Abstract
Blockade of fatty acid synthase (FASN), a key enzyme involved in de novo lipogenesis, results in robust death of ovarian cancer cells. However, known FASN inhibitors have proven to be poor therapeutic agents due to their ability to induce cachexia. Therefore, we sought to identify additional targets in the pathway linking FASN inhibition and cell death whose modulation might kill ovarian cancer cells without the attendant side effects. Here, we show that the initiator caspase-2 is required for robust death of ovarian cancer cells induced by FASN inhibitors. REDD1 (also known as Rtp801 or DDIT4), a known mTOR inhibitor previously implicated in the response to FASN inhibition, is a novel caspase-2 regulator in this pathway. REDD1 induction is compromised in ovarian cancer cells that do not respond to FASN inhibition. Inhibition of FASN induced an ATF4-dependent transcriptional induction of REDD1; downregulation of REDD1 prevented orlistat-induced activation of caspase-2, as monitored by its cleavage, proteolytic activity, and dimerization. Abrogation of REDD1-mediated suppression of mTOR by TSC2 RNAi protected FASN inhibitor-sensitive ovarian cancer cells (OVCA 420 cells) from orlistat-induced death. Conversely, suppression of mTOR with the chemical inhibitors PP242 or rapamycin sensitized DOV13, an ovarian cancer cell line incapable of inducing REDD1, to orlistat-induced cell death through caspase-2. These findings indicate that REDD1 positively controls caspase-2-dependent cell death of ovarian cancer cells by inhibiting mTOR, placing mTOR as a novel upstream regulator of caspase-2 and supporting the possibility of manipulating mTOR to enhance caspase-2 activation in ovarian cancer.
Keywords: lipogenesis, FASN, caspase-2, REDD1, ER stress, ovarian cancer, mTOR, ATF4
INTRODUCTION
Altered cellular metabolism is emerging as a hallmark of cancer and has been shown to support several steps along the pathway of cancer development.1–3 In addition to aberrant glucose metabolism, as exemplified by the Warburg effect, accelerated de novo fatty acid synthesis is frequently observed in human malignancies. Elevated lipogenesis may provide one avenue for fulfilling the demand of cancers for increased genesis of membranes during unrestrained growth.4–6 Indeed, inhibition of fatty acid synthase (FASN) has been shown to trigger ER stress in tumor cells,7 while FASN inhibitors, such as orlistat and C75, have been found to produce antitumor effects in a variety of cancers, including ovarian cancers [reviewed in ref. 5].
Interestingly, several recent studies suggest a “lipid addiction” phenotype for ovarian cancers. For example, FASN levels are upregulated in 80% of ovarian carcinoma samples and correlate with poor prognosis.8, 9 FABP4, a lipid chaperone, has been shown to be upregulated in ovarian-derived metastases to enable the uptake of exogenous lipids as an energy source.10 Most importantly, blockade of de novo lipid synthesis with FASN inhibitors has been shown to be suppressive for ovarian cancer in vitro and in vivo by inhibiting cancer proliferation and stimulating apoptosis.11–13
Apoptosis is executed by caspases, a family of cysteine proteases. Although caspase-2 is the second member to be discovered, its biological role remains enigmatic, in part due to the lack of an obvious phenotype in caspase-2 knockout mice under unstressed conditions.14 Interestingly, genetic deletion of caspase-2 has been recently found to shorten mouse life span, accelerate the development of age-related traits15 and prompt tumorigenesis in mouse models of leukemia16, 17 and carcinoma,18 suggesting a protective role for caspase-2 in ageing and tumorigenesis. Moreover, previous studies in our laboratory demonstrated that glucose-6-phosphate blocks caspase-2 activation and the subsequent Xenopus oocyte death induced by nutrient deprivation, revealing that caspase-2 is capable of coordinating glucose metabolism and cell death.19, 20
A variety of physiological stresses have been shown to activate caspase-2 [reviewed in ref. 21]. Of clinical interest, several common drugs used in chemotherapy, such as paclitaxel and cisplatin, appear to induce apoptosis, at least in part, through caspase-2.22, 23 Caspase-2 can engage the intrinsic apoptotic pathway by cleaving Bid, and thereby induces Bax/Bak-dependent mitochondrial outer membrane permeabilization (MOMP), cytochrome c release, and subsequent cell death.24, 25 Caspase-2 has also been show to mediate the activation of caspase-8 and the extrinsic apoptotic pathway in ceramide- and TRAIL-induced cell death.26, 27 Similar to other initiator caspases, the inert caspase-2 monomer is activated by dimerization, and subsequent intramolecular cleavage further stabilizes its proteolytic activities.28 A p53 inducible protein, PIDD, with the aid of RAIDD, has been suggested to mediate caspase-2 dimerization by forming an activating platform, namely the PIDDosome.29 Nevertheless, several recent studies indicate that caspase-2 may be activated in a PIDD-independent manner,30, 31 and a previously developed bimolecular fluorescence complementation (BiFC) assay that measures the activating dimerization of caspase-2 also provides the means to identify novel modulators that control caspase-2 dimerization, such as Hsp90α.32
Here we report the identification of REDD1 as a novel caspase-2 regulator that facilities caspase-2 dimerization/activation upon the suppression of de novo lipogenesis. REDD1 was transcriptionally upregulated through an ATF4-dependent pathway to permit caspase-2 dimerization following FASN inhibition. Interestingly, abrogation of REDD1-mediated mTOR inhibition by TSC2 RNAi prevented orlistat-induced cell death, whereas pretreatment with mTOR inhibitors reversed the resistance of REDD1-deficient ovarian cancer cells to orlistat by activating caspase-2. Collectively, these data demonstrated the involvement of caspase-2 in ovarian cancer cell death induced by FASN inhibition and identified REDD1 as a novel caspase-2 regulator that controls caspase-2 dimerization through the ATF4-REDD1-TSC2-mTOR axis.
