Abstract
Inhibitory neurons make up a significant fraction of the neurons within the preBötzinger Complex (preBötC), a site critical for mammalian eupneic breathing. The role of glycinergic preBötC neurons in respiratory rhythmogenesis in mice was investigated by optogenetically-targeted excitation or inhibition. Channelrhodopsin-2 (ChR2) or Archaerhodopsin (Arch) was expressed in glycinergic preBötC neurons of glycine transporter 2 (GlyT2)-Cre mice. In ChR2-transfected mice, brief inspiratory-phase bilateral photostimulation targeting the preBötC prematurely terminated inspiration, whereas expiratory-phase photostimulation delayed the onset of the next inspiration. Prolonged photostimulation produced apneas lasting as long as the light pulse. Inspiratory-phase photoinhibition in Arch-transfected mice during inspiration increased tidal volume without altering inspiratory duration, whereas expiratory-phase photoinhibition shortened the latency until the next inspiration. During persistent apneas, prolonged photoinhibition restored rhythmic breathing. We conclude that glycinergic preBötC neurons modulate inspiratory pattern and are important for reflex apneas but that the rhythm can persist after significant dampening of their activity.
Keywords: breathing, preBötzinger Complex, optogenetics, respiratory rhythm, adeno-associated virus, inhibition
Introduction
Breathing in mammals is a complex behavior that must work (nearly) continuously from birth throughout life and can be rapidly modified, even transiently stopped, to support a wide range of physiological and functional demands. The preBötC, in the ventrolateral medulla, is a critical locus for respiratory rhythmogenesis in the intact eupneic mammal1 and in reduced rodent en bloc and slice preparations2. The preBötC contains intermingled heterogeneous populations of excitatory and inhibitory neurons3, 4. Excitatory preBötC neurons are critical components of the respiratory central pattern generator (CPG)1, 5–7. However, the role of inhibitory preBötC neurons is not well understood. Although strong inhibitory currents are observed in intracellular recordings of presumptive preBötC neurons in deeply anesthetized adult cats and rodents8, 9, respiratory rhythm persists after bilateral blockade of fast inhibitory neurotransmission in the preBötC in anesthetized spontaneously breathing adult rats10. Since there is a substantial population of glycinergic preBötC neurons3, some of which are phasically active during inspiration11, we wanted to determine their contribution to breathing movements by examining the effects of rapid, acute perturbations of their ongoing activity in vivo.
We utilized optogenetic tools to probe the functional contribution of glycinergic preBötC neurons to respiratory rhythm and pattern. We used viral transfection to express Channelrhodopsin-2 (ChR2) or Archaerhodopsin (Arch) in glycinergic preBötC neurons by locally injecting cre recombinase-dependent viruses into the preBötC of transgenic mice expressing Cre driven by the glycine transporter 2 (GlyT2) promoter12–14. Since the GlyT2 promoter is specific to glycinergic neurons15, this intersectional strategy allowed us to selectively activate and silence these inhibitory neurons in intact, awake or anesthetized, spontaneously breathing mice. We found that perturbing glycinergic neuronal activity in the preBötC profoundly modulated the amplitude and timing of inspiratory motor output and expiratory period with no indications that they were critical for rhythmogenesis.
Results
Targeting opsin expression to preBötC GlyT2 neurons (Fig. 1)
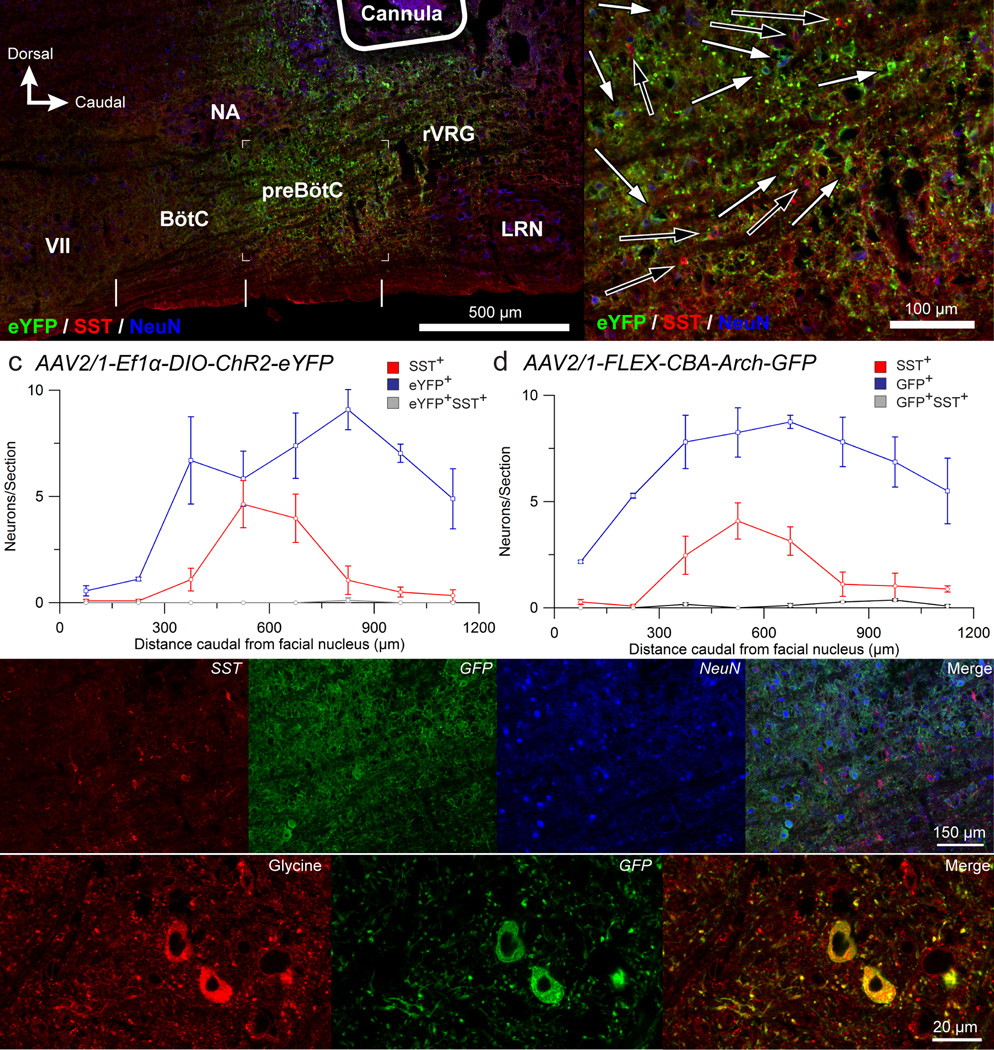
Figure 1. Cre-dependent ChR2 or Arch expression targeted to preBötC GlyT2 neurons.
