Abstract
For decades, theta rhythms (~5–10 Hz) have been thought to play a critical role in memory processing in the entorhinal-hippocampal network. However, recent evidence suggests that successful memory performance also requires coupling of ~30–100 Hz gamma rhythms to particular phases of the theta cycle. Recent insights imply ways in which theta-gamma coupling may facilitate transfer of information throughout the entorhinal-hippocampal network. Activating gamma-modulated cell assemblies at a particular theta phase may allow the network to produce a more powerful output by ensuring that distributed cells fire closely in time. I hypothesize that such a mechanism would serve to facilitate either memory encoding or memory retrieval, depending on which type of gamma rhythms are recruited.
Introduction
Theta rhythms occur prominently in the hippocampus and entorhinal cortex during active behaviors [1] and play a key role in memory processing [2,3]. Within each hippocampal theta cycle, bursts of gamma oscillations emerge [4,5], raising the possibility that such theta-gamma interactions are a critical component of mnemonic operations. Theta-nested gamma oscillations may allow the hippocampal-entorhinal network to temporally organize sequences of events within each theta cycle [6–9].
Interactions between theta and gamma oscillations can be assessed by measuring theta-gamma ‘coupling’. The most commonly studied form of theta-gamma coupling is theta phase-gamma amplitude coupling, which is defined as a reliable emergence of gamma oscillations at a particular phase of the underlying theta rhythms. Such coupling could arise because gamma-generating circuitry gets activated at a particular theta phase or because interneurons that fire at a particular theta phase disrupt mechanisms of gamma generation.
Here I review recent studies that indicate that coupling between theta and gamma oscillations plays an important role in functions of the entorhinal-hippocampal network. I will first describe recent studies suggesting that theta-gamma coupling enhances memory processing. I will then discuss recent results that shed light on the question of how theta-gamma coupling arises. Finally, I will present possible ways in which theta-gamma coupling may promote effective memory processing in the entorhinal-hippocampal network.
Theta-gamma coupling and memory operations in the entorhinal-hippocampal network
A relatively early study investigating the link between hippocampal theta-gamma coupling and memory performance was performed in humans implanted with depth electrodes for treatment of temporal lobe epilepsy [10]. In a word recognition paradigm, coupling between theta phase and ~45 Hz gamma power was selectively enhanced when patients successfully remembered previously presented words. This finding suggested that recruitment of gamma oscillations at a particular theta phase facilitated retrieval of memories of earlier experiences. Later, consistent results were reported in rats. In an initial important study, animals learned that rewards were associated with two different stimuli in two different contexts [11]. In hippocampal subfield CA3, coupling between theta phase and ~40 Hz gamma amplitude improved as animals learned the task. This theta-gamma interaction occurred during exploration prior to the stimulus choice, a time when animals presumably retrieved their memory of the correct response associated with a particular environment. More recently, Igarashi and colleagues showed that ~20–40 Hz oscillations in hippocampal subfield CA1 became more tightly locked to theta phase as animals learned odor-place associations [12]. This cross-frequency coupling was observed during the odor-sampling period of the task when animals presumably remembered which reward site was associated with a given odor. In another recent study, ~30–45 Hz gamma power in CA1 increased at the point in a delayed spatial alternation task when animals needed to remember which side to choose [13]. Taken together, these results support the hypothesis that coupling between theta phase and ~30–45 Hz gamma facilitates retrieval of previously learned memories.
It is noteworthy that each of the studies mentioned above described effects in the ~25–45 Hz range, i.e., the lower frequency end of the band traditionally defined as gamma. This ‘slow gamma’ activity synchronizes CA1 with inputs from CA3 [14–17], a region that is believed to play a key role in memory retrieval [18–20]. Thus, it is possible that slow gamma reflects a state in which the entorhinal-hippocampal network is optimally primed to retrieve, rather than encode, memories. If the hippocampus exhibits slow gamma during memory retrieval, then entorhinal inputs may need to synchronize with slow gamma in order for sensory cues to trigger memory retrieval, as occurred in the Igarashi et al. study [12]. These interactions across regions are likely to involve both theta and slow gamma, with slow gamma activating cell assemblies that represent a particular memory within a given theta cycle. Consistent with this notion, co-modulation of theta and ~30–50 Hz slow gamma power was several times higher during spatial memory retrieval than during exploration prior to memory encoding in a study by Shirvalkar and colleagues [21]. Moreover, co-modulation of theta and slow gamma power was higher during successful trials than during error trials.