RESULTS
Caspase-2 controls ovarian cancer cell death induced by inhibition of FASN
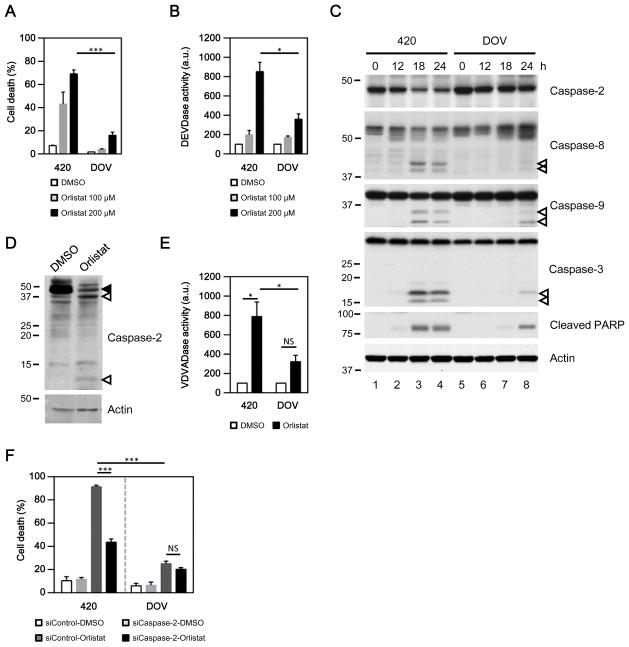
Our previous work linked caspase-2 to oocyte apoptosis upon nutrient deprivation;19, 20, 33 here we sought to explore caspase-2’s metabolic role in another paradigm. We hypothesized that caspase-2 might be involved in cancer cell death induced by the inhibition of de novo fatty acid synthesis. To test this hypothesis, we treated ovarian cancer cell lines OVCA420 (420) and DOV13 (DOV) with orlistat, an FDA approved drug capable of inhibiting FASN. Orlistat induced a dose-dependent increase in cell death (Fig. 1A) and effector caspase activities (Fig. 1B) in both ovarian cancer cell lines, consistent with its reported death-inducing effects for cancers of other origin. Notably, orlistat treatment caused significantly higher cell death and effector caspase activities in 420 cells, whereas its effects in DOV cells were relatively marginal (Fig. 1A & B, black bars). Similarly, orlistat-induced cleavage of caspases and PARP (an indicator for the activation of caspases) was much more pronounced in the 420 than in the DOV line (Fig. 1C, lane 3 vs. 7 and 4 vs. 8). The appearance of cleaved caspase-8 and -9 suggested that inhibition of FASN may activate both the intrinsic and extrinsic apoptotic pathways (Fig. 1C, lane 3 and 4). Note that these data are in contrast to a previous report in MDA-MB-435 melanoma cancer cells where caspase-8, but not caspase-9 cleavage was observed following FASN inhibition.34
Fig. 1. Blockade of de novo lipid synthesis activates caspase-2.
(A–B) FASN inhibitor orlistat induced strong cell death (A) and effector caspase (DEVDase) activities (B) in the ovarian cancer cell line OVCA420 (420), but only had marginal effects in DOV13 (DOV) cells (*p<0.05; ***p<0.001; a.u., arbitrary units). (C) Activation of caspases induced by FASN inhibition was monitored at the indicated time points by the loss of full-length caspase-2, the appearance of cleaved caspase-8, -9, -3 (empty arrows), and caspase-mediated proteolysis of PARP. A rat monoclonal caspase-2 antibody (11B4) was used. (D) Orlistat induced the appearance of cleaved caspase-2 in 420 cells as monitored by a rabbit polyclonal caspase-2 antibody (Solid and empty arrows indicate full-length and cleaved caspase-2, respectively). (E) FASN inhibition activated the proteolytic cleavage of VDVAD-pNA (caspase-2 preferred substrate) in 420 (*p<0.05), but not in DOV cells (NS, not significant; p=0.08; a.u., arbitrary units). (F) Downregulation of caspase-2 effectively reduced cell death induced by orlistat in 420, but not in DOV cells (***p<0.001; NS, not significant, p=0.137).
Interestingly, the levels of full-length caspase-2 were concurrently decreased as other caspases became activated in 420 cells (Fig. 1C, lane 3 and 4), suggesting that caspase-2 might be involved in the death signaling. To examine this possibility, cleavage of caspase-2 was carefully examined with a polyclonal antibody capable of recognizing caspase-2 cleavage products. As shown in Fig. 1D, orlistat, but not DMSO (vehicle), readily induced the appearance of caspase-2 cleavage products (empty arrows), accompanied with a loss of its full-length form (solid arrow) in 420 cells. However, no clear loss of full-length caspase-2 or the appearance of its truncated species was detected in orlistat-treated DOV cells within the same time frame (Fig. 1C and data not shown). Concomitantly, orlistat stimulated a dramatic increase in the proteolytic activity of caspase-2-like activity in 420 cells, but caused only a minor increase in activity against the caspase-2 preferred substrate VDVAD-pNA in DOV cells (Fig. 1E).
To evaluate a possible role for caspase-2 in orlistat-induced cell death, caspase-2 was downregulated by RNAi. The efficacies of different siRNAs were tested by immunoblotting and shown in Supplementary Fig. S1A. Knockdown of caspase-2 with an siRNA targeted against the 3′-UTR region of its transcript (siCaspase-2) greatly reduced the robust 420 cell death induced by orlistat (Fig. 1F, left panel). Similarly, 420 cells transfected with siCaspase-2 ORF, an independent siRNA directed against the coding region of the caspase-2 transcript, were resistant to orlistat-induced death as well (Supplementary Fig. S1B). By contrast, orlistat only caused limited cell death in DOV cells, and caspase-2 deficiency had no significant effect (Fig. 1F, right panel), likely due to the inability of orlistat to stimulate capsase-2 in this line (Fig. 1E). Together, these data indicated that inhibition of FASN was capable of activating caspase-2, and this activation was correlated with orlistat-induced cell death.