(a) Representative confocal mosaic micrograph of sagittal brainstem section of GlyT2-cre+ mouse showing the extent of eYFP+ and Sst+ neurons after injection of AAV2/1-Ef1α-DIO-ChR2-eYFP into preBötC (n = 5). Vertical white lines at bottom mark approximate rostral-caudal boundaries of Bötzinger Complex (BötC) and preBötzinger Complex (preBötC). Outline of cannula tip placement at top right. No labeled somas were found in the brainstem outside the boundaries of this micrograph. Abbreviations: VII: facial nucleus; NA: nucleus ambiguus; LRN: lateral reticular nucleus. (b) High magnification micrograph of bracketed segment in (a) showing eYFP+ preBötC neurons (white arrows) intermingled with Sst+ neurons (blue arrows). (c, d) Distribution of eYFP/GFP+ (blue line), marking location of ChR2- (c; n = 3) or Arch- (d; n = 3) expressing neurons and Sst+ (red line) neurons relative to caudal boundary of facial nucleus. Error bars: mean ± s.e.m. (e) Representative single channel and overlay confocal micrographs showing eYFP+ preBötC neurons (green) after AAV2/1-Ef1α-DIO-ChR2-eYFP injection. Section also labeled for Sst (red) and NeuN (blue) immunoreactivity. (f) Single channel and overlay confocal micrographs (n = 3) showing eYFP+ preBötC neurons (green) with glycine immunoreactivity (red).
To express ChR2 or Arch in glycinergic preBötC neurons, we used adeno-associated viruses (AAV 2/1) encoding either ChR2-eYFP driven by the constitutive promoter Ef1α in a double floxed inverted open reading frame configuration (DIO-ChR2)16 or Arch-GFP driven by the constitutive chicken β-actin (CBA) promoter in a FLEX switch (FLEX-Arch). We injected either virus bilaterally into the preBötC of transgenic mice expressing Cre recombinase under the GlyT2 promoter (Fig. 1a, b). These injections produced protein expression in preBötC neurons that was detectable post hoc, with some expression in neighboring neurons outside the preBötC including in the ventral respiratory column (VRC) in the rostral-caudal direction, particularly caudal to the preBötC (Fig 1a–d). We found no evidence of transfected neurons at more distant brainstem sites including those with established projections to preBötC, e.g., nucleus of the solitary tract (NTS), parabrachial nucleus. The peak density of transfected neurons was caudal to the facial nucleus (VII) by 750 – 900 µm in ChR2-transfected mice and 600 – 750 µm in Arch-transfected mice and ventral to the compact nucleus ambiguus, which predominately overlapped with the rostrocaudal distribution of somatostatin (Sst)-expressing glutamatergic neurons1, 4 (Fig. 1c, d) that demark the preBötC4. Within the preBötC, transfected neurons intermingled but rarely colocalized with Sst immunoreactivity in either DIO-ChR2 (0.3 ± 0.6 out of 45.5 ± 13.4 Sst+ neurons; n = 3) or FLEX-Arch (2.0 ± 0.0 out of 36.3 ± 9.8 Sst+ neurons; n = 3) transfected mice (Fig. 1e). EYFP/GFP expression colocalized with glycine immunoreactivity in both sets of mice (Fig. 1f).
To efficiently deliver light specifically into the preBötC, we implanted optical cannulae bilaterally from a dorsal approach, about 200 – 400 µm dorsal to the preBötC (Fig 1a–b). By placing the cannulae close to the preBötC, we ensured that the pool of light-responsive neuronal somas able to affect breathing were preBötC GlyT2 neurons. As delineated in the DISCUSSION, the most parsimonious explanation of our results is that the light-induced responses were due to activation of preBötC GlyT2 neurons.
Brief photostimulation of ChR2-transfected neurons (Fig. 2)
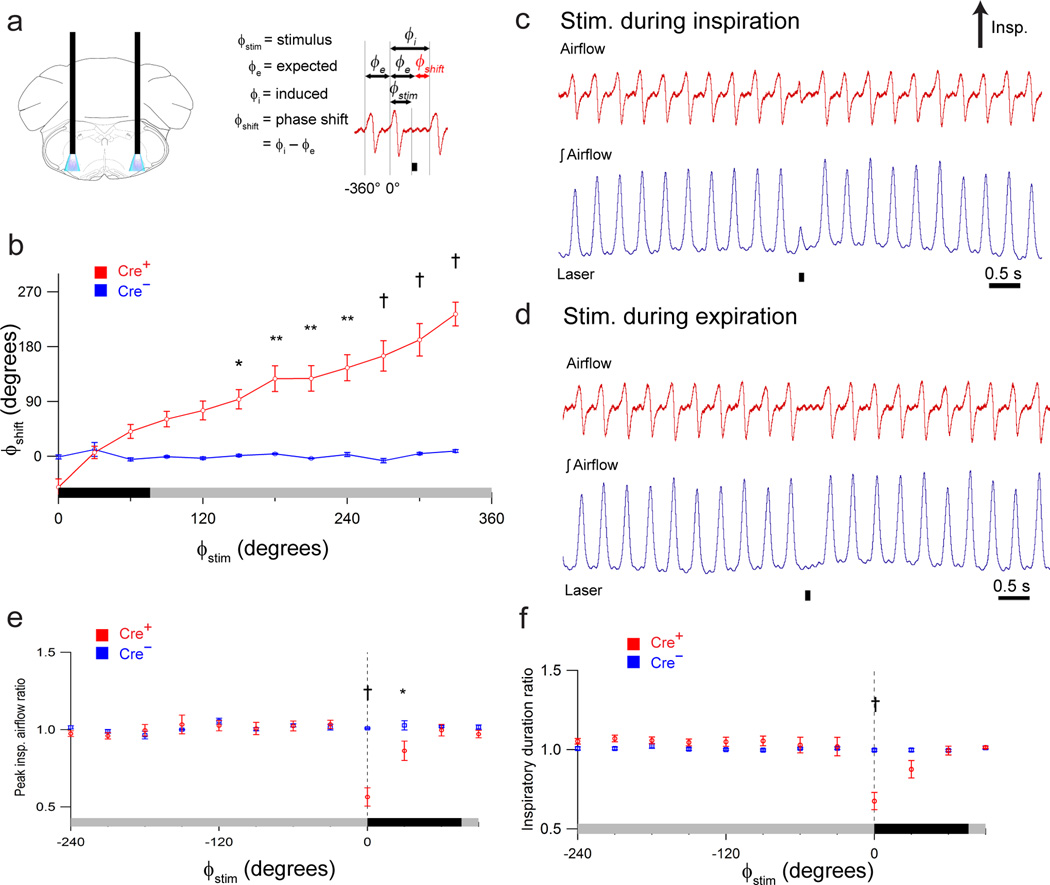
Figure 2. Photostimulation of preBötC GlyT2 neurons depresses breathing.
