is defined here as protease-resistant cpm out of cpm bound. Shown are the mean values of triplicate samples with standard error. Data are representative of four similar experiments. EM analysis of HSV uptake by CHO-nectin-1 (C) cells or CHO cells (D). HSV-1 KOS (MOI of 50) was bound to cells for 2 h at 4°C followed by a shift to 37°C for 3 min, and then cells were processed for EM. Magnification, ×90,000. Enveloped virions are 150 to 200 nm in diameter. (E) Uptake of infectious HSV from the cell surface. HSV-1 KOS was bound to CHO-nectin-1 cells for 2 h at 4°C (MOI of 0.5). Cells were washed with PBS and incubated at 37°C, and extracellular virus was inactivated by acid treatment at the indicated times. At 8 h p.i. cells were fixed and random fields of 400 to 500 cells per sample were evaluated. Total cell number was evaluated by nuclear staining with DAPI, and infected cells were detected by immunofluorescence with an anti-HSV polyclonal antibody. Maximum infectivity was set to 100%.
Endocytic uptake of HSV is independent of gD receptors.
CHO cells are inherently resistant to HSV infection. Expression of a gD receptor, such as nectin-1, is required for HSV to initiate virus-induced gene expression in CHO cells (16, 28). Interaction with gD-binding receptors is a well-characterized event during HSV entry (7, 8, 47). However, the precise role of these entry receptors in initiating HSV infection remains unclear.
We explored the possibility that gD receptors might mediate the virus uptake step. The rate of 35S-HSV uptake from the surface of CHO-nectin-1 cells (Fig. 1A) was compared to that of wild-type CHO cells, which lack any known gD receptors. Uptake of HSV into CHO cells was rapid and similar to that demonstrated for CHO-nectin-1 cells, with a t1/2 of ∼9 min (Fig. 1A). To compare the efficiency of uptake, the amount of radiolabeled virus taken up by the two cell types by 30 min was measured. CHO-nectin-1 and CHO cells endocytosed 49% and 57% of bound virus, respectively (Fig. 1B), indicating similar, efficient uptake of HSV. Thus, the presence of a known entry receptor, such as nectin-1, does not promote endocytic uptake of HSV into CHO cells. Rather, a gD receptor is required at another step or steps in the infectious process, such as anchoring the virus to a cellular membrane and/or penetration from an endocytic compartment into the cytosol.
Transmission electron microscopy (EM) was used to confirm that uptake of HSV by endocytosis is fast and independent of gD receptor. By 3 min after infection, intact, enveloped herpesvirions were detected in intracellular vesicles adjacent to the plasma membrane of both CHO-nectin-1 cells and CHO cells (Fig. 1C and D). Virions were present in smooth-walled vesicles in both cells, but from this analysis we could not determine whether these carrier vesicles were identical. Fusion of HSV with the plasma membrane of either CHO cell line was not detected. By contrast, when infected Vero cells were analyzed at 3 min p.i., many virions fusing with the plasma membrane were observed but virion-containing vesicles were not seen (data not shown). This series of experiments indicates that endocytic uptake of HSV from the cell surface is rapid, efficient, and not dependent on any of the known entry receptors.
Endocytic uptake of infectious HSV is rapid.
The above studies of radiolabeled HSV and the EM analyses revealed that enveloped virions were endocytosed but could not distinguish whether the particles were infectious or noninfectious. To measure specifically the rate of uptake of infectious particles, virus was first bound to CHO-nectin-1 cells at 4°C. At various times after warming of cultures to 37°C, extracellular virus was inactivated. Cultures were incubated for a total of 8 h, and cells that expressed newly synthesized viral antigen were quantitated by immunofluorescence microscopy. The acquisition of viral resistance to inactivation, which reflects uptake by endocytosis, was fast (t1/2 of ∼8 min) (Fig. 1E). More than 80% of infectious HSV particles were endocytosed by 30 min. Thus, the rate of uptake of infectious virus, like that of the bulk of virus particles, was rapid in CHO-nectin-1 cells. As expected (28), when wild-type CHO cells lacking any known gD receptors were infected under identical conditions, no newly synthesized viral antigen was detected (data not shown). Thus, by the criteria tested here, whether or not an HSV particle has the potential to complete eventually the infectious process has no discernible bearing on its initial uptake.
Endocytic trafficking of HSV.
Following uptake, HSV or its subvirion components must use cellular transport machinery to traverse the cytosol and to ultimately arrive at the nuclear membrane. To investigate the kinetics of intracellular transport of HSV, the infectivity of virions taken up by CHO-nectin-1 cells was determined. At various times after infection, virus particles that were still bound to the cell surface and had not been endocytosed were inactivated. Cell lysates were prepared and assayed for plaque formation. In this assay, particles that are competent to form plaques should represent enveloped, infectious virions that were protected within intracellular vesicles.