Caspase-2 acts as the dominant initiator caspase in cell death induced by suppression of FASN
Caspase-2 has been implicated in both the intrinsic and extrinsic apoptotic pathways.24–27 Consistent with its established role, caspase-2 was capable of cleaving Bid in response to inhibition of FASN. Suppression of caspase-2 with a chemical inhibitor zVDVAD-fmk blocked the orlistat-induced loss of full-length Bid (Fig. 2A, upper panel) and the appearance of truncated/activated Bid in 420 cells (Fig. 2A, middle panel). Cleaved Bid has been shown to cause MOMP and mitochondrial release of cytochrome c by activating Bax/Bak.24, 25 Indeed, active Bax and Bak were detected in orlistat-treated 420 cells by immunoprecipitation using antibodies specific to their active forms (Fig. 2B). Bax/Bak-mediated MOMP appeared to be essential for orlistat-induced cell death, since knockdown of either Bax or Bak significantly impaired 420 cell death and the efflux of cytochrome c from mitochondria into the cytoplasm (Supplementary Fig. S2). Notably, orlistat-induced Bax activation was greatly suppressed in cells transfected with caspase-2 siRNA (Fig. 2C), suggesting a significant role for caspase-2 in triggering the intrinsic apoptotic pathway following the blockade of FASN. Moreover, activation of caspase-3 & -8 and cleavage of the effector caspase substrate, PARP, were almost completely suppressed by caspase-2 deficiency in orlistat-treated 420 cells (Fig. 2D, lane 2 vs. 4), whereas downregulation of caspase-8 partially prevented caspase-3 activation, but failed to inhibit loss of full-length caspase-2 (Fig. 2D, lane 2 vs. 6). These data suggest either that caspase-2 is upstream of both caspase-8 and Bax/Bak-mediated MOMP or that caspase-8 cleavage results from caspase 3 activation that occurs downstream of caspase-2-dependent MOMP. In either case, caspase-2 activation does not appear to depend on caspase-8, consistent with a dominant and apical role for caspase-2 in triggering the caspase cascade following the suppression of FASN.
Fig. 2. Orlistat-induced activation of the caspase cascade depends on caspase-2.
(A) Cotreatment with the caspase-2 inhibitor zVDVAD-fmk prevented orlistat from inducing a decrease in full-length Bid (short exposure) and the appearance of truncated Bid (long exposure; solid and empty arrows indicate full-length and cleaved Bid, respectively). (B) Inhibition of FASN by orlistat activated Bax and Bak. Active Bax or Bak were immunoprecipitated (IP) from the whole cell lysate (WCL) and detected as described in the Materials and Methods. (C) Downregulation of caspase-2 by RNAi dampened orlistat-induced Bax activation. (D) Inhibition of caspase-2 suppressed the activation of the caspase cascade (Solid and empty arrows indicate full-length and cleaved caspases, respectively). The loss of caspase-2 was revealed by a monoclonal caspase-2 antibody 11B4. 420 cell lysates were collected 12 h (A–C) or 24 h (D) post treatment.
Induction of REDD1 by ATF4 is required to activate caspase-2 after FASN inhibition
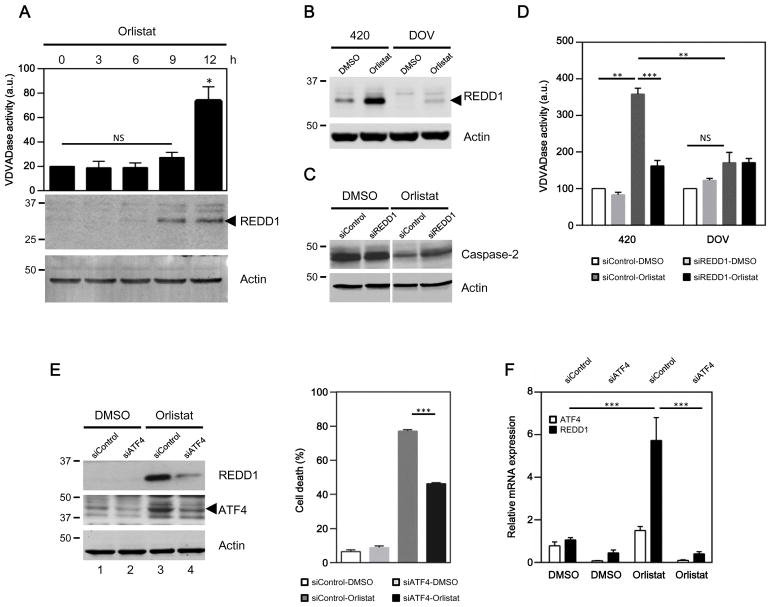
To gain more mechanistic insight into how suppression of FASN activates caspase-2, we examined the involvement of several proteins that have been implicated in cell death triggered by metabolic stresses, such as REDD1, a 2-deoxyglucose-inducible protein involved in neuronal loss in Parkinson’s disease and cigarette smoke-induced pulmonary pathology.35–37 Basal expression levels of REDD1 were too scarce to detect in most ovarian cancer cell lines (Fig. 3A & B) and patient biopsies examined (Supplementary Fig. S3A); however, REDD1 was readily induced transcriptionally after orlistat treatment (Supplementary Fig. S3B). Among cell death regulators we examined, REDD1 showed the strongest transcriptional induction by orlistat in 420 cells (followed by PUMA and ATF4). Temporal examination of REDD1 induction after orlistat treatment showed that REDD1 levels were elevated prior to an increase in the cellular proteolytic activity against VDVAD-pNA (a caspase-2 preferred substrate) in 420 cells (Fig. 3A). This induction was not suppressed by the pan-caspase inhibitor zVAD-fmk (Fig. 3B), indicating that REDD1 induction was unlikely to be a consequence of caspase activation. In contrast, orlistat only induced limited levels of REDD1 in DOV cells (Fig. 3B). Given the fact that REDD1 rose prior to the increase in caspase-2-like activity and that the induction levels of REDD1 correlated with caspase-2 activation, we reasoned that REDD1 might be required to trigger caspase-2 after FASN inhibition. In support of this notion, orlistat-induced cleavage (Fig. 3C) and caspase-2-like activities (Fig. 3D, left panel) were largely suppressed in 420 cells transfected with REDD1 siRNA.
Fig. 3. Inhibition of FASN activates caspase-2 through REDD1.
(A) REDD1 was upregulated prior to caspase-2 activation. 420 lysates were collected at the indicated time points and divided into two portions to examine cellular caspase-2-like (VDVADase) activities and REDD1 levels (*p<0.05; NS, not significant). (B) Caspase activities were dispensable for REDD1 induction by orlistat. REDD1 levels were monitored in 420 and DOV cells treated with DMSO or orlistat in the presence of zVAD-fmk. REDD1 is indicated by an arrow. (C) REDD1 siRNA prevented caspase-2 cleavage resulting from FASN inhibition in 420 cells. The loss of caspase-2 was revealed by a monoclonal caspase-2 antibody 11B4. (D) REDD1 deficiency suppressed caspase-2 activities induced after FASN inhibition. Orlistat induced strong caspase-2-like activities in 420 cells transfected with control siRNA, but this induction was largely suppressed in cells that received REDD1 siRNA (left panel; **p<0.01; ***p<0.001). The same treatment with orlistat only caused negligible VDVADase activity in cells incapable of inducing REDD1 (right panel; NS, not significant, p=0.98). (E) REDD1 induction and the subsequent cell death induced by FASN inhibition was abrogated in 420 cells by siATF4 (***p<0.001). (F) 420 cells were treated as in (E), and indicated mRNA levels were analyzed by a semi-quantitative PCR. Orlistat significantly increased REDD1 mRNA levels in cells transfected with control siRNA (black bars, siControl, DMSO vs. Orlistat), but not in ATF4 deficient cells (black bars, orlistat-treated cells, siControl vs. siATF4) (***p<0.001).