(a) Left: Schematic depicting bilateral placement of optical cannulae targeting preBötC. Right: schematic airflow trace depicting definitions of stimulus phase (ϕstim), expected phase (ϕe), induced phase (ϕi), and phase shift (ϕshift), demarcated with horizontal arrows, relative to the reference cycle that spans 0° – 360°; the control cycle preceding reference cycle spans phase 360° – 0°. Black bar below trace is laser-on period that defines the start of ϕstim. ϕe is the period of the previous control respiratory cycle, and ϕshift is the difference between ϕe and ϕi. (b) Shift in respiratory phase (ϕshift) resulting from bilateral photostimulation (100 ms pulse) of preBötC GlyT2 neurons in GlyT2-cre+ (Cre+, red; n = 5) and GlyT2-cre− (Cre−, blue; n = 5) mice. Stimulus phase (ϕstim) is depicted on x-axis (b, e, f) with inspiration (black bar) defined from 0° – 72°, with gray bar: (b) the subsequent expiration defined from 72° – 360° or (e, f) the preceding expiration defined from −288° – 0°. P values: 150 – 180°: P = 0.007; 180 – 210°: P = 2×10−5; 210 – 240°: P = 4×10−6; 240 – 270°: P = 4×10−7; 270 – 300°: P = 1×10−9; 300 – 330°: P = 5×10−10; 330 – 360°: P = 4×10−10. (c, d) Representative airflow and tidal volume (airflow) traces illustrating effect of photostimulation (100 ms pulse; black bar beneath trace) during inspiration (c) and expiration (d). (e, f) Comparison of ratio of peak inspiratory airflow (e; 0 – 30°: P = 4×10−10; 30 – 60°: P = 0.02) or inspiratory duration (f; 0 – 30°: P = 4×10−10) in GlyT2-cre+ (Cre+, red) and GlyT2-cre− (Cre−, blue) anesthetized mice. Dotted vertical line (e, f) at 0° indicates onset of inspiration. Error bars, mean ± s.e.m. Statistical significance was determined with a one-way ANOVA and pair-wise comparisons were made with Tukey’s HSD test. * P < 0.05; ** P < 0.001; † P < 10−8.
In anesthetized Cre+ mice, brief bilateral photostimulation (100 ms pulse; 473 nm; Fig. 2a) of ChR2-transfected preBötC GlyT2 neurons generated respiratory phase-dependent changes in breathing (n = 5). As revealed by phase response curves (see Methods and Fig. 2a right), ChR2 activation affected respiratory cycle timing, peak inspiratory airflow, and inspiratory duration (Fig. 2b–f).
Bilateral photostimulation during early inspiration (ϕstim: 0° – 30°; see Fig. 2a right) resulted in premature inspiratory burst termination, i.e., a breath with truncated peak inspiratory airflow (decreased to 56.4 ± 5.9% of control; P = 10−10; n = 5; Fig. 2c, e) and shortened inspiratory duration (decreased to 67.5 ± 5.4% of control; P = 10−10; n = 5; Fig. 2c, f). In contrast, stimulation during expiration (ϕstim: 180° – 360°) delayed the onset of the subsequent inspiration, with the strongest effect during the late expiratory (ϕstim: 330° – 360°; often referred to as the preinspiratory (pre-I)) phase (Fig. 2b, d) with a 233° ± 20° shift (P = 2×10−10; n = 5), while stimulations falling earlier in expiration, i.e., (ϕstim: 150° – 180°), produced a smaller 94° ± 16° shift (P = 0.007; n = 5; Fig. 2b). Following this phase shift, the next inspiration had no significant change in amplitude (101 ± 3% of control; P = 1; n = 5) or duration (104 ± 3% of control; P = 1; n = 5).
Prolonged photostimulation of ChR2-transfected neurons (Fig. 3)
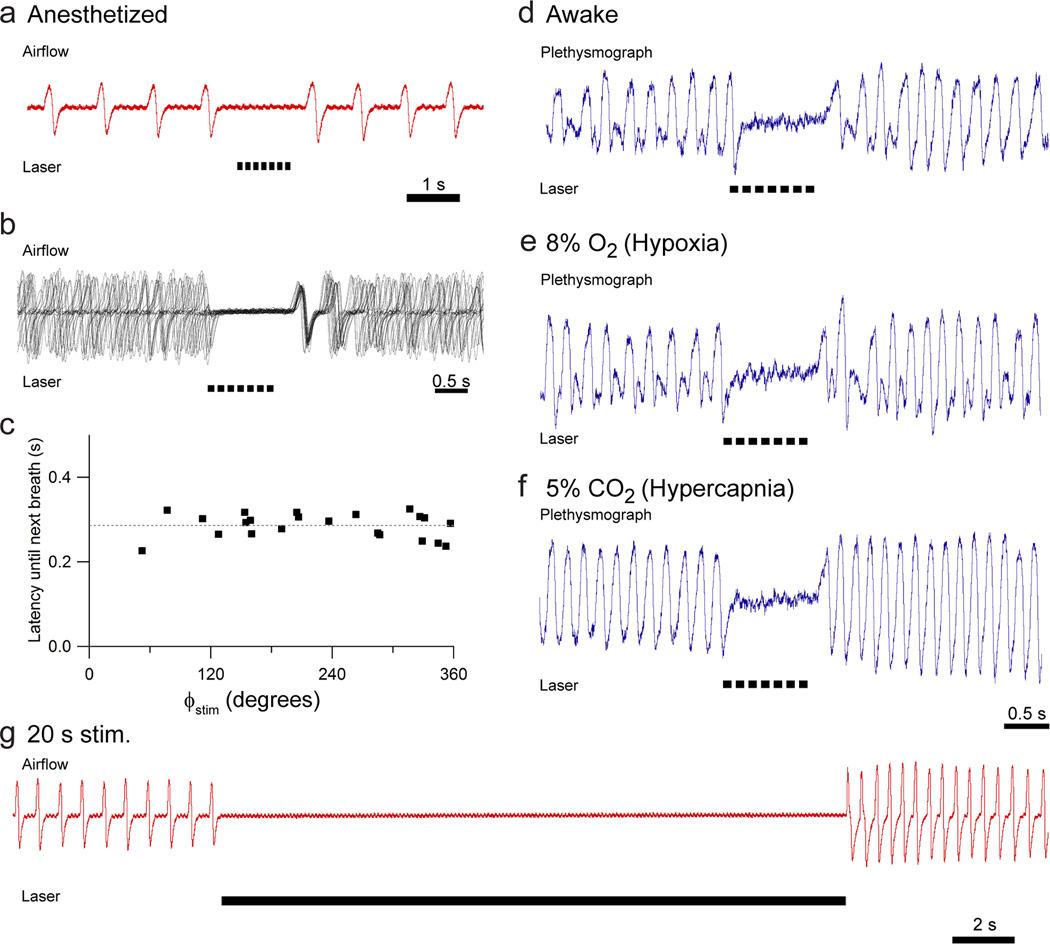
Figure 3. Prolonged photostimulation of preBötC GlyT2 neurons results in apnea.