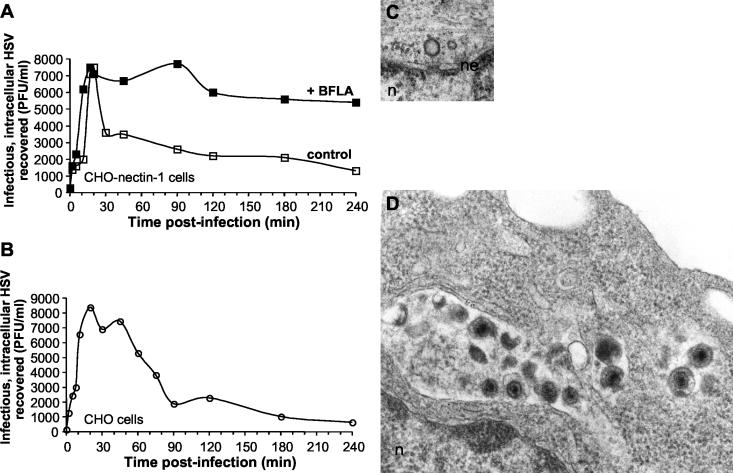
In CHO-nectin-1 cells, recovery of intracellular, infectious virus increased steadily for up to about 20 min p.i. (Fig. 2A). This initial, rapid accumulation of HSV in endocytic vesicles is similar to the rate at which particles are taken up from the surface (Fig. 1). Moreover, previous EM analyses of infected CHO-nectin-1 cells revealed intact, enveloped virions in smooth-walled vesicles at 20 min p.i (33). By 30 min after infection there was a sharp decline in recovery of infectious virus (Fig. 2A). This decline suggests that by this time HSV begins to lose its envelope, while its capsid penetrates from an endocytic compartment into the cytosol. By 4 h p.i., ∼80% of intracellular virions had lost their infectivity, yet ∼20% infectivity remained. It is not known whether this residual virus would, in time, have completed the infectious process.
FIG. 2.
Intracellular trafficking of HSV. HSV-1 KOS was bound to CHO-nectin-1 cells (A) or CHO cells (B) for 2 h at 4°C. Cultures were shifted to 37°C in the presence or absence of 200 nM BFLA. At the indicated time p.i., following inactivation of extracellular virus, cells were lysed by two cycles of freezing and thawing. The numbers of infectious, intracellular particles were assayed by plaque titration on Vero cells. Data shown are representative of at least three independent experiments. For ultrastructural analysis, HSV-1 KOS (MOI of 50) was bound to cells for 2 h at 4°C followed by a shift to 37°C for 1 h, and then samples were processed for EM. (C) Naked, empty viral capsid docked at the nuclear pore complex of a CHO-nectin-1 cell. (D) Accumulation of degraded HSV particles in CHO cells. Enveloped virions are 150 to 200 nm, and capsids are ∼100 nm in diameter. Magnification, 90,000×. n, nucleus; ne, nuclear envelope.
Having shown that most endocytosed virions retain their infectivity for at least 20 to 30 min, we sought to understand the process by which their capsids are released. Our hypothesis was that enveloped particles release their capsids into the cytosol upon arrival in an acidic compartment (33). To this end, we studied the effects of bafilomycin A1 (BFLA), a lysosomotropic agent that elevates endosomal pH by inhibiting vacuolar H+-ATPase (38). It was previously shown that BFLA inhibits the transport of incoming HSV to the nuclei of CHO-nectin-1 cells (33). This inhibition is partially reversible (data not shown).
BFLA treatment had no effect on recovery of infectious virus within the cell for up to 20 min p.i. (Fig. 2A). This suggests that this agent does not alter uptake or early trafficking of HSV. However, treatment with BFLA completely inhibited the loss of particle infectivity. Nearly all of the infectivity recovered within cells at 20 min p.i. persisted for as long as 4 h p.i. in the presence of the agent (Fig. 2A). This finding is consistent with the trapping of enveloped, infectious virions in neutralized endocytic vesicles. It also indicates that sustained exposure to the abnormally elevated intravesicular pH did not destroy virion infectivity.
In the absence of gD receptors, HSV undergoes lysosomal degradation.