Since REDD1 induction appeared to be important for caspase-2 activation, we sought to elucidate how orlistat upregulates REDD1 levels. Several transcription factors, including HIF-1, ATF4 and C/EBP, have been shown to mediate REDD1 induction in response to various stimuli.36, 38, 39 Among them, the transcription factor ATF4 has been implicated in the induction of REDD1 in response to glucose deprivation.39 We found that downregulation of ATF4 drastically suppressed induction of REDD1 by orlistat and the subsequent cell death in 420 cells (Fig. 3E). As shown in Fig. 3F, REDD1 mRNA was increased by ~ 6-fold following orlistat treatment in control 420 cells (black bars, siControl, DMSO vs. Orlistat), whereas this induction was almost completely abrogated by ATF4 deficiency (black bars, orlistat-treated cells, siControl vs. siATF4). There data suggest that the increase in REDD1 protein following FASN inhibition most likely occurs via an ATF4-mediated transcriptional induction. Interestingly, DOV cells, which expressed much less REDD1 (Fig. 3B), were found to express significantly less ATF4 before and after orlistat treatment (Supplementary Fig. S3C), suggesting a plausible explanation for the differences in REDD1 induction levels and sensitivity to orlistat between 420 and DOV cells.
REDD1 suppresses mTOR to enable caspase-2-dependent ovarian cancer cell death following FASN inhibition
REDD1 has been found to impinge on mTOR activity by activating TSC2 after hypoxia.40, 41 Consistent with previous reports, REDD1 induction in orlistat-treated 420 cells was accompanied by a decrease in mTOR activity, as revealed by the dephosphorylation of 4EBP1, a well-characterized mTOR target (Fig. 4A, lane 1–4). Downregulation of REDD1 by RNAi prevented mTOR activities from falling after orlistat treatment (Fig. 4A, lane 4 vs. 8). TSC2 deficiency rendered cells refractory to orlistat (Fig. 4B, C and Supplementary Fig. S4A), suggesting that the suppression of mTOR might be critical for orlistat to induce cell death in ovarian cancer cells. To test this hypothesis, we examined the role of mTOR suppression in cellular sensitivity to orlistat. In REDD1-deficient 420 cells, in which mTOR inhibition was compromised (Fig. 4A, lane 4 vs. 8), cell death was significantly reduced (Fig. 4D, orlistat-treated siControl vs. siREDD1, white bars). Importantly, pharmacological suppression of mTOR with synthetic inhibitors, PP242 or rapamycin, restored cell death in REDD1-deficient 420 cells (Fig. 4D, orlistat-treated siREDD1, white bar vs. grey and black bars), indicating that REDD1 facilitated orlistat-induced cell death at least in part by blocking mTOR.
Fig. 4. REDD1 suppresses mTOR to control ovarian cell death induced by orlistat.
(A) Orlistat caused time-dependent induction of REDD1 (indicated by an arrow; an asterisk shows a non-specific signal), decreased phosphorylated 4EBP1 at S65, and induced a downshift of the 4EBP1 proteins in control 420 cells (lane 1–4). Knockdown of REDD1 by siRNA ameliorated the dephosphorylation of 4EBP1 at S65 (lane 3 vs.7 and 4 vs. 8) and preserved the slowly migrating/hyper-phosphorylated species (arrows) detected by the 4EBP1 antibody (lane 4 vs. 8). (B–C) Downregulation of TSC2 by siRNA (B) or shRNA (C) suppressed orlistat-induced 420 cell death (*p<0.05; **p<0.01). (D) Inhibition of mTOR by PP242 or rapamycin restored the sensitivity to orlistat in REDD1 deficient 420 cells (**p<0.01; ***p<0.001). (E) Suppression of mTOR by PP242 or rapamycin greatly enhanced DOV cell death induced by orlistat through a caspase-2-dependent pathway (*p<0.05; ***p<0.001; NS, not significant).
To examine whether this finding could be applied to reverse the resistance of cells inherently lacking the REDD1 induction machinery, DOV cells were treated with orlistat in the presence and absence of mTOR inhibitors. As indicated by the phosphorylation at 4EBP1 S65, DOV cells exhibited a higher basal mTOR activity than 420 cells (Supplementary Fig. S4B, upper panel, lane 1 vs. 3), inversely correlated with basal REDD1 expression pattern in these two cell lines (Fig. 3B). Orlistat completely removed phosphorylation at 4EBP1 S65 in 420 cells, whereas residual phosphorylation remained in DOV 24 h post orlistat treatment (Supplementary Fig. S4B, upper panel, lane 2 vs. 4), indicating mTOR was still active. The degree of PARP cleavage following orlistat treatment correlated with mTOR suppression, and was more evident in 420 cells (Supplementary Fig. S4B, middle panel, lane 2 vs. 4). Although PP242 treatment did not directly result in PARP cleavage in DOV cells (Supplementary Fig. S4B, lane 6), cotreatment of PP242 with orlistat removed residual mTOR activity and enhanced the cleavage of PARP (Supplementary Fig. S4B, lane 4 vs. 5), suggesting that orlistat-induced caspase activities could be enhanced in DOV cells when mTOR was suppressed, whereas mTOR inhibition was not sufficient to trigger cell death. Accordingly, inhibition of mTOR with either PP242 or rapamycin sensitized DOV to orlistat-induced death (Fig. 4E, orlistat-treated siControl, white bar vs. grey and black bars). However, this sensitization was greatly reduced by caspase-2 deficiency (Fig. 4E, orlistat-treated siCaspase-2, white bar vs. grey and black bars). A similar trend was observed in DOV cells transfected with another caspase-2 siRNA (Supplementary Fig. S4C). These data demonstrate that REDD1 suppressed mTOR to stimulate orlistat-induced cell death via caspase-2 activation.