(a) Representative airflow trace illustrating effect of 1 s pulse train of photostimulation (black bars beneath trace; 7× 100ms pulses with a 50 ms interpulse interval; n = 5). (b) Overlay of airflow traces aligned to the laser onset at various phases of respiratory cycle (7× 100 ms pulses with a 50 ms interpulse interval) reveals that the next breath occurred at a fairly constant delay after the laser shuts off. (c) Representative graph of latency to next breath as a function of initial phase of photostimulation. Dotted line indicates mean latency. (d, e, f) Photostimulation with a 1 s pulse train (7× 100 ms pulses with 50 ms interpulse interval) of awake, behaving mice in a plethysmograph under eupneic (d; n = 5), hypoxic (e; n = 5), or hypercapnic (f; n = 5) states. (g) Airflow during bilateral 20 s pulse train of light (100 ms pulses with 50 ms interpulse interval; n = 3) in anesthetized mouse.
In anesthetized ChR2-transfected mice, a 1 s photostimulus (7 × 100 ms pulses, 50 ms interpulse interval) at any time during the respiratory cycle stopped breathing, i.e., produced an apnea, which continued until after the light shut off (Fig. 3a; n = 5). The subsequent respiratory cycle began at a fairly constant delay after the laser turned off regardless of stimulus phase (Fig. 3b, c). The duration of this delay was unique to each mouse (300 – 860 ms, min-max; n = 3), presumably due to differences in the precise position of the virus injection and optical fiber placement. When tested in awake mice, a 1 s pulse train consistently produced apneas (Fig. 3d; n = 5). We further tested photostimulus responses in anesthetized mice when ventilation was increased during 5 min of either hypoxic (8% O2, 92% N2; n = 5) or hypercapnic (5% CO2 in room air; n = 5) inspired gas mixtures, finding that ChR2 activation still consistently produced apnea in either of these conditions of increased respiratory drive (Fig. 3e, f). Prolonged stimulation (up to 20 s pulse trains; n = 3) produced an apnea that persisted until the laser turned off (Fig. 3g).
Photoinhibition of Arch-transfected preBötC GlyT2 neurons (Fig. 4)
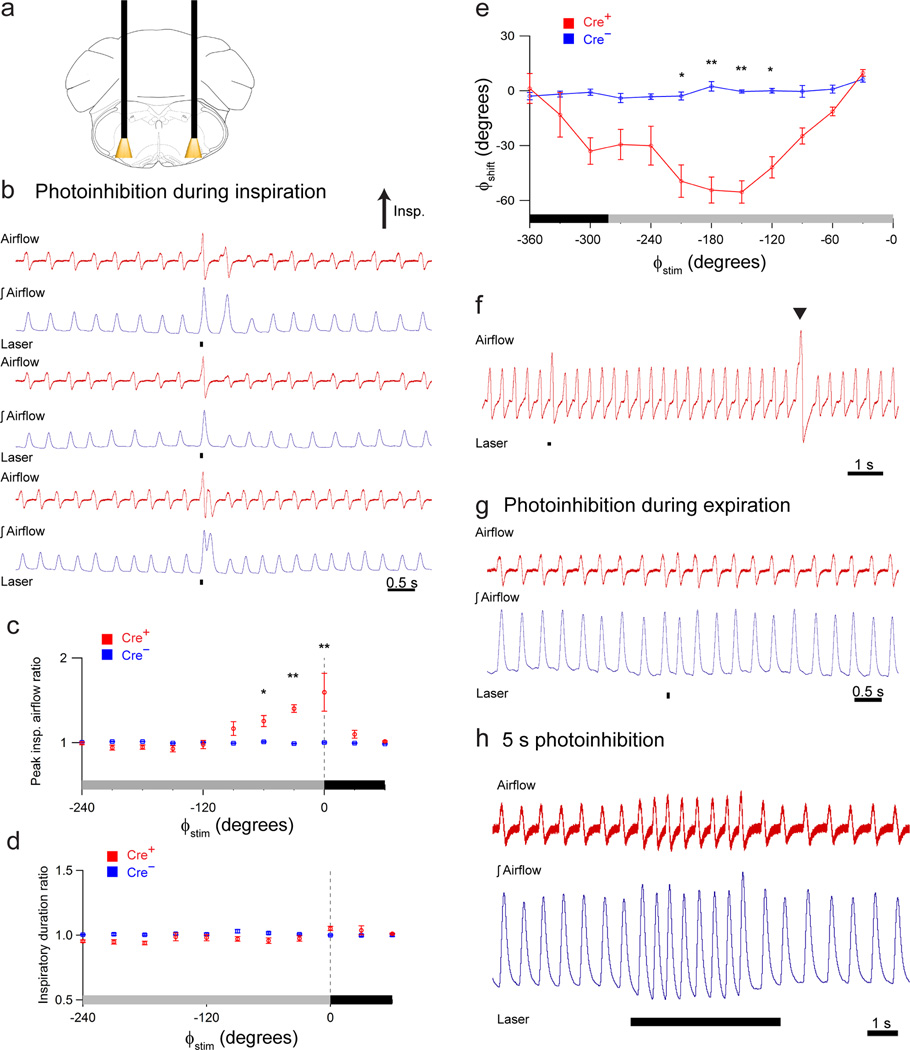
Figure 4. Photoinhibition of preBötC GlyT2 neurons augments breathing.
(a) Schematic depicting bilateral placement of optical cannulae targeting preBötC. (b) Representative airflow and tidal volume (∫airflow) traces (3 pairs) illustrating effect of brief photoinhibition (100 ms pulse; black bar) during inspiration. (c, d) Comparison of photoinhibited cycle to prior control cycle as a function of stimulus phase (ϕstim), for ratio of peak inspiratory airflow (c; n = 7; 0 – 30°: P = 2×10−7; 300 – 330°: P = 0.01; 330 – 360°: P = 5×107) or inspiratory duration (d; n = 7; all P > 0.05) in Cre+ (red) and Cre− (blue) anesthetized mice. Respiratory stimulus phase (ϕstim) on x-axis (c, d, e) with inspiration (black) defined from 0° to 78° (−360° to −282°) and expiration (gray) defined from 78° to 360° (-282° to 0°). The dotted vertical line (c, d) at 0° indicates onset of inspiration. (e) Shift in respiratory phase (ϕshift) resulting from preBötC-targeted laser pulses (100 ms pulse) in GlyT2-cre+ (Cre+, red; n = 7) and GlyT2-cre− (Cre−, blue; n = 5) mice. P values: 150 – 180°: P = 0.002; 180 – 210°: P = 2×10−5; 210 – 240°: P = 8×10−6; 240 – 270°: P = 0.01. (f) Representative light response in response to photoinhibition (black bar) compared to endogenous sigh (arrow). (g) Representative airflow and tidal volume (∫airflow) traces illustrating effect of brief photoinhibition (100 ms pulse; black bar) during expiration. (h) Representative airflow and tidal volume traces illustrating that longer photostimulation (5 s continuous pulse) increased peak inspiratory airflow and frequency (n = 3). Error bars, mean ± s.e.m. Statistical significance was determined with a one-way ANOVA and pair-wise comparisons were made with Tukey’s HSD test. * P < 0.05; ** P < 0.001; † P < 10−8.