Another recent study suggested that theta-modulated slow gamma reflects a memory retrieval mode in the hippocampal network. During theta cycles exhibiting slow gamma activity, place cell ensembles represented locations ahead of the animal’s actual location [22]. Such ‘prospective’ firing may occur as cells retrieve stored memory representations of upcoming locations.
Yet, some results do not support the hypothesis that theta-modulated slow gamma is involved in memory retrieval. Trimper and colleagues reported that ~30–50 Hz gamma coherence between CA3 and CA1 was increased in rats exploring novel objects [23], a behavior during which memory encoding, not memory retrieval, would be expected to occur. Moreover, the observed increases in slow gamma coherence were greater for objects that were subsequently remembered well compared to objects that were remembered poorly. However, in this study, rats experienced many trials containing novel objects in the same set of locations. This led Trimper et al. to speculate that the slow gamma coherence reflected recollection that different objects had been previously presented in those locations. Still, another study showed that slow gamma power in CA1 was greater on a novel W-maze compared to a familiar W-maze [17]. Again though, it is possible that rats retrieved their memory of the general W-maze paradigm in the new maze, considering that novel maze exploration was immediately preceded by a session in the familiar maze. Whatever the case may be, these results challenge the hypothesis that slow gamma plays a role in memory retrieval and demonstrate that much work remains to be done to understand the functional significance of slow gamma.
Theta is not only coupled with slow gamma rhythms in the entorhinal-hippocampal network but also with fast gamma rhythms (~60–100 Hz) at a different theta phase [14,17,24,25]. During periods of fast gamma, CA1 is preferentially synchronized with inputs from the medial entorhinal cortex (MEC) [14]. Several recent studies suggested functional roles for such theta-modulated fast gamma coupling in the entorhinal-hippocampal network. Cabral and colleagues found that CA1 fast gamma power was more strongly locked to theta phase in mice using a place-based strategy, rather than a sequence memory-based strategy, to solve a mnemonic task [26]. Mice must pay attention to environmental stimuli, such as landmarks, when using a place-based strategy. Under these conditions, theta-modulated fast gamma may facilitate transmission of current sensory information from the entorhinal cortex to the hippocampus. The hippocampus also needs to access current sensory information when encoding new memories, and thus fast gamma coupling between the hippocampus and entorhinal cortex may support memory encoding. A recent study showed that fast, but not slow, gamma power in the MEC was reduced by the drug scopolamine, which blocks memory encoding but not memory retrieval [24]. Indirect support for the hypothesis that fast gamma is involved in memory encoding was provided by a study of place cell firing during theta-modulated slow and fast gamma states [22]. During theta-modulated fast gamma, individual place cells fired late in their place fields, and place cell ensembles preferentially coded locations in the recent past. Such ‘retrospective’ activity in the hippocampus may occur in response to persistent firing in entorhinal cortex [27,28] and may provide the repetitive activation necessary to drive memory encoding [29].
However, a more recent study challenges the hypothesis that theta-modulated fast gamma facilitates encoding of current information. Yamamoto and colleagues found that fast gamma phase synchrony between CA1 and MEC increased at the choice point of a delayed non-match-to-place task [25], a location where memory retrieval is expected, not memory encoding. Moreover, such increases were not seen when the animal made an incorrect choice, and correct task performance was disrupted when fast gamma was suppressed at the choice point. The authors suppressed fast gamma by silencing MEC inputs to CA1. Thus, although these results support the conclusion that MEC inputs drive fast gamma in CA1, they are inconsistent with the hypothesis that fast gamma promotes memory encoding. Clearly, more studies are needed to better understand the functional significance of fast gamma in the entorhinal-hippocampal network.