REDD1 controls caspase-2 dimerization
Since our data supported a role for REDD1 in controlling caspase-2 activation following FASN inhibition, we wished to determine whether REDD1 could modulate caspase-2 dimerization, the first step in its activation. To this end, we adapted a recently developed bimolecular fluorescence complementation (BiFC) assay for caspase-232 using HeLa Tet-Off cells stably transfected with an inducible vector encoding a pair of caspase-2 BiFC constructs. Cells treated with either paclitaxel, a known caspase-2 activating drug, or orlistat emitted BiFC fluorescence, validating our experimental system and indicating that caspase-2 was activated following FASN inhibition (Fig. 5A, left panels). Notably, REDD1 was specifically induced by orlistat, but not by other caspase-2 activating drugs, even though they all caused similar levels of cell death (Fig. 5B). Importantly, orlistat-induced BiFC signals were largely suppressed in REDD1 deficient cells (Fig. 5A, right panels). To quantify this effect, BiFC cells transfected with two different REDD1 siRNAs were treated with orlistat in the presence or absence of Doxycycline (Dox) to modulate the expression of BiFC constructs. As shown in Fig. 5C, orlistat increased the BiFC fluorescence positive population in control cells (white bars, DMSO vs. Orlistat). However, this induction was greatly dampened by two independent REDD1 siRNAs (Orlistat-treated, white bar vs. grey and black bars), and the suppressive effects of each siRNA correlated with its ability to reduce orlistat-induced REDD1 (Fig. 5C, right panel). Cotreatment with Dox blocked the induction of fluorescence, indicating that the signals were specifically from caspase-2 BiFC constructs (Fig. 5C, orlistat+Dox-treated group). In concert with our previous examination of the cleavage and activity of caspase-2 (Fig. 3C, D), these data demonstrated that REDD1 was essential for caspase-2 dimerization and activation following the inhibition of FASN.
Fig. 5. Inhibition of FASN stimulates caspase-2 dimerization through REDD1.
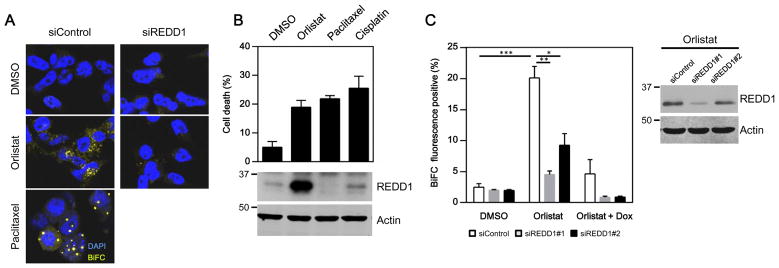
(A) Caspase-2 dimerization was monitored by a BiFC-based assay and recorded by photomicrographs taken with a confocal microscope. Paclitaxel, a known caspae-2 activator, was used as a positive control, whereas solvent (DMSO) was used as a negative control. Orlistat induced caspase-2 BiFC signals (yellow) in control BiFC cells but not in those transfected with REDD1 siRNA. DAPI (blue) stained the nucleus. (B) REDD1 expression was specifically induced upon FASN inhibition. BiFC cells were analyzed after indicated treatments by flow cytometry for cell death, and then lysed to measure REDD1 levels by western blotting. Actin was used as a loading control. (C) Orlistat induced BiFC signals in control BiFC cells, whereas this induction was largely suppressed by two different REDD1 siRNAs (middle, orlistat-treated group) or by doxycycline (Dox) (right, orlistat+Dox-treated group) (*p<0.05; **p<0.01; ***p<0.001). The efficacy of REDD1 siRNA was examined by western blotting.
DISCUSSION
Accumulating evidence indicates that dysregulated lipid metabolism supports the progression of ovarian cancer, representing a potential vulnerability for the development of diagnostic or therapeutic interventions to combat this leading cause of cancer-related death in women.42, 43 Experiments with inhibitors targeting FASN, a key enzyme mediating de novo lipogenesis, have encouragingly revealed their anticancer effects in cultured ovarian cancer cells or a xenograft mouse model.11–13 However, how repression of FASN reduces the survival of ovarian cancer cells is still not fully elucidated. Here, we demonstrate that REDD1 is transcriptionally induced by ATF4 following FASN inhibition, permitting the dimerization/activation of caspase-2, which in turn functions as the apical caspase to trigger cell death through a pathway involving MOMP. It appears that caspase-2 may also act upstream of caspase-8 to promote caspase-8 activation as caspase-8 cleavage following orlistat treatment was reduced by caspase-2 deficiency.
In an attempt to explore caspase-2’s role in cellular metabolism, we found that caspase-2 was activated in ovarian cancer cells following FASN inhibition, broadening caspase-2’s role in coordinating cellular energy status and death. Downregulation of caspase-2 by RNAi significantly ameliorated ovarian cancer cell death induced by orlistat. Besides caspase-2, other apoptotic regulators have been suggested to mediate the tumoricidal effects of FASN inhibition. Downregulation of FASN in breast cancer cells stimulates the expression of pro-apoptotic proteins, such as BNIP3, TRAIL, and DAPK2,44 whereas in melanoma, suppression of FASN reduces anti-apoptotic Bcl2 family proteins45 and activates caspase-8.34 It is not yet clear if there are cancer type/subtype specific signaling pathways or if particular pathways dominate in some cancer types. Our observations suggest that caspase-2 may act upstream of caspase-8 and induce MOMP through the Bid-Bax/Bak axis after FASN inhibition, at least in the case of ovarian cancer. Further investigation will help elucidate the functional hierarchy of caspase-2 and other apoptotic components in distinct cancer settings.
As we and others have previously shown that caspase-2 activation is suppressed by prodomain phosphorylations at S157 and S164,19, 27 we monitored how FASN inhibition modulated phosphorylation of the caspase-2 prodomain. However, phosphorylations at S157 and S164 appeared unchanged (data not shown). Instead, we found that REDD1 was induced by orlistat prior to caspase-2 activation, and that downregulation of this stress-inducible protein prevented the activation of caspase-2, as assessed by its cleavage, proteolytic activities, and dimerization. Interestingly, although both orlistat and paclitaxel activated caspase-2, the intracellular distribution pattern of the induced BiFC signal (an indicator of caspase-2 dimerization) was distinct, suggesting that FASN inhibition might activate caspase-2 through a platform distinct from that engaged by paclitaxel. In support of this notion, REDD1 was induced following the inhibition of FASN, but not by other caspase-2 activating agents employed, including paclitaxel.