The recovery of infectious, intracellular particles was used to monitor the trafficking of HSV in wild-type CHO cells, which lack any known gD receptors and do not support successful infection (28). In these cells, as in CHO-nectin-1 cells (Fig. 2A), there was a steady increase in yield of infectious virions for the first 20 min (Fig. 2B). This reflects enveloped virions that were rapidly taken up into the cell and accumulated in vesicles. Thus, by the approaches used here, there are no discernible differences between CHO and CHO-nectin-1 cells regarding HSV binding to the plasma membrane, uptake from the cell surface, or early intracellular trafficking. However, demonstration that endocytic transport is identical in the presence or absence of gD receptor awaits identification of the compartments through which HSV is transported. Maximal recovery of intracellular, infectious virus from CHO cells declined from 30 min to 90 min p.i. (Fig. 2B). This decline occurred at a reproducibly slower rate than that seen with CHO-nectin-1 cells (Fig. 2A). Because infectious virions do not complete the entry process in CHO cells, the loss of infectivity here was presumed to represent gradual degradation of virions and not penetration into the cytosol.
Indeed, at 1 h p.i. multiple damaged virions were detected by EM in large vesicles or lysosomes of wild-type CHO cells (Fig. 2D). Unenveloped capsids were not detected in the cytosol. By contrast, in CHO-nectin-1 cells, virions were not found in lysosomes at 1 h p.i. Instead, intact virions were seen in endocytic vesicles, and unenveloped capsids were present in the cytosol of these CHO-nectin-1 cells. Figure 2C shows a capsid docked at the nuclear pore complex with a penton vertex facing the nucleus of a CHO-nectin-1 cell. The absence of an electron-dense core indicates that the viral DNA had been injected into the nucleus by 1 h p.i. Similar images of capsid docking have been reported following infection of Vero cells (3, 45). Together, these results indicate that HSV initiates productive infection of CHO-nectin-1 cells by traveling an endocytic pathway that terminates at the lysosome. In the absence of virus interaction with a gD receptor, at the cell surface and/or inside an endocytic compartment, enveloped virions cannot penetrate the host cell membrane and are delivered to lysosomes for degradation.
Wortmannin inhibits HSV entry into CHO-nectin-1 and HeLa cells.
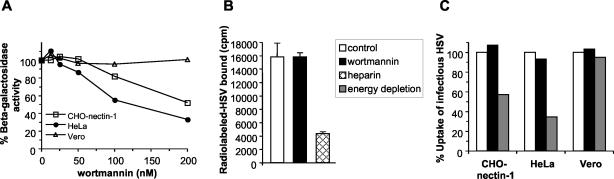
Endocytic pathways utilized for entry by viruses such as adenovirus (25), influenza virus (42), and HHV-8 (30) are dependent on cellular kinase activities. To probe further the mechanism utilized by HSV to initiate infection, the effect of protein kinase inhibitors was assessed. Wortmannin is a fungal metabolite that inhibits the mammalian PI 3-kinases (49). We found that treatment of CHO-nectin-1 cells or HeLa cells with wortmannin inhibited HSV entry, as measured by virus-induced expression of β-galactosidase at 6 h p.i (Fig. 3A). Specifically, entry was inhibited by ∼50% at a concentration of 200 nM. In contrast, wortmannin treatment of Vero cells had no effect on HSV entry in this assay (Fig. 3A). Treatment with calphostin C, an inhibitor of various isoforms of protein kinase C, had no effect on HSV entry in any of the cell types tested (data not shown). Under the conditions tested, the kinase inhibitors had no cytotoxic effects (data not shown).
FIG. 3.
Effect of wortmannin on HSV entry. (A) CHO-nectin-1, HeLa, or Vero cells were pretreated with the indicated concentrations of wortmannin for 30 min. Cells were infected with the HSV-1 strain KOS (for CHO-nectin-1 cells) or lacZ+ KOS 7134 (for HeLa and Vero cells) at an MOI of 1 for 6 h in the continued presence of agent. Entry was measured as the percentage of β-galactosidase activity relative to that obtained in the absence of wortmannin. (B) Effect of wortmannin on virus binding to cells. 35S-labeled HSV was added to CHO-nectin-1 cells (MOI of 3) for 2 h at 4°C in the presence of 200 nM wortmannin or 100 μg of heparin/ml. Cells were washed three times, and then cell-associated cpm of detergent lysates were determined. Each value is the mean and standard deviation of triplicate determinations. The legend applies to both panels B and C. (C) Effect of wortmannin on uptake of infectious virus. HSV was bound to CHO-nectin-1, HeLa, or Vero cells at 4°C for 2 h. Cells were washed and then treated for 15 min at 4°C with medium containing 200 nM wortmannin or with sodium azide and 2-deoxy-d-glucose (energy depletion medium). Cultures were shifted to 37°C in the presence of these agents for 1 h to allow virus to be taken up by endocytosis (CHO-nectin-1 and HeLa cells) or to penetrate directly from the surface (Vero cells). Inhibitor was removed, and extracellular virus was acid inactivated. Cultures were returned to 37°C for 7 h in normal medium without inhibitors. Cells were fixed, and random fields of 400 to 500 cells were evaluated per sample. Total cell number was evaluated by nuclear staining with DAPI, and infected cells were detected by immunofluorescence with an anti-HSV polyclonal antibody. One-hundred percent uptake was taken as the level of infection detected in control untreated cultures. Each value represents the mean of two or three independent experiments.