Mechanisms of theta-gamma coupling in the entorhinal-hippocampal network
In addition to theta phase-gamma amplitude coupling, phase-phase coupling has also been reported between theta and gamma [30]. Coupling with theta phase was seen for both ~30–50 Hz (defined here as ‘slow’) and ~60–90 Hz (defined here as ‘fast’ but defined in [30] as ‘midfrequency’) gamma phase in CA1. This phase-phase coupling suggests that gamma-generating interneurons become active at a particular theta phase, emitting trains of gamma frequency spikes that produce gamma rhythmic inhibitory postsynaptic potentials in pyramidal cells. Other recent results provide support for this mechanism. Whole cell recordings from dentate gyrus granule cells in vivo revealed theta rhythmic excitatory postsynaptic currents (EPSCs), which originated from the entorhinal cortex, and gamma rhythmic inhibitory postsynaptic currents (IPSCs) within each theta cycle [31]. Additionally, Pastoll and colleagues [32] found that theta frequency stimulation of MEC layer II in vitro triggered theta rhythms and ~85 Hz fast gamma oscillations that coupled to a specific part of the theta cycle. This fast gamma activity corresponded to rhythmic IPSCs in stellate cells. These results suggest that theta-mediated excitation activates gamma-generating interneurons at a particular part of the theta cycle.
It remains unclear whether similar or different mechanisms trigger theta-modulated slow and fast gamma rhythms. Gamma frequency in rats was found to increase with running speed [33] (but see [34] for different results in mice), as was the frequency of interneuron spiking [33]. These findings suggest that similar mechanisms generate slow and fast gamma in rats, with speed-modulated inputs controlling gamma frequency. However, other results support the hypothesis that different mechanisms generate slow and fast gamma. Gamma in the ~30–50 Hz and ~50–90 Hz ranges was found to be associated with current source density (CSD) analysis-defined sinks in stratum radiatum and stratum lacunosum-moleculare, respectively [30]. These results support the conclusion that slow gamma is driven by CA3 inputs and fast gamma is driven by entorhinal inputs. However, a more recent CSD study by Lastoczi and Klausberger reported that ~30 Hz gamma oscillations were dominant in stratum lacunosum-moleculare, not stratum radiatum [35]. Spikes from projection neurons in layer III of MEC were phase-locked to the gamma activity in stratum lacunosum-moleculare, suggesting that this activity was driven by MEC [35]. The reason for the frequency differences between these results and the earlier results [30] may be due to the use of anesthesia in the majority of experiments in the Lastoczi and Klausberger study [35]. In line with this idea, example recordings obtained by Lastoczi and Klausberger from an anesthesia-free mouse showed ~70 Hz gamma in stratum lacunosum-moleculare (see Figure 1C in [35]). Additionally, the authors recorded multiple interneurons in the apical dendritic region of CA1. Some of these interneurons fired phase-locked to ~20–45 Hz oscillations, while others phase-locked to ~60–100 Hz oscillations. These results support the hypothesis that different classes of interneurons in CA1 drive slow and fast gamma activity. These different gamma-generating interneuron classes are likely activated by different inputs arriving at different theta phases. Recent evidence raises the possibility that interneurons activated during slow gamma from CA3 inhibit fast gamma from MEC. Specifically, OLM interneurons in CA1, which are activated by CA3, inhibit inputs from entorhinal cortex while at the same time disinhibiting inputs from CA3 [36].