REDD1 was initially identified as a developmentally-regulated protein expressed under the control of p53.46 Subsequent studies extended REDD1’s role in stress sensing by showing its induction by various stimuli, such as hypoxia, glucose deprivation, and cigarette smoke-induced oxidative stress; several transcription factors, including HIF-1, ATF4 and C/EBP have been implicated in REDD1 induction in response to these stimuli.36, 38, 39 Although the basal expression levels of REDD1 in ovarian cancer biopsies or cell lines that we examined were very limited, REDD1 protein levels were drastically upregulated in ovarian cancer cells that were sensitive to FASN inhibition, consistent with an increase in its mRNA observed by us and in a reported screen for genes upregulated in MDA-MB-435 cells following knockdown of FASN.34 This induction appeared to be dependent on ATF4, as the induction levels of ATF4 correlated with the expression levels of REDD1, while deficiency of this ER stress-related transcription factor reduced both the mRNA and protein levels of REDD1, suppressing the subsequent cell death following orlistat treatment. Interestingly, inhibition of FASN has been shown to trigger ER stress in cancer cells, with unclear functional consequences.7 Our findings, therefore, provide a potential molecular link between ER stress and cell death by showing that ATF4-induced REDD1 induction promotes caspase-2 activation. This suggests that the status of the ER functions may control the susceptibility of ovarian cancer cells to death following FASN inhibition.
Consistent with its reported function in hypoxia,40, 41 we found that REDD1 induced by orlistat also caused a suppression of mTOR in ovarian cancer cells. Inhibition of mTOR with PP242 or rapamycin restored the sensitivity of REDD1 deficient 420 cells, and even overcame the lack of REDD1 induction machinery in refractory cells, such as DOV, by stimulating caspase-2-dependent cell death after orlistat treatment, indicating mTOR inhibition is a proximal determinant that controls cell death after FASN inhibition by permitting caspase-2 dimerization and activation. Therefore, the persistent mTOR activity and resistance to orlistat observed in DOV cells could be, at least in part, attributed to the lower steady-state levels of ATF4 and REDD1 and/or their weak induction after FASN inhibition.
Given the fact that mTOR is capable of stimulating FASN expression through SREBP,47 in theory REDD1-mediated inhibition of mTOR could further inhibit lipogenesis by reducing the expression of FASN. However, FASN protein levels remained constant throughout the course of our experiments (data not shown). Meanwhile, since mTOR inhibitors did not directly trigger cell death, but sensitized DOV cells to orlistat-induced PARP cleavage and death, our data collectively indicate that mTOR inhibition is essential, though not sufficient, to activate caspase-2 to initiate the death signaling in ovarian cancer cells after FASN inhibition. Precisely, how REDD1-mediated mTOR suppression facilitates caspase-2 activation is currently under investigation. Nonetheless, these data indicate, for the first time, that mTOR activity controls caspase-2 activation, and provide supportive rationale for the use of mTOR inhibitors to enhance caspase-2-mediated cell death in ovarian cancer treatment.
MATERIALS AND METHODS
Ovarian cancer biopsies and cell culture
Ovarian cancer biopsies and cell lines OVCA420 (420) and DOV13 (DOV) were kindly provided by Dr. Susan K. Murphy and Dr. Andrew Berchuck (Duke University, Durham, NC, USA), and grown at 37°C in RPMI 1640 media supplemented with 10% FBS. The purity of both lines was analyzed and confirmed using the PowerPlex 18D kit (Promega, Madison, WI, USA) for polymorphic short tandem repeat (STR) markers (The Duke University DNA Analysis Facility, NC, USA). Cells were treated with 100 or 200 μM orlistat or DMSO (vehicle) and then collected for assays 18–24 h post treatment or at indicated time points. Caspase-2 specific inhibitor zVDVAD-fmk (Enzo, Farmingdale, NY, USA) was utilized at 50 μM when indicated, while 50 μM pan-caspase inhibitor zVAD-fmk (Enzo) was cotreated with orlistat to examine REDD1 expression, unless otherwise specified. To suppress mTOR, 4 μM PP242 and 10 μM (420) or 5 μM (DOV) rapamycin were cotreated with orlistat as indicated. Cell death and caspase activity were measured as previously described by flow cytometry or using the substrates specific for different caspases [Ac-VDVAD-pNA (Caspase-2) or Ac-DEVD-pNA (caspase-3/7), Enzo] 48. To monitor mitochondrial release of cytochrome c, treated cells were incubated in digitonin-based buffer (250 mM sucrose, 20 mM Tris, pH 7.4, 10 mM KCl, 5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 × Roche complete protease inhibitor, 250 μg/mL digitonin) on ice for 10 min; cytosolic and mitochondrial fractions were then separated by centrifugation. Otherwise, cells were lysed as previously described for immunoblotting.48
RNA interference
Cells were transfected with 50 nM siRNA twice on two consecutive days by Lipofectamine RNAiMAX reagents (Invitrogen, Grand Island, NY, USA), and assayed 48 h after the first transfection. The sources and sequences of siRNA could be found in the Supplementary information. To generate a stable TSC2 knockdown cell line, plasmids encoding scramble shRNA (Addgene, Cambridge, MA, USA, plasmid 1864) or TSC2 shRNA (Addgene, plasmid 15478) were used to generate lentivirus. 420 cells were infected with the virus and selected with 2 μg/mL puromycin. Knockdown efficiency was examined by immunoblotting.
Reagents and antibodies
Orlistat and cisplatin were from Sigma, St. Louis, MO, USA or Cayman Chemical, Ann Arbor, MI, USA; paclitaxel and rapamycin were from LC Laboratories, Woburn, MA, USA; PP242 was from Active Biochem, Maplewood, NJ, USA. Antibodies were purchased from Abcam, Cambridge, MA, USA (Polyclonal caspase-2 from rabbit, Actin, tubulin), Enzo (Monoclonal caspase-2 from rat, 11B4), BD Pharmingen, San Jose, CA, USA (Active Bax 6A7, cytochrome c), Santa Cruz, Dallas, TX, USA (Actin), Proteintech (REDD1, ATF4), CalBiochem, Billerica, MA, USA (Active Bak Ab-1), Cell Signaling, Danvers, MA, USA (Caspase-8, Caspase-9, Caspase-3, cleaved PARP, Bid, Bax, Bak, COX IV, pS65 4EBP1, total 4EBP1, TSC2), and specified in the Supplementary information.