Preinspiratory or early inspiratory bilateral photoinhibition (ϕstim: −120° – 15°) of Arch-transfected preBötC GlyT2 neurons (100 ms pulse; 593 nm; Fig 4a; n=7) increased peak inspiratory airflow (137.0 ± 3.8% of control; P = 2×10−9; n=7) and tidal volume (Fig. 4b, c) without a significant change in inspiratory duration (103.0 ± 0.9% of control; P=1; n=7; Fig. 4b, d) or respiratory phase (P = 1; n = 7; Fig. 4e). Compared to these photoinhibition-augmented breaths, sighs, i.e., larger breaths endogenously generated periodically to hyperinflate the lungs, had greater amplitudes (Fig. 4f). Sighs had peak inspiratory airflows that were 173 ± 15% of control (P = 10−5; n = 7) that was also statistically different from photoinhibition-induced augmented breaths (P = 0.02; n = 7). Bilateral photoinhibition during midexpiration (ϕstim: −210° – −120°) produced a shift in respiratory phase manifested by an earlier onset of the subsequent inspiration (52.3° ± 6.2° phase shift; P = 8×10−6; n = 7; Fig. 4e, g), with the strongest effect at 180° – −150°. Prolonged photoinhibition (5 s pulse) increased respiratory frequency but the effect was transient and did not last for the full duration of the laser pulse (Fig. 4h; n = 3).
Arch activation rescues breathing during a reflex apnea (Fig. 5)
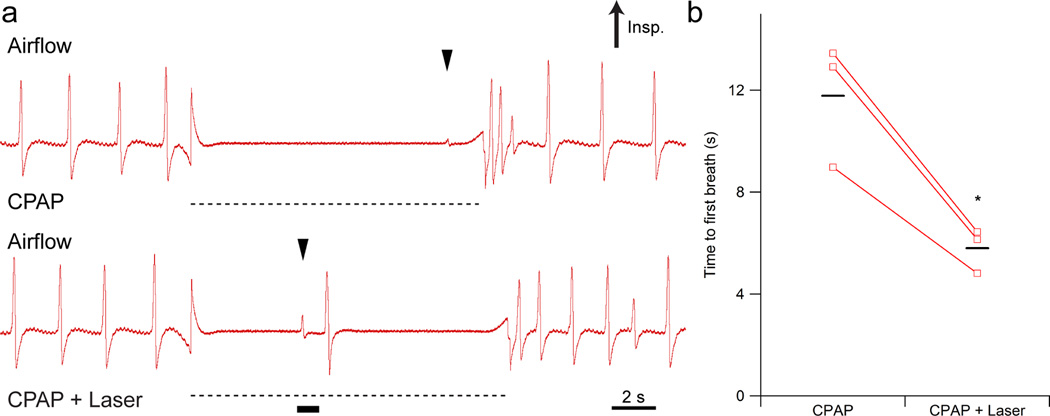
Figure 5. Photoinhibition of preBötC GlyT2 neurons during a reflex apnea rescues breathing.
(a) Breuer-Hering lung inflation reflex (BHIR) triggered by continuous positive airway pressure (CPAP; ~4 cm H2O; dotted black line) induced apnea in a tracheotomized, anesthetized Arch-transfected mouse in control (top) and bilateral photoinhibition (bottom, black bar) conditions. Top: After onset of BHIR, first breathe was at ~11 sec (black arrow). Bottom: 1s pulse applied 5.5 ± 0.5 s from onset of BHIR produced a first breath (indicated with a black arrow) 5.8 ± 0.5 s after the start of reflex, i.e., a 300 ms delay from the laser onset, versus an expected first breath at 11.8 ± 1.4 s, as seen during control (CPAP only) periods. (b) Duration of induced apnea, measured from onset of BHIR to onset of the next inspiration, in CPAP only and CPAP + laser conditions. Means are indicated with black horizontal lines (n = 3; P = 3×10−6). Statistical significance was determined with an unpaired t-test. * P < 10−5.
A critical role of inhibition in the preBötC in controlling respiratory pattern is likely in the production of apneas10, such as during swallowing or breathholding. To determine whether preBötC GlyT2 neurons participate in generating apneas, we photoinhibited these neurons during Breuer-Hering lung inflation reflex (BHIR)-induced apneas10. The lungs were inflated with sufficient continuous positive airway pressure (CPAP) to produce apneas that lasted ~11 s (~4 cm H2O) during control periods (Fig. 5a, top). Photoinhibiting Arch-transfected preBötC GlyT2 neurons (1 s pulse) broke the apnea with one or two breaths (Fig. 5a, bottom; n = 3). Laser onset at 5.5 ± 0.5 s after onset of CPAP (CPAP + Laser) induced a first breath at 5.8 ± 0.5 s, i.e., a 300 ms delay from the laser onset versus an expected first breath at 11.8 ± 1.4 s during control (CPAP only) periods (ANOVA; P = 3×10−6; Fig. 5b; n = 3).
Single-unit recording in ChR2- and Arch-transfected mice (Fig. 6)
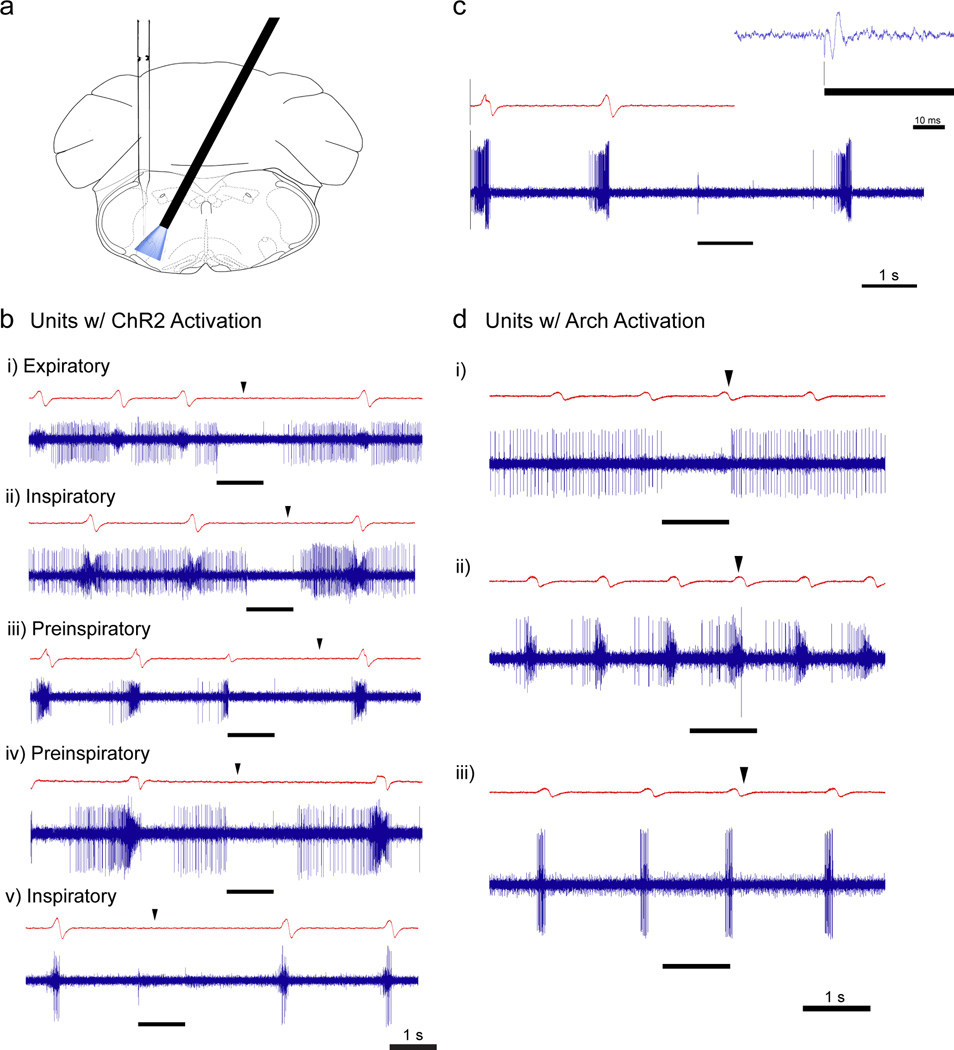
Figure 6. Unit recording with concurrent ChR2 or Arch activation.