Conclusions
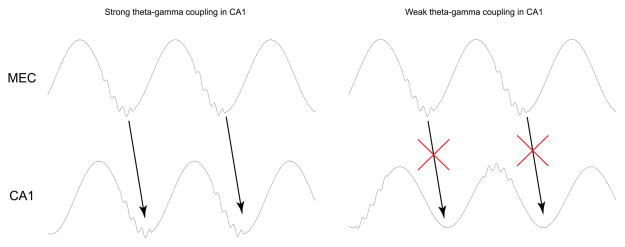
The above-described studies provide evidence that theta-gamma coupling facilitates memory operations in the entorhinal-hippocampal network and suggest a mechanism for how such coupling arises. But the question remains as to why strong coupling between theta phase and gamma amplitude would improve memory processing. If theta-modulated slow and fast gamma serve separate memory retrieval and encoding functions, as hypothesized above, then ensuring that slow and fast gamma episodes occur on different theta phases may prevent interference between memory retrieval and encoding [37]. It will be important to determine whether slow and fast gamma play important roles in retrieval and encoding processes or merely correlate with retrieval and encoding due to their relationships to theta phase. In any case, locking fast gamma to a particular theta phase could ensure that the entorhinal cortex and hippocampus are in the fast gamma mode at the same time, considering that theta is phase-synchronized across the regions [38] (Figure 1). Maximal firing probability of cells in the superficial layers of entorhinal cortex occurs immediately after the theta phase associated with maximal gamma power [39]. CA1 place cells have also been reported to exhibit maximal firing immediately after the theta phase associated with maximal fast gamma power [14]. Thus, theta-fast gamma coupling in MEC and CA1 may allow entorhinal spikes to arrive when hippocampal cells are likely to respond. Also, fast gamma synchronization of MEC spikes may produce a more powerful output from MEC and thereby increase spiking in CA1, as has been shown to occur during gamma coordination of V1 inputs to V2 [40].
Figure 1.
A cartoon illustrating how coordinated theta-gamma phase coupling between MEC and CA1 could affect information transfer across the regions. Note alignment of gamma activity across the regions when strong theta phase-gamma power coupling is seen in CA1 (left). Under these conditions, effective interregional communication is expected, considering that cells in the regions are highly active immediately after ~80 Hz gamma episodes [14,30]. Weak theta-gamma coupling in CA1, as depicted in the panel on the right, would be expected to detrimentally affect communication between the regions.
However, direct demonstrations that coordinated theta-gamma coupling improves entorhinal-hippocampal operations are lacking. Thus, it may be the case that memory-related changes in theta-gamma coupling simply mirror memory-related increases in gamma power and phase synchrony [41]. Novel approaches (e.g., optogenetics) should allow researchers to disturb the relationship between gamma power and theta phase without affecting theta or gamma power. Such a manipulation would enable researchers to directly determine how coupling between theta and gamma affects neuronal signaling and memory operations in the entorhinal-hippocampal network.
Highlights.
Theta-gamma coupling supports memory processes in the entorhinal-hippocampal network
Theta-modulated slow gamma may promote memory retrieval
Theta-modulated fast gamma may facilitate memory encoding
Theta-nested gamma reflects gamma rhythmic inhibition and theta rhythmic excitation
Acknowledgments
I thank Sean G. Trettel for assistance with preparation of this manuscript. Funding was provided by the Esther A. and Joseph Klingenstein Fund, the Alfred P. Sloan Foundation, grant P30 MH089900 from NIMH, and grant N00014-14-1-0322 from ONR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
* indicates paper of special interest from the past 2 years.