Bimolecular fluorescence complementation (BiFC)
The BiFC assay was adapted from Bouchier-Hayes et al.,32 and described in detail in the Supplementary information. The BiFC signals were recorded by Leica SP5 confocal microscopy or flow cytometry. To examine REDD1 induction, a similar experiment was performed, and cells were treated with DMSO (vehicle), 200 μM orlistat, 10 μM paclitaxel, or 10 μM cisplatin for 24 h. Treated cells were assayed by flow cytometry for cell death, or lysed to examine REDD1 induction by immunoblotting. Pan-caspase inhibitor q-VD-OPh (10 μM) was used in concert with pro-apoptotic drugs to suppress cell death.
Immunoprecipitation of active Bax/Bak
Active Bax and Bak were detected as described previously.49, 50 Immunoprecipitants collected with active Bax or Bak antibodies were resolved by a SDS-PAGE gel and detected by immunoblotting with antibodies against total Bax or Bak.
Statistical analysis
Data from flow cytometry-based experiments and caspase assays were shown as mean±sem; qPCR results were shown as mean±sd. Data were analyzed by unpaired two-tailed Student’s t-test.
Supplementary Material
Acknowledgments
We thank Drs. Susan Murphy and Andrew Berchuck for de-identified human ovarian cancer biopsy samples, and the members of the Kornbluth laboratory for sharing reagents and discussion. This work was supported by R01 GM080333 to SK.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
C.-S.Y. and S.K. developed the concept and wrote the manuscript. C.-S. Y. and K.M. designed, performed experiments and analyzed data, with help from A.C.R. for the BiFC assays, from N.-J.H. for immunoprecipitation of active Bax/Bak, from B.H. for detection of caspase-2 phosphorylation and from L.Z. for qPCR.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012 Nov 15;491(7424):364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 3.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008 Sep 5;134(5):703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Santos CR, Schulze A. Lipid metabolism in cancer. The FEBS journal. 2012 Aug;279(15):2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 5.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature reviews Cancer. 2007 Oct;7(10):763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 6.Currie E, Schulze A, Zechner R, Walther TC, Farese RV., Jr Cellular fatty acid metabolism and cancer. Cell metabolism. 2013 Aug 6;18(2):153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer research. 2007 Feb 1;67( 3):1262–1269. doi: 10.1158/0008-5472.CAN-06-1794. [DOI] [PubMed] [Google Scholar]
- 8.Alo PL, Visca P, Framarino ML, Botti C, Monaco S, Sebastiani V, et al. Immunohistochemical study of fatty acid synthase in ovarian neoplasms. Oncology reports. 2000 Nov-Dec;7(6):1383–1388. doi: 10.3892/or.7.6.1383. [DOI] [PubMed] [Google Scholar]
- 9.Gansler TS, Hardman W, 3rd, Hunt DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Human pathology. 1997 Jun;28(6):686–692. doi: 10.1016/s0046-8177(97)90177-5. [DOI] [PubMed] [Google Scholar]
- 10.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature medicine. 2011;17(11):1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, et al. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer research. 2007 Apr 1;67(7):2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- 12.Rahman MT, Nakayama K, Rahman M, Katagiri H, Katagiri A, Ishibashi T, et al. Fatty acid synthase expression associated with NAC1 is a potential therapeutic target in ovarian clear cell carcinomas. British journal of cancer. 2012 Jul 10;107(2):300–307. doi: 10.1038/bjc.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomek K, Wagner R, Varga F, Singer CF, Karlic H, Grunt TW. Blockade of fatty acid synthase induces ubiquitination and degradation of phosphoinositide-3-kinase signaling proteins in ovarian cancer. Molecular cancer research : MCR. 2011 Dec;9(12):1767–1779. doi: 10.1158/1541-7786.MCR-10-0467. [DOI] [PubMed] [Google Scholar]
- 14.Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, et al. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes & development. 1998 May 1;12( 9):1304–1314. doi: 10.1101/gad.12.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Padalecki SS, Chaudhuri AR, De Waal E, Goins BA, Grubbs B, et al. Caspase-2 deficiency enhances aging-related traits in mice. Mechanisms of ageing and development. 2007 Feb;128(2):213–221. doi: 10.1016/j.mad.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puccini J, Shalini S, Voss AK, Gatei M, Wilson CH, Hiwase DK, et al. Loss of caspase-2 augments lymphomagenesis and enhances genomic instability in Atm-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2013 Dec 3;110(49):19920–19925. doi: 10.1073/pnas.1311947110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho LH, Taylor R, Dorstyn L, Cakouros D, Bouillet P, Kumar S. A tumor suppressor function for caspase-2. Proceedings of the National Academy of Sciences of the United States of America. 2009 Mar 31;106(13):5336–5341. doi: 10.1073/pnas.0811928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons MJ, McCormick L, Janke L, Howard A, Bouchier-Hayes L, Green DR. Genetic deletion of caspase-2 accelerates MMTV/c-neu-driven mammary carcinogenesis in mice. Cell death and differentiation. 2013 Sep;20(9):1174–1182. doi: 10.1038/cdd.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen JL, Thompson JW, Lindblom KR, Johnson ES, Yang CS, Lilley LR, et al. A biotin switch-based proteomics approach identifies 14-3-3zeta as a target of Sirt1 in the metabolic regulation of caspase-2. Molecular cell. 2011 Sep 2;43(5):834–842. doi: 10.1016/j.molcel.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nutt LK, Margolis SS, Jensen M, Herman CE, Dunphy WG, Rathmell JC, et al. Metabolic regulation of oocyte cell death through the CaMKII-mediated phosphorylation of caspase-2. Cell. 2005 Oct 7;123(1):89–103. doi: 10.1016/j.cell.2005.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouchier-Hayes L. The role of caspase-2 in stress-induced apoptosis. Journal of cellular and molecular medicine. 2010 Jun;14(6A):1212–1224. doi: 10.1111/j.1582-4934.2010.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho LH, Read SH, Dorstyn L, Lambrusco L, Kumar S. Caspase-2 is required for cell death induced by cytoskeletal disruption. Oncogene. 2008 May 29;27(24):3393–3404. doi: 10.1038/sj.onc.1211005. [DOI] [PubMed] [Google Scholar]
- 23.Vakifahmetoglu H, Olsson M, Tamm C, Heidari N, Orrenius S, Zhivotovsky B. DNA damage induces two distinct modes of cell death in ovarian carcinomas. Cell death and differentiation. 2008 Mar;15(3):555–566. doi: 10.1038/sj.cdd.4402286. [DOI] [PubMed] [Google Scholar]