(a) Schematic showing cannulae implantation at a 27° angle and vertical electrode placement to record from units within the cone of light (473 nm or 593 nm) in opsin-transfected mice. (b) Representative airflow traces (red) and respiratory-modulated units (blue; i) expiratory, ii) inspiratory, iii) pre-inspiratory, iv) pre-inspiratory, v) inspiratory) showing strong inhibition during 1s continuous laser pulse (black bar). Black arrows indicate the expected onset of inspiration following photostimulation. In total, 18 neurons were recorded from 3 mice. (c) Representative recording of multiple units following photostimulation of ChR2-expressing preBötC GlyT2 neurons with airflow trace. Inset: Shorter time-scale at onset of continuous laser pulse (black line) showing large field potential within ~2 msec. Vertical black line shows alignment of laser onset and initial artifact on trace. (d) Response of preBötC neurons to photoinhibition of Arch-expressing preBötC GlyT2 neurons with 1 s continuous laser pulse (black line). Black arrows indicate the expected onset of inspiration following photoinhibition as measured on the airflow trace. In total, 12 neurons were recorded from 2 mice.
In ChR2-transfected mice, we unilaterally photostimulated (1 s pulse) and recorded from preBötC neurons within the cone of light (n = 18 in 3 mice; Fig. 6a). Unilateral ChR2 photostimulation of preBötC GlyT2 neurons was sufficiently strong to recreate the effects observed from bilateral photostimulation, i.e., a persistent apnea during the photostimulation period, e.g., Fig 2g. In total, we recorded 18 neurons, of which 8 were respiratory-modulated (5 neurons were pre-I, 2 were inspiratory, and 1 was expiratory; Fig. 6b) and 10 neurons fired tonically. All of the recorded respiratory-modulated neurons and all but two of the tonically firing neurons were silenced during photostimulation. The onset of the next inspiration following photostimulation was delayed relative to the expected phase (black arrows in Fig. 6b depict expected onset of inspiration). Pre-I neurons could be silenced at any point in the respiratory cycle. When silenced early enough in the preinspiratory phase, initial preBötC activity was not followed by burst firing, motor output, or the onset of inspiratory airflow (Fig. 6b.iv). The delay between the laser shutting off and the onset of the next breath (described above; see Fig. 3b) was also seen in the preBötC neuron firing pattern. While recording within the preBötC, we consistently observed a short-lasting multi-unit field potential after laser onset, consistent with (transient) activation of neighboring GlyT2 neurons (Fig. 6c inset).
In Arch-transfected mice, we recorded from preBötC neurons during unilateral photoinhibition (1 s pulse; n = 12 units in 2 mice), finding 7 respiratory-modulated neurons (2 pre-I neurons, 2 inspiratory neurons, 1 post-I (or early-E) neuron, and 1 expiratory neuron) (Fig. 6d) and 5 tonic neurons. Unilateral photoinhibition generated shifts in the respiratory cycle, comparable to that observed during bilateral photoinhibition, but did not alter peak inspiratory airflow. During photoinhibition, preBötC neurons exhibited a shorter interval between inspirations, consistent with changes in the breathing pattern (black arrow s in Fig. 6d depict expected inspiratory onset following photoinhibition). We did not find any neurons that increased their firing during delivery of light pulses, which may be reflective of unilateral photoinhibition not altering inspiratory amplitude. We also found 2 tonic neurons that were silenced in response to light from a baseline firing rate of ~16 Hz, e.g., Fig. 6d.i. Following a 1 s light pulse, these neurons exhibited rebound firing after the laser shut off. The remaining neurons did not change their firing pattern or phase relationship to breathing during photoinhibition.
Discussion
Inhibition is an essential element of most neural circuits in the mammalian nervous system. While GlyT2+ inhibitory neurons are present in the preBötC and in slices in vitro, ~20% of these neurons are inspiratory-modulated and ~50% are tonic (we do not know what percentage in vivo are respiratory-modulated, tonic or silent, and under which physiological conditions)3, 11, their functional role in the generation of respiratory rhythm and pattern is not well understood and is disputed10, 17. Strong inhibitory currents are observed in intracellular recordings of preBötC neurons in deeply anesthetized rats and cats8. While a normal rhythmic breathing pattern remains in the absence of conventional postsynaptic inhibition in the preBötC10, 18, inhibitory modulation plays important roles in preBötC dynamics8. By making temporally precise brief bilateral perturbations of preBötC GlyT2 neurons, we probed their role in the generation of respiratory pattern in spontaneously breathing adult mice.
We utilized conditional gene targeting for restricting protein expression to inhibitory preBötC neurons. Since the GlyT2 promoter is specific to glycinergic neurons15, by locally injecting cre recombinase-dependent viruses into the preBötC of transgenic mice expressing Cre driven by the GlyT2 promoter12–14, we could selectively activate and silence these inhibitory neurons specifically in the preBötC of intact, awake or anesthetized, spontaneously breathing mice. To verify this, we examined the overlap of transfected neurons with known markers of excitatory or inhibitory neurons. We found that transfected neurons rarely colocalized with Sst, which demarcates a subset of excitatory preBötC neurons, and colocalized with glycine immunoreactivity, which identifies glycinergic neurons.