The studies described above used the β-galactosidase reporter assay, which documents viral entry by quantifying gene expression at 6 h p.i. To define more precisely the step at which PI 3-kinase activity is needed for the HSV entry process, we tested the effect of inhibitors on virion binding and uptake. To measure binding, radiolabeled HSV was bound to CHO-nectin-1 cells in the presence of wortmannin. The inhibitor did not alter virus binding to cells (Fig. 3B). As a positive control (41), virus binding was inhibited in the presence of heparin (Fig. 3B).
We next addressed whether PI 3-kinase is required for uptake of infectious HSV from the cell surface. HSV was bound to cells at 4°C, and then the cultures were shifted to 37°C in the presence or absence of inhibitors for 1 h. Following inactivation of extracellular virus and removal of any inhibitors, immunofluorescence microscopy was used to score infected cells at 8 h p.i. Thus, only infectious particles that were taken up during the 1-h incubation would be detected. In this assay, uptake of infectious HSV from the surfaces of CHO-nectin-1, HeLa, or control Vero cells was not affected by wortmannin (Fig. 3C). As a positive control (33), depletion of cellular energy specifically inhibited the endocytic uptake of HSV in CHO-nectin-1 and HeLa cells but had no effect on virus uptake in Vero cells (Fig. 3C), which occurs via direct penetration at the cell surface.
Inhibitors of PI 3-kinase block transport of incoming HSV to the nucleus.
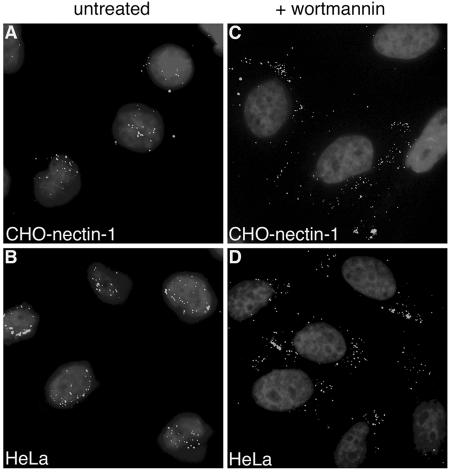
Because wortmannin had no effect on HSV binding or uptake yet inhibited the more-downstream event of gene expression, we examined an intermediate step in the entry process: transport of incoming virus to the nuclear membrane. HSV must traverse the cytosol, and its capsid ultimately docks at the nuclear pore complex. In several cell types the HSV capsid was shown to arrive at the nucleus by 1 to 2.5 h p.i (3, 33, 45) (Fig. 2C). We determined that, at 2.5 h p.i. in the presence of cycloheximide, particles of HSV strain K26GFP, which have GFP fused to the VP26 capsid protein, were distributed primarily at the nuclear periphery of CHO-nectin-1 (Fig. 4A), HeLa (Fig. 4B), or Vero cells (data not shown). In the presence of 10 nM wortmannin (Fig. 4C and D) or 25 μM LY294002, a structurally distinct inhibitor of PI 3-kinase (data not shown), incoming virions were distributed throughout the cytoplasm of HeLa and CHO-nectin-1 cells and were not predominantly localized adjacent to the nucleus. In contrast, in untreated Vero cells, as previously reported (33), or in Vero cells treated with wortmannin (data not shown), particles were predominantly at the nuclear periphery. Similar results were obtained for all cell types tested when wortmannin and LY294002 concentrations as high as 200 nM and 100 μM, respectively, were used (data not shown). These results suggest that cellular PI 3-kinase activity is required for proper trafficking of HSV to the nuclear periphery.
FIG. 4.
Effect of PI 3-kinase inhibitors on the cellular distribution of incoming virions. HSV-1 KOS K26GFP (MOI of 20) was bound to CHO-nectin-1 (A and C) or HeLa (B and D) cells for 2 h at 4°C. Cells were washed with PBS and then warmed in medium containing no agent (A and B) or 10 nM wortmannin (C and D). Infection proceeded for 2.5 h in the presence of cycloheximide. Cells were washed with PBS and fixed in 3% paraformaldehyde. Nuclei were counterstained with DAPI. Punctate fluorescence indicates HSV particles. Cells were viewed with a 63× oil immersion objective.