- 24.Bonzon C, Bouchier-Hayes L, Pagliari LJ, Green DR, Newmeyer DD. Caspase-2-induced apoptosis requires bid cleavage: a physiological role for bid in heat shock-induced death. Molecular biology of the cell. 2006 May;17(5):2150–2157. doi: 10.1091/mbc.E05-12-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES. Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. The Journal of biological chemistry. 2002 Apr 19;277(16):13430–13437. doi: 10.1074/jbc.M108029200. [DOI] [PubMed] [Google Scholar]
- 26.Lin CF, Chen CL, Chang WT, Jan MS, Hsu LJ, Wu RH, et al. Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramideand etoposide-induced apoptosis. The Journal of biological chemistry. 2004 Sep 24;279(39):40755–40761. doi: 10.1074/jbc.M404726200. [DOI] [PubMed] [Google Scholar]
- 27.Shin S, Lee Y, Kim W, Ko H, Choi H, Kim K. Caspase-2 primes cancer cells for TRAIL-mediated apoptosis by processing procaspase-8. The EMBO journal. 2005 Oct 19;24( 20):3532–3542. doi: 10.1038/sj.emboj.7600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baliga BC, Read SH, Kumar S. The biochemical mechanism of caspase-2 activation. Cell death and differentiation. 2004 Nov;11(11):1234–1241. doi: 10.1038/sj.cdd.4401492. [DOI] [PubMed] [Google Scholar]
- 29.Tinel A, Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004 May 7;304(5672):843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- 30.Ribe EM, Jean YY, Goldstein RL, Manzl C, Stefanis L, Villunger A, et al. Neuronal caspase 2 activity and function requires RAIDD, but not PIDD. The Biochemical journal. 2012 Jun 15;444(3):591–599. doi: 10.1042/BJ20111588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manzl C, Krumschnabel G, Bock F, Sohm B, Labi V, Baumgartner F, et al. Caspase-2 activation in the absence of PIDDosome formation. The Journal of cell biology. 2009 Apr 20;185(2):291–303. doi: 10.1083/jcb.200811105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouchier-Hayes L, Oberst A, McStay GP, Connell S, Tait SW, Dillon CP, et al. Characterization of cytoplasmic caspase-2 activation by induced proximity. Molecular cell. 2009 Sep 24;35(6):830–840. doi: 10.1016/j.molcel.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nutt LK, Buchakjian MR, Gan E, Darbandi R, Yoon SY, Wu JQ, et al. Metabolic control of oocyte apoptosis mediated by 14-3-3zeta-regulated dephosphorylation of caspase-2. Developmental cell. 2009 Jun;16(6):856–866. doi: 10.1016/j.devcel.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knowles LM, Yang C, Osterman A, Smith JW. Inhibition of fatty-acid synthase induces caspase-8-mediated tumor cell apoptosis by up-regulating DDIT4. The Journal of biological chemistry. 2008 Nov 14;283(46):31378–31384. doi: 10.1074/jbc.M803384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malagelada C, Ryu EJ, Biswas SC, Jackson-Lewis V, Greene LA. RTP801 is elevated in Parkinson brain substantia nigral neurons and mediates death in cellular models of Parkinson’s disease by a mechanism involving mammalian target of rapamycin inactivation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006 Sep 27;26(39):9996–10005. doi: 10.1523/JNEUROSCI.3292-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida T, Mett I, Bhunia AK, Bowman J, Perez M, Zhang L, et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nature medicine. 2010 Jul;16(7):767–773. doi: 10.1038/nm.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Molecular and cellular biology. 2005 Jul;25(14):5834–5845. doi: 10.1128/MCB.25.14.5834-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, et al. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Molecular and cellular biology. 2002 Apr;22(7):2283–2293. doi: 10.1128/MCB.22.7.2283-2293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin HO, Seo SK, Kim YS, Woo SH, Lee KH, Yi JY, et al. TXNIP potentiates Redd1-induced mTOR suppression through stabilization of Redd1. Oncogene. 2011 Sep 1;30( 35):3792–3801. doi: 10.1038/onc.2011.102. [DOI] [PubMed] [Google Scholar]
- 40.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes & development. 2004 Dec 1;18(23):2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes & development. 2008 Jan 15;22(2):239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pyragius CE, Fuller M, Ricciardelli C, Oehler MK. Aberrant lipid metabolism: an emerging diagnostic and therapeutic target in ovarian cancer. International journal of molecular sciences. 2013;14(4):7742–7756. doi: 10.3390/ijms14047742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tania M, Khan MA, Song Y. Association of lipid metabolism with ovarian cancer. Current oncology. 2010 Oct;17(5):6–11. doi: 10.3747/co.v17i5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bandyopadhyay S, Zhan R, Wang Y, Pai SK, Hirota S, Hosobe S, et al. Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer research. 2006 Jun 1;66(11):5934–5940. doi: 10.1158/0008-5472.CAN-05-3197. [DOI] [PubMed] [Google Scholar]
- 45.Ho TS, Ho YP, Wong WY, Chi-Ming Chiu L, Wong YS, Eng-Choon Ooi V. Fatty acid synthase inhibitors cerulenin and C75 retard growth and induce caspase-dependent apoptosis in human melanoma A-375 cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2007 Oct;61(9):578–587. doi: 10.1016/j.biopha.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 46.Ellisen LW, Ramsayer KD, Johannessen CM, Yang A, Beppu H, Minda K, et al. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Molecular cell. 2002 Nov;10(5):995–1005. doi: 10.1016/s1097-2765(02)00706-2. [DOI] [PubMed] [Google Scholar]
- 47.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell metabolism. 2008 Sep;8(3):224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang CS, Thomenius MJ, Gan EC, Tang W, Freel CD, Merritt TJ, et al. Metabolic regulation of Drosophila apoptosis through inhibitory phosphorylation of Dronc. The EMBO journal. 2010 Sep 15;29(18):3196–3207. doi: 10.1038/emboj.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang NJ, Zhang L, Tang W, Chen C, Yang CS, Kornbluth S. The Trim39 ubiquitin ligase inhibits APC/CCdh1-mediated degradation of the Bax activator MOAP-1. The Journal of cell biology. 2012 Apr 30;197(3):361–367. doi: 10.1083/jcb.201111141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Upreti M, Chu R, Galitovskaya E, Smart SK, Chambers TC. Key role for Bak activation and Bak-Bax interaction in the apoptotic response to vinblastine. Molecular cancer therapeutics. 2008 Jul;7(7):2224–2232. doi: 10.1158/1535-7163.MCT-07-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.