Our injection sites were centered on the preBötC with some GlyT2+ neuronal somas transfected rostrally including in the BötC, which also contains substantial numbers of respiratory-modulated inhibitory, including glycinergic, neurons19–21, caudally in the rostral ventral respiratory group that contains bulbospinal inspiratory neurons projecting to phrenic and intercostal motoneurons, and dorsally in the adjacent reticular formation. Peak densities of transfected neurons substantially overlapped with the distribution of Sst+ neurons relative to the caudal boundary of the facial nucleus, indicating that we successfully hit the preBötC. Optical cannulae were placed 200 – 400 µm dorsal to the caudal end of the preBötC to limit the light path to mainly target the somas of preBötC neurons and avoid the somas of transfected neurons outside the preBötC. However, opsins are not localized to any one neuronal domain (including expression in synaptic terminals), so there is the possibility that light affected synaptic terminals in the preBötC that originated from transfected somas adjacent to its boundaries. Insofar as these axons and presynaptic terminals arose from somas in the preBötC (including from the contralateral side), the results reflect the effects of perturbing preBötC GlyT2 neuron excitability. Of concern are effects that may have resulted from stimulating axons and presynaptic terminals of GlyT2 neurons with somas outside but synapsing within the preBötC. At the rostrocaudal level of the preBötC, neurons adjacent to the VRC do not have any respiratory-modulated activity22, and have no established monosynaptic projections to the preBötC23, 24. Thus, we consider the likelihood that glycinergic neurons at this level outside the preBötC could have contributed to the observed responses to be small. Caudal to the preBötC in the VRC there are inspiratory neurons, mostly bulbospinal, with no known monosynaptic rostral projections to the preBötC23, 24. Rostral to the preBötC in the VRC is the BötC, which contains considerable numbers of GlyT2+ neurons that appear to project to the preBötC; if photoactivation (ChR2) or photoinhibition (ARCH) affected their terminals, then they could contribute to responses evoked when light was confined to the preBötC. By aiming at the caudal preBötC with our injection and cannulae placement, we avoided most, but not all, BötC neuronal somas, but may have affected some of their putative transfected terminals in the preBötC. If BötC and preBötC GlyT2 neurons constitute a functionally homogeneous population (which is presently unknown) we have no concern here. If they represent two distinct populations with different effects, then our interpretation of the source of the observed effects, i.e., preBötC and/or BötC, would need to be parceled out. The most parsimonious explanation for the results we report is that light activated responses are predominately from perturbations affecting preBötC GlyT2 neurons including their terminals, and we frame the subsequent discussion accordingly.
Brief photoinhibition of Arch-expressing preBötC GlyT2 neurons during the preinspiratory or inspiratory phases produced significantly larger inspirations with no change in inspiratory duration. Thus, at least a subset of transfected GlyT2 neurons is endogenously active during (pre)inspiration and is affecting the amplitude of inspiratory outflow without an obligatory effect on its termination. If these neurons were essential to rhythmogenesis, one would predict that their photoinhibition during inspiration would also increase inspiratory duration. This was not the case when silencing a subset of these neurons sufficient to substantially increase inspiratory amplitude (Fig 4b–d). We suggest that these data further support previous observations that rhythmogenesis per se is not particularly sensitive to changes in inhibition10. When photoinhibition occurred during mid-expiration, expiratory duration was shortened. Thus, withdrawal of inhibition during mid-expiration allows preBötC (pre)inspiratory activity to initiate earlier and/or spread through the network more rapidly25, resulting in inspiration being triggered sooner than expected. These data are consistent with the interpretation that these neurons can modulate respiratory timing.
Brief photostimulation of ChR2-expressing preBötC GlyT2 neurons when delivered during the inspiratory phase prematurely terminated inspiration, i.e., shorter inspiratory durations and smaller peak inspiratory airflows. Photostimulation during expiration, including the preinspiratory phase, lengthened that phase, significantly delaying the subsequent inspiration. In response to longer stimuli, this effect was sufficiently strong to produce apnea in anesthetized or awake mice at rest, as well as when ventilation was increased by hypoxic or hypercapnic challenges. We found that respiration resumed with a consistent latency after the laser shut off regardless of the phase of photostimulation, which indicates that activation of preBötC GlyT2 neurons can reset the respiratory cycle.
Activation of preBötC GlyT2 neurons inhibits other respiratory-modulated preBötC neurons. We found 7 (pre)inspiratory-modulated neurons in total and all were silenced by ChR2 activation, at any point in the respiratory cycle. Silencing preinspiratory neurons prior to the start of inspiration coincided with typical preinspiratory spiking activity that was not followed by an inspiratory burst. That initial preinspiratory activity can occur without a subsequent burst is consistent with our hypothesis that inspiratory burst initiation and patterning are separate components of a two-stage process that can be experimentally decoupled25. We conclude that preBötC GlyT2 neurons can modulate the activity of rhythmogenic preBötC neurons and, when activated, can prematurely terminate an inspiratory burst, but that rhythmogenesis is not particularly sensitive to changes in inhibition.
We suggest that one essential role of preBötC GlyT2 inhibitory neurons is to modulate basic breathing parameters such as inspiratory flow, tidal volume, inspiratory and expiratory duration, and respiratory frequency, irrespective of any role in rhythmogenesis per se. By modulating the activity of preBötC GlyT2 neurons, we were able to, in effect, tune the dynamics of the preBötC network.
We did not isolate any neurons that were activated by photostimulation. There are several possibilities: i) Our sample size was too small. ii) Photostimulation activated a substantial number of preBötC GlyT2 neurons that were silent under control conditions and therefore we did not isolate them prior to stimulation. At present, we do not know the fraction of preBötC GlyT2 neurons that are respiratory-modulated or tonically active in anesthetized spontaneously breathing mice, and we have to allow the possibility that this fraction is small. iii) Laser light caused rapid, potent, and as long as the light was on, continual release of glycine from synaptic terminals of the transfected neurons. This would rapidly terminate the activity of all preBötC, including glycinergic, neurons. Thus, glycine could continue to be released during the entire duration of stimulation while all neurons were silent. The multi-unit field potential observed after laser onset may, instead, be the signature of transient activation of nearby GlyT2 neurons (Fig. 6c, inset).
Numerous models of central pattern generation focus on inhibition as a key component in the generation of rhythmic behaviors26–29; for breathing, inspiratory burst termination and/or phase transitions are often postulated to be mediated by postsynaptic actions of inhibitory neurons9, 30, 31. One recent model postulates that interactions between excitatory preBötC neurons and inhibitory subpopulations within the preBötC and BötC are essential to generate a normal respiratory rhythm17. However, while profound changes in breathing result from slow or relatively rapid onset perturbations to excitatory preBötC subpopulations1, 5, a normal respiratory rhythm persists in anesthetized adult rats after substantial blockade of inhibitory neurotransmission in preBötC and BötC by local injection of GABAA and glycine antagonists10. Our data are consistent with this result. We did not observe any breakdown in eupneic breathing when silencing a subset of preBötC GlyT2 neurons that was nonetheless sufficient to substantially increase inspiratory amplitude (Fig. 4b–d). Whether silencing a larger percentage of the preBötC GlyT2 neurons affect rhythmogenesis per se remains to be determined. Thus, we consider it unlikely that preBötC inhibitory neurons are playing an obligatory role in terminating inspiration.