Envelope glycoprotein requirements for HSV infection via endocytosis.
Thus far we have shown that HSV infection of HeLa and CHO cells, but not Vero cells, requires active endocytosis, intracellular low pH, and PI 3-kinase activity (33) (Fig. 3 and 4). HSV envelope glycoproteins gB, gD, and gH-gL are required for penetration of the Vero plasma membrane (5, 14, 23, 39). We therefore determined whether these same viral glycoproteins are required for HSV infection via the endocytic route. That different sets of glycoproteins could be required in each HSV entry pathway was suggested by observations with the gammaherpesvirus Epstein-Barr virus (EBV). EBV utilizes both endosomal and nonendosomal pathways in different cell types and requires a distinct subset of glycoproteins for each process (32, 48, 50).
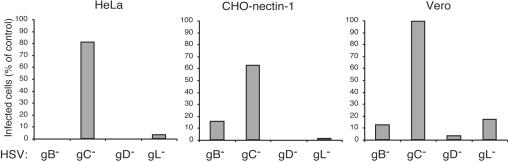
Mutant viruses lacking the individual envelope glycoprotein gB, gC, gD, or gL were assayed for infectivity on HeLa, CHO-nectin-1, and Vero cells. gL is required for processing and incorporation of gH into virions, so the gL-negative HSV lacks both gH and gL in the envelope (39). Viruses lacking gB, gD, or gH-gL were virtually noninfectious in that they were defective in producing newly synthesized viral antigen when added to HeLa or CHO-nectin-1 cells (Fig. 5A and B). Thus, these glycoproteins are essential for infectivity via the endocytic pathway. The gC gene is dispensable for HSV replication (19, 40). As expected, HSV lacking gC infected both HeLa and CHO-nectin-1 cells effectively. The panel of null mutants added to Vero cells had a similar pattern of infectivity (Fig. 5C), as expected from previous studies with these cells (5, 14, 23, 39).
FIG. 5.
HSV glycoprotein requirements for infection of cells that support distinct entry pathways. HeLa, CHO-nectin-1, or Vero cells were infected with HSV-1 strain KOS mutants devoid of gB, gC, gD, or gL for 10 h. Total cell number and HSV antigen-positive cells were quantitated by immunofluorescence microscopy. Four-hundred to 500 cells per sample were evaluated. One-hundred percent represents the infectivity of an equivalent number of particles of the corresponding complemented virus.
gB, gD, and gH-gL are required for transport of incoming HSV to the CHO-nectin-1 cell nucleus.
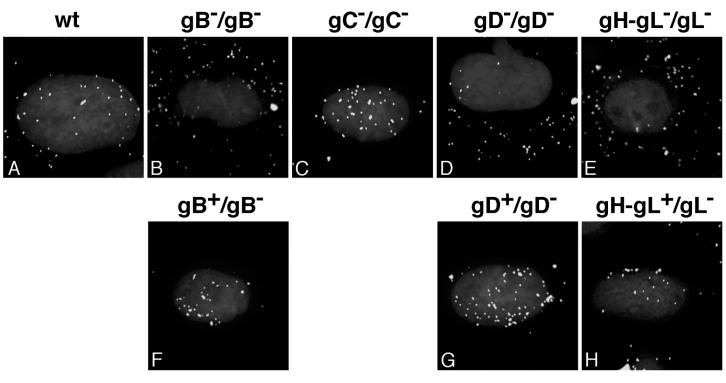
Having shown that gB, gD, and gH-gL are essential for infection by the endocytic route, we next determined whether these glycoproteins are required for delivery of virions to the nuclear periphery. To do so, indirect immunofluorescence microscopy was used to monitor the transport of incoming mutant virus to the nuclei of CHO-nectin-1 cells. At 2.5 h in the presence of cycloheximide to prevent viral protein synthesis, wild-type HSV particles were detected at the nucleus (Fig. 6A). However, HSV mutants lacking the individual glycoprotein gB, gD, or gH-gL were distributed throughout the cytoplasm (Fig. 6B, D, and E). In contrast, both wild-type and HSV-lacking gC were detected at the nuclear periphery (Fig. 6A and C). Similar results were obtained when the panel of mutant viruses was added to HeLa cells (data not shown). Mutant viruses grown on the appropriate glycoprotein-expressing rescue cell lines carry a full set of glycoproteins in the envelope. HSV mutants complemented in this way with gB, gD, or gH-gL localized to the nucleus by 2.5 h (Fig. 6F to H). Together the results indicate that gB, gD, gH, and gL are required for HSV entry via the endocytic pathway, whereas gC is dispensable.