** indicates paper of outstanding interest from the past 2 years.
- 1.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26(4):407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 2.Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15(7):827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- 3.Colgin LL. Mechanisms and functions of theta rhythms. Annu Rev Neurosci. 2013;36:295–312. doi: 10.1146/annurev-neuro-062012-170330. [DOI] [PubMed] [Google Scholar]
- 4.Buzsaki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287(2):139–171. doi: 10.1016/0165-0173(83)90037-1. [DOI] [PubMed] [Google Scholar]
- 5.Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15(1 Pt 1):47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisman JE, Idiart MA. Storage of 7 ± 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 7.Senior TJ, Huxter JR, Allen K, O’Neill J, Csicsvari J. Gamma oscillatory firing reveals distinct populations of pyramidal cells in the CA1 region of the hippocampus. J Neurosci. 2008;28:2274–2286. doi: 10.1523/JNEUROSCI.4669-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Gupta AS, van der Meer MA, Touretzky DS, Redish AD. Segmentation of spatial experience by hippocampal theta sequences. Nat Neurosci. 2012;15:1032–1039. doi: 10.1038/nn.3138. This study showed that sequences of place cells within a theta cycle preferentially represent locations that correspond to paths between task-relevant landmarks. The duration of the theta period increased with increasing path length. The number of gamma cycles within a theta cycle also increased as path length increased. These findings support the hypothesis that cell assemblies representing sequences of locations are activated across successive gamma cycles within a theta cycle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisman JE, Jensen O. The theta-gamma neural code. Neuron. 2013;77:1002–1016. doi: 10.1016/j.neuron.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mormann F, Fell J, Axmacher N, Weber B, Lehnertz K, Elger CE, Fernandez G. Phase/amplitude reset and theta-gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15:890–900. doi: 10.1002/hipo.20117. [DOI] [PubMed] [Google Scholar]
- 11.Tort AB, Komorowski RW, Manns JR, Kopell NJ, Eichenbaum H. Theta-gamma coupling increases during the learning of item-context associations. Proc Natl Acad Sci USA. 2009;106:20942–20947. doi: 10.1073/pnas.0911331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Igarashi KM, Lu L, Colgin LL, Moser MB, Moser EI. Coordination of entorhinal-hippocampal ensemble activity during associative learning. Nature. 2014;510:143–147. doi: 10.1038/nature13162. This study measured ~20–40 Hz oscillatory coupling across CA1 and lateral entorhinal cortex (LEC) as rats learned an odor-place association. In the task, animals were presented with an olfactory cue that signaled where they needed to go to receive a reward. In well-trained animals, ~20–40 Hz activity was coherent across LEC and distal CA1, the part of CA1 that predominantly receives LEC inputs. Coherence was maximal during the second half of the cue-sampling period, a time when memory retrieval would be expected to occur. This strong coherence developed as the animal learned how to perform the task correctly and was not observed during error trials. Cross-frequency coupling between theta and ~20–40 Hz oscillations in distal CA1 also developed with learning, as did phase-locking of LEC and CA1 spikes to ~20–40 Hz oscillations. Moreover, after learning, LEC and CA1 firing patterns discriminated between different odors, and the development of this odor selectivity was correlated with the development of ~20–40 Hz coupling between LEC and CA1. These findings suggest that LEC-CA1 coupling in the ~20–40 Hz range plays an important role in cued memory retrieval. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M, Nishida H, Redish AD, Lauwereyns J. Theta phase shift in spike timing and modulation of gamma oscillation: a dynamic code for spatial alternation during fixation in rat hippocampal area CA1. J Neurophysiol. 2014;111:1601–1614. doi: 10.1152/jn.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462(7271):353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- 15.Carr MF, Karlsson MP, Frank LM. Transient slow gamma synchrony underlies hippocampal memory replay. Neuron. 2012;75:700–713. doi: 10.1016/j.neuron.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colgin LL. Slow gamma takes the reins in replay. Neuron. 2012;75:549–550. doi: 10.1016/j.neuron.