Photoinhibition of preBötC GlyT2 neurons increased respiratory drive, producing larger breaths at a higher frequency. Thus, the preBötC in vivo with diminished preBötC GlyT2 neuronal activity but otherwise intact continued to generate a eupneic breathing rhythm10. Photoinhibition did not change the firing of (pre)inspiratory neurons, suggestive of weak or absent Arch expression in these neurons. We do not know from our experiments whether these neurons were excitatory, i.e., non-GlyT2, or simply failed to express sufficient protein. Since we did not observe increased activity in (pre)inspiratory neurons, we suggest either unilateral photoinhibition of preBötC GlyT2 is not sufficient to induce changes in individual (pre)inspiratory neurons or the increased respiratory drive results from an increase in the total number of active neurons, not the number of spikes per se.
Precisely timed apneas are an essential component of many vital, complex oropharyngeal movements, e.g., swallowing, vocalization, suckling, and chewing, and there are many powerful reflex apneas, such as the BHIR or following superior laryngeal nerve activation32–34 that can be triggered by tracheal obstruction. We hypothesize that inhibitory neurons play an important role in the generation of central apneas10. In support of this hypothesis and refining the experimental protocol to target the preBötC, we found that prolonged photostimulation of preBötC GlyT2 neurons initiated at any point in the respiratory cycle can produce apnea. Moreover, photoinhibition of preBötC GlyT2 neurons can counteract a reflex-induced apnea generated by the BHIR (Fig. 5a).
In summary, we employed a virus-based strategy to selectively introduce ChR2 or Arch into inhibitory preBötC neurons. By delivering light into the preBötC via chronically implanted optical cannulae in transfected anesthetized or awake mice, we have shown that preBötC GlyT2 neuronal activity modulates the respiratory output, may be critical in the manifestation and control of apneas essential for normal behavior, but does not appear to be an essential component underlying respiratory rhythmogenesis. Finally, the phase-response curves for photostimulation and photoinhibition represent a new class of data whose reproduction represents an essential test for any model purporting to explain respiratory rhythmogenesis.
Methods
Animals
Animal use was in accordance with the guidelines approved by the UCLA Institutional Animal Care and Use Committee. Animals were housed in a vivarium under a 12 hr light cycle with free access to food and water. All experiments were done on adult male GlyT2-Cre mice (24–30 g; C57Bl6 background; average post-surgery duration of ~30 days) with peripheral nerves, e.g., vagus, carotid sinus nerve intact, that were 10–14 weeks of age at the time of AAV injection. Prior to surgery, animals were housed in groups of variable size up to 5 mice per cage; after surgery, animals were housed individually as required by our study protocols. Mice breathed spontaneously. The GlyT2-Cre mice were kindly provided by H.U. Zeilhofer, University of Zurich, Switzerland.
Viral vector design
The viral constructs AAV2/1-Ef1α-DIO-ChR2-eYFP (Addgene plasmid 20298; provided by K. Deisseroth, Stanford University, Palo Alto, CA)16 and AAV2/1-flex-CBA-Arch-GFP (Penn Vector P2432; provided by E. Boyden, Massachusetts Institute of Technology, Cambridge, MA)13 were produced by the University of Pennsylvania Gene Therapy Program Vector Core. We used a ChR2 variant with the H134R mutation, which produces a twofold increase in the steady-state current as compared to the wildtype variant of ChR235, 36.
Microinjection
Mice were anesthetized with isoflurane (4% for induction and 2% for maintenance) and placed in a stereotaxic apparatus (David Kopf Instruments) with Bregma and Lambda skull landmarks level. Two holes were drilled in the skull at predefined coordinates relative to Bregma to allow the insertion of glass pipettes containing a virus solution (ChR2: 5 – 10×1012 GC/ml; Arch: 6 – 10×1012 GC/ml) connected to a pressure ejection system (Picospritzer II; Parker Hannafin) targeted to the preBötC. Once situated, 100–200 nl of per side was injected into the preBötC. Injections were made 6.80 mm caudal to Bregma, 1.20 mm lateral to the midline, and 4.65 mm ventral from the dorsal surface of the brain. Coordinates were determined from microinjections (n = 7) of red fluorescent microspheres (1.0 µm diameter, 5% solution; Invitrogen) into calculated coordinates based on a mouse brain atlas37 and adjusted based on the distance from preBötC, as determined by the location of Sst immunoreactive neurons4. The pipette was left in place for 5 min after injection to minimize backflow. The wound was closed with 5–0 non-absorbable sutures. The mice were returned to their home cage and allowed 2–3 weeks to recover, allowing time for sufficient levels of protein to express. For controls, negative littermates (those not expressing Cre recombinase) received virus injections as described above; as expected, there was no virus expression. Microinjections of cre-dependent virus were performed with the investigator blind to whether the animal was Cre+ or Cre−.
Implantation of fiber optics
Mice were placed into the stereotaxic frame and fiber-optic cannulae with a 1.25 mm stainless steel ferrule (200 µm fiber; Doric Lenses), held by a stereotaxic adaptor (Doric Lenses), were implanted bilaterally to a depth 200–400 µm dorsal to the preBötC, based on predetermined coordinates. The cannulae were glued to the skull using Metabond™ (Parkell) and the wound was sealed by applying a layer of clear dental acrylic (Lang Dental).
Photostimulation and photoinhibition
Branching optical fibers (200 µm fiber; Doric Lenses) with 1.25 mm stainless steel ferrules as ends were connected to the implanted cannulae via plastic sleeves. The back end of the fiber was connected to a 473nm/593nm dual wavelength laser (OptoDuet Laser; IkeCool) via an optical rotary joint (Doric Lenses). Light pulses were controlled by a pulse generator (Pulsemaster A300 Generator; WPI). Anesthetized mice were placed on a heating pad and in front of a nose cone connected to a flowmeter (GM Instruments) to record airflow. Awake mice were placed in a plethysmograph chamber (Buxco) connected to a pressure transducer (F.W. Kirk Company) with the optical fibers exiting the chamber through a small hole.
Breuer-Hering inflation reflex (BHIR)
The BHIR was induced in anesthetized Arch transfected mice (FLEX-Arch) by inflating the lungs with continuous positive airway pressure (CPAP). The pressure was adjusted to yield an apnea that lasted for ~10 – 15s (~4 cm H2O) before being “broken” by breaths reappearing despite the CPAP. We applied one control period of CPAP, waited for 30–60s, and then applied a second period of CPAP combined with a 1s pulse of light (593nm) delivered bilaterally into the preBötC. We measured both the amount of time it took for breathing to resume relative to the start of the reflex and the onset of the laser pulse.
Single-unit recording
To record neuronal activity, transfected mice were placed in the stereotaxic frame as described above. An optical cannula was implanted at a 27° angle from vertical, tilted across the midline from right to left, down to preBötC and glued to the skull with Metabond (Parkell). A window was drilled through the skull of sufficient size to allow the recording electrode free range of movement to record on the left side of the brain. Respiratory-