FIG. 6.
HSV glycoprotein requirements for transport of particles to the nuclear periphery. CHO-nectin-1 cells were infected with ∼108 particles of HSV-1 wild-type (wt) KOS (A), gB null (B), gC null (C), gD null (D), gH-gL null (E), complemented gB null (F), complemented gD null (G), or complemented gH-gL null (H) for 2.5 h in the presence of cycloheximide. The envelope content/genome content of the infecting virus is indicated above each panel. Following fixation, cells were probed with anti-HSV VP5 antibody and Alexa Fluor 488-conjugated secondary antibody to visualize individual particles. Nuclei were counterstained with DAPI. Cells were viewed with a 100× oil immersion objective.
DISCUSSION
HSV successfully enters some cultured cells (e.g., HeLa and CHO-nectin-1 cells) via an endocytic process that has not been well characterized. Using biochemical, imaging, and virological approaches, we provide an increasingly detailed picture of the initial events that lead to infection of these cells. Following initial binding to the cell surface, intact, enveloped HSV is endocytosed in the absence of any of the known gD-binding entry receptors. Infectious virus then travels the lysosome-terminal endocytic pathway, and, in cells that express a gD receptor, HSV escapes or penetrates into the cytosol. In addition, the viral envelope glycoproteins gB, gD, and gH-gL are needed for entry via this pathway. HSV also requires intracellular low pH and wortmannin-sensitive trafficking for successful endocytic infection.
Uptake of HSV by endocytosis.
The cumulative data indicate that uptake of enveloped, infectious HSV by endocytosis is an important step for successful entry into certain cell types. It has been suggested recently that entry of HHV-8 also occurs via a pH-dependent, endocytic mechanism (1, 22). When cell surface uptake is blocked, both HSV uptake and virus-induced gene expression is reduced in HeLa and CHO-nectin-1 cells (Fig. 3C) (33). The viral and cellular factors responsible for uptake remain to be determined. Uptake of HSV from the surface of CHO cells is rapid and efficient, which is reminiscent of penetration from the surface of Vero cells. Thus, regardless of the entry pathway that is ultimately utilized, HSV invades the cell within minutes of initial contact.
Trafficking and the role of gD receptors in endocytic entry.
The classical endocytic system traffics both ligands and receptors by a common pathway (11). Following arrival in the early endosome, receptors are sorted to recycling endosomes and ligands are sorted to late endosomes and ultimately lysosomes. Evidence is provided that the infectious endosomal entry pathway used by HSV is one that terminates at the lysosome. However, the possibility that HSV follows the recycling pathway has not been ruled out.
The intracellular trafficking of HSV in CHO cells during the first ∼20 min of infection is similar in the presence or absence of gD receptor (Fig. 2). This suggests that vesicular transport of HSV to the site of penetration occurs independently of the known gD receptors. The expression of a gD receptor such as nectin-1, however, likely allows the virus to escape the lysosome-terminal pathway before the virus is degraded. This is consistent with the appearance of HSV in vesicles in gD-expressing cells in which incoming virus likely does not have access to occupied receptors and is presumably degraded (6). Based on the cumulative data, we propose that interaction with a gD receptor is required for HSV penetration from an acidic, prelysosomal compartment, likely an endosome.
In CHO cells that express nectin-1, nectin-2, or HveA, virus binding to these gD receptors can be detected at the cell surface (A. V. Nicola and S. E. Straus, unpublished data). It is not known whether HSV remains bound to its receptor during transit through the endocytic pathway or whether a transient interaction of virion gD with its receptor at the cell surface is sufficient to prime the virus for subsequent penetration from an endocytic compartment. Successful penetration of HSV from an intracellular membrane may require additional cellular factors, such as low pH (33). Notably, a receptor-primed, low pH-triggered entry mechanism has been proposed for avian leukosis virus (29) and major group human rhinoviruses (35).
PI 3-kinase activity in intracellular trafficking of HSV.