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Kemere C, Carr MF, Karlsson MP, Frank LM. Rapid and continuous modulation of hippocampal network state during exploration of new places. PLoS One. 2013;8:e73114. doi: 10.1371/journal.pone.0073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutherland RJ, Whishaw IQ, Kolb B. A behavioural analysis of spatial localization following electrolytic, kainate- or colchicine-induced damage to the hippocampal formation in the rat. Behav Brain Res. 1983;7:133–153. doi: 10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- 19.Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- 20.Steffenach HA, Sloviter RS, Moser EI, Moser MB. Impaired retention of spatial memory after transection of longitudinally oriented axons of hippocampal CA3 pyramidal cells. Proc Natl Acad Sci USA. 2002;99:3194–3198. doi: 10.1073/pnas.042700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirvalkar PR, Rapp PR, Shapiro ML. Bidirectional changes to hippocampal theta-gamma comodulation predict memory for recent spatial episodes. Proc Natl Acad Sci USA. 2010;107:7054–7059. doi: 10.1073/pnas.0911184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Bieri KW, Bobbitt KN, Colgin LL. Slow and fast gamma rhythms coordinate different spatial coding modes in hippocampal place cells. Neuron. 2014;82:670–681. doi: 10.1016/j.neuron.2014.03.013. This study investigated whether place cells code spatial information differently during slow and fast gamma. The paper first investigated spatial coding at the single cell level using the paradigm of ‘prospective’ and ‘retrospective’ coding within a place field. Prospective coding was defined as periods when place cell spikes occurred primarily as a rat entered a place field. Retrospective coding was defined as periods when spikes occurred mostly in the later part of the place field. Slow gamma power and phase-locking of interneuron spikes was significantly heightened during prospective coding. In contrast, fast gamma power and interneuron phase-locking was significantly higher during retrospective coding. Spatial coding was also investigated at the place cell ensemble level. Ensembles of place cells coded upcoming locations during slow gamma-associated theta cycles and coded recently visited locations during fast gamma-associated theta cycles. The findings support the hypothesis that slow and fast gamma constitute distinct spatial coding modes in the hippocampal network. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trimper JB, Stefanescu RA, Manns JR. Recognition memory and theta-gamma interactions in the hippocampus. Hippocampus. 2014;24:341–353. doi: 10.1002/hipo.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Newman EL, Gillet SN, Climer JR, Hasselmo ME. Cholinergic blockade reduces theta-gamma phase amplitude coupling and speed modulation of theta frequency consistent with behavioral effects on encoding. J Neurosci. 2013;33:19635–19646. doi: 10.1523/JNEUROSCI.2586-13.2013. This study showed that injections of the muscarinic antagonist scopolamine suppressed fast gamma but not slow gamma in the MEC. Scopolamine also affected theta-fast gamma coupling such that fast gamma episodes occurred at a later theta phase after drug administration. This finding suggests that cholinergic inputs are important for fast gamma generation during theta. The findings may also provide a mechanism for scopolamine’s detrimental effects on memory encoding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto J, Suh J, Takeuchi D, Tonegawa S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell. 2014;157:845–857. doi: 10.1016/j.cell.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 26**.Cabral HO, Vinck M, Fouquet C, Pennartz CM, Rondi-Reig L, Battaglia FP. Oscillatory dynamics and place field maps reflect hippocampal ensemble processing of sequence and place memory under NMDA receptor control. Neuron. 2014;81:402–415. doi: 10.1016/j.neuron.2013.11.010. In this study, mice were trained on a starmaze task. On this task, mice could either use a sequence memory-based strategy or a place-based strategy. The ratio of fast gamma to slow gamma was found to increase when mice employed the place-based strategy. This effect was not observed in mice lacking NMDA receptors in CA1. Also, coupling between theta and fast gamma was significantly enhanced during trials when mice relied on the place-based strategy. These results support the hypothesis that fast gamma facilitates transfer of spatial information from MEC to CA1. [DOI] [PubMed] [Google Scholar]