HSV activates both NF-κB (2, 36) and FAK (9) early in infection. Both molecules can be downstream effectors of PI 3-kinase (49), but the role of this lipid kinase in HSV entry has not been assessed directly. We show that pharmacologic disruption of endogenous PI 3-kinase activity selectively blocked a step in entry that occurred after surface binding and uptake but prior to capsid arrival at the nucleus and virus-induced gene expression (Fig. 3 and 4). This is evidence that entry by endocytosis is molecularly distinct from the entry process in Vero cells. Because PI 3-kinase regulates membrane trafficking events, we suspect that activated PI 3-kinase promotes a vesicle trafficking event that is important for delivery of HSV to the nuclear periphery. However, it is not clear whether, in the presence of wortmannin, HSV retains its envelope or whether it is inside vesicles. Also, a direct effect of kinase inhibitors on penetration of capsids from endosomes into the cytosol cannot be ruled out. One attractive possibility is that HSV stimulates cellular PI-3 kinase activity, thereby facilitating efficient infection of cells that support an endocytic entry pathway.
Glycoprotein requirements for endocytic entry.
Our results suggest that HSV utilizes similar viral glycoproteins to enter via endocytic and nonendocytic pathways. A common fusion apparatus of gB, gD, and gH-gL may be active during entry into all cell types. This would differ from EBV, which uses a distinct subset of glycoproteins to enter epithelial cells by direct penetration and B cells via endocytosis (32, 48, 50). Another possibility is that the viral requirements may be similar for the two routes, but different domains of a critical protein(s) may be involved in a particular pathway. Several other membrane glycoproteins, such as gC, gE, gG, and gI, are nonessential for Vero cell entry (46). We show that gC is not required for entry by endocytosis, but the roles of other “nonessential” HSV proteins in endocytic entry need to be investigated.
In conclusion, we have defined the time course and several critical features of steps in HSV entry via endocytosis. In vitro, HSV infects a wide variety of cultured cells and utilizes at least two distinct strategies to initiate entry into them. Targets of HSV in the human host are also quite diverse and include cells of the mucosal epithelia and neurons of the peripheral nervous system. Efforts are under way to evaluate the routes of HSV entry into such primary human cells. This will allow the two HSV entry routes to be studied in a more physiologically relevant context.
Acknowledgments
We thank C. Brandt, G. Cohen, P. Desai, R. Eisenberg, D. Johnson, S. Person, P. Schaffer, and P. Spear for generous gifts of reagents and Jeffrey Cohen and Qingxue Li for critical reading of the manuscript.
REFERENCES
- 1.Akula, S. M., P. P. Naranatt, N. S. Walia, F. Z. Wang, B. Fegley, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis. J. Virol. 77:7978-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amici, C., G. Belardo, A. Rossi, and M. G. Santoro. 2001. Activation of I kappa B kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 276:28759-28766. [DOI] [PubMed] [Google Scholar]
- 3.Batterson, W., D. Furlong, and B. Roizman. 1983. Molecular genetics of herpes simplex virus. VIII. Further characterization of a temperature-sensitive mutant defective in release of viral DNA and in other stages of the viral reproductive cycle. J. Virol. 45:397-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai, W., and P. A. Schaffer. 1991. A cellular function can enhance gene expression and plating efficiency of a mutant defective in the gene for ICP0, a transactivating protein of herpes simplex virus type 1. J. Virol. 65:4078-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai, W. H., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campadelli-Fiume, G., M. Arsenakis, F. Farabegoli, and B. Roizman. 1988. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J. Virol. 62:159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10:305-319. [DOI] [PubMed] [Google Scholar]
- 8.Carfi, A., S. H. Willis, J. C. Whitbeck, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and D. C. Wiley. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol. Cell 8:169-179. [DOI] [PubMed] [Google Scholar]
- 9.Cheshenko, N., B. Del Rosario, C. Woda, D. Marcellino, L. M. Satlin, and B. C. Herold. 2003. Herpes simplex virus triggers activation of calcium-signaling pathways. J. Cell Biol. 163:283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, G. H., M. Ponce de Leon, and C. Nichols. 1972. Isolation of a herpes simplex virus-specific antigenic fraction which stimulates the production of neutralizing antibody. J. Virol. 10:1021-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 422:37-44. [DOI] [PubMed] [Google Scholar]
- 12.Dean, H. J., M. S. Warner, S. S. Terhune, R. M. Johnson, and P. G. Spear. 1995. Viral determinants of the variable sensitivity of herpes simplex virus strains to gD-mediated interference. J. Virol. 69:5171-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai, P., and S. Person. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 72:7563-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller, A. O., and P. G. Spear. 1987. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc. Natl. Acad. Sci. USA 84:5454-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 17.Gompels, U., and A. Minson. 1986. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology 153:230-247. [DOI] [PubMed] [Google Scholar]
- 18.Helenius, A., J. Kartenbeck, K. Simons, and E. Fries. 1980. On the entry of Semliki forest virus into BHK-21 cells. J. Cell Biol. 84:404-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herold, B. C., D. WuDunn, N. Soltys, and P. G. Spear. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 65:1090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Highlander, S. L., S. L. Sutherland, P. J. Gage, D. C. Johnson, M. Levine, and J. C. Glorioso. 1987. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J. Virol. 61:3356-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, A. S., and R. R. Wagner. 1964. Penetration of herpes simplex virus into human epidermoid cells. Proc. Soc. Exp. Biol. Med. 116:863-869. [DOI] [PubMed] [Google Scholar]
- 22.Inoue, N., J. Winter, R. B. Lal, M. K. Offermann, and S. Koyano. 2003. Characterization of entry mechanisms of human herpesvirus 8 by using an Rta-dependent reporter cell line. J. Virol. 77:8147-8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, D. C., and M. W. Ligas. 1988. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J. Virol. 62:4605-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koyama, A. H., and T. Uchida. 1987. The mode of entry of herpes simplex virus type 1 into Vero cells. Microbiol. Immunol. 31:123-130. [DOI] [PubMed] [Google Scholar]
- 25.Li, E., D. Stupack, R. Klemke, D. A. Cheresh, and G. R. Nemerow. 1998. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J. Virol. 72:2055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martys, J. L., C. Wjasow, D. M. Gangi, M. C. Kielian, T. E. McGraw, and J. M. Backer. 1996. Wortmannin-sensitive trafficking pathways in Chinese hamster ovary cells. Differential effects on endocytosis and lysosomal sorting. J. Biol. Chem. 271:10953-10962. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 29.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 30.Naranatt, P. P., S. M. Akula, C. A. Zien, H. H. Krishnan, and B. Chandran. 2003. Kaposi's sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-zeta-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J. Virol. 77:1524-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navarro, D., P. Paz, and L. Pereira. 1992. Domains of herpes simplex virus I glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology 186:99-112. [DOI] [PubMed] [Google Scholar]
- 32.Nemerow, G. R., and N. R. Cooper. 1984. Early events in the infection of human B lymphocytes by Epstein-Barr virus: the internalization process. Virology 132:186-198. [DOI] [PubMed] [Google Scholar]
- 33.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicola, A. V., M. Ponce de Leon, R. Xu, W. Hou, J. C. Whitbeck, C. Krummenacher, R. I. Montgomery, P. G. Spear, R. J. Eisenberg, and G. H. Cohen. 1998. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J. Virol. 72:3595-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nurani, G., B. Lindqvist, and J. M. Casasnovas. 2003. Receptor priming of major group human rhinoviruses for uncoating and entry at mild low-pH environments. J. Virol. 77:11985-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel, A., J. Hanson, T. I. McLean, J. Olgiate, M. Hilton, W. E. Miller, and S. L. Bachenheimer. 1998. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology 247:212-222. [DOI] [PubMed] [Google Scholar]
- 37.Peng, T., M. Ponce-de-Leon, H. Jiang, G. Dubin, J. M. Lubinski, R. J. Eisenberg, and G. H. Cohen. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J. Virol. 72:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez, L., and L. Carrasco. 1994. Involvement of the vacuolar H(+)-ATPase in animal virus entry. J. Gen. Virol. 75:2595-2606. [DOI] [PubMed] [Google Scholar]
- 39.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruyechan, W. T., L. S. Morse, D. M. Knipe, and B. Roizman. 1979. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J. Virol. 29:677-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shukla, D., and P. G. Spear. 2001. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Investig. 108:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sieczkarski, S. B., H. A. Brown, and G. R. Whittaker. 2003. Role of protein kinase C βII in influenza virus entry via late endosomes. J. Virol. 77:460-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith, G. A., and L. W. Enquist. 2002. Break ins and break outs: viral interactions with the cytoskeleton of mammalian cells. Annu. Rev. Cell Dev. Biol. 18:135-161. [DOI] [PubMed] [Google Scholar]
- 44.Sodeik, B. 2000. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 8:465-472. [DOI] [PubMed] [Google Scholar]
- 45.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spear, P. G. 1993. Membrane fusion induced by herpes simplex virus, p. 201-232. In J. Bentz (ed.), Viral fusion mechanisms. CRC Press, Inc., Boca Raton, Fla.
- 47.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 48.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanhaesebroeck, B., S. J. Leevers, K. Ahmadi, J. Timms, R. Katso, P. C. Driscoll, R. Woscholski, P. J. Parker, and M. D. Waterfield. 2001. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70:535-602. [DOI] [PubMed] [Google Scholar]
- 50.Wang, X., W. J. Kenyon, Q. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou, G., V. Galvan, G. Campadelli-Fiume, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]