- 27.Klink R, Alonso A. Muscarinic modulation of the oscillatory and repetitive firing properties of entorhinal cortex layer II neurons. J Neurophysiol. 1997;77:1813–1828. doi: 10.1152/jn.1997.77.4.1813. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida M, Fransen E, Hasselmo ME. mGluR-dependent persistent firing in entorhinal cortex layer III neurons. Eur J Neurosci. 2008;28:1116–1126. doi: 10.1111/j.1460-9568.2008.06409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Almeida L, Idiart M, Villavicencio A, Lisman J. Alternating predictive and short-term memory modes of entorhinal grid cells. Hippocampus. 2012;22:1647–1651. doi: 10.1002/hipo.22030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsaki G. Cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. J Neurosci. 2012;32:423–435. doi: 10.1523/JNEUROSCI.4122-11.2012. This paper defines three different frequency bands of gamma activity in CA1 that exhibit different current source density profiles and different relationships to theta. ~30–50 Hz activity was termed ‘slow gamma’ and exhibited a maximal sink in stratum radiatum. ~60–90 Hz activity was termed ‘midfrequency gamma’ and was associated with a large sink in stratum lacunosum-moleculare. ~90–150 Hz activity was defined as ‘fast gamma/epsilon’. This epsilon band had its largest sink in the pyramidal cell layer and was largely associated with spiking. The amplitude of activity in each of these frequency bands was modulated by theta phase, but each frequency band exhibited maximal power at a different theta phase. Additionally, phase-phase coupling was observed between theta and ~30–50 Hz gamma and between theta and ~60–90 Hz gamma but not between theta and epsilon. These results suggest that two distinct variants of gamma rhythms exist in the hippocampal network. One shows a peak frequency ~40 Hz and likely originates from CA3, and the other has a peak frequency ~75 Hz and likely is driven by the entorhinal cortex. Activity in the epsilon range appears to reflect spiking from neurons close to the recording site. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Pernia-Andrade AJ, Jonas P. Theta-gamma-modulated synaptic currents in hippocampal granule cells in vivo define a mechanism for network oscillations. Neuron. 2014;81:140–152. doi: 10.1016/j.neuron.2013.09.046. In this elegant study, whole cell recordings were obtained from granule cells in the dentate gyrus of anesthetized and awake rats. The results showed excitatory postsynaptic currents in granule cells that were coherent with theta oscillations in the local field potential (LFP) and were reduced by inactivation of the entorhinal cortex. Inhibitory postsynaptic currents were also observed and were coherent with gamma rhythms in the LFP. The findings indicate that theta-modulated gamma rhythms in the dentate gyrus reflect gamma rhythmic inhibitory currents nested within theta cycles initiated by excitatory input from the entorhinal cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastoll H, Solanka L, van Rossum MC, Nolan MF. Feedback inhibition enables theta-nested gamma oscillations and grid firing fields. Neuron. 2013;77:141–154. doi: 10.1016/j.neuron.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 33**.Ahmed OJ, Mehta MR. Running speed alters the frequency of hippocampal gamma oscillations. J Neurosci. 2012;32:7373–7383. doi: 10.1523/JNEUROSCI.5110-11.2012. This paper shows that the frequency of hippocampal gamma in rats increases with increasing running speed. This finding suggests that gamma frequency increases at high running speeds allow for faster transitions across cell assemblies coding sequences of locations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z, Resnik E, McFarland JM, Sakmann B, Mehta MR. Speed Controls the Amplitude and Timing of the Hippocampal Gamma Rhythm. PLoS ONE. 2011;6(6):e21408. doi: 10.1371/journal.pone.0021408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lasztoczi B, Klausberger T. Layer-specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron. 2014;81:1126–1139. doi: 10.1016/j.neuron.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Leao RN, Mikulovic S, Leao KE, Munguba H, Gezelius H, Enjin A, Patra K, Eriksson A, Loew LM, Tort ABL, Kullander K. OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat Neurosci. 2012;15(11):1524–1530. doi: 10.1038/nn.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14:793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell SJ, Ranck JB. Generation of theta rhythm in medial entorhinal cortex of freely moving rats. Brain Res. 1980;189:49–66. doi: 10.1016/0006-8993(80)90006-2. [DOI] [PubMed] [Google Scholar]
- 39.Quilichini P, Sirota A, Buzsaki G. Intrinsic circuit organization and theta-gamma oscillation dynamics in the entorhinal cortex of the rat. J Neurosci. 2010;30:11128–11142. doi: 10.1523/JNEUROSCI.1327-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia X, Tanabe S, Kohn A. Gamma and the coordination of spiking activity in early visual cortex. Neuron. 2013;77:762–774. doi: 10.1016/j.neuron.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci USA. 2007;104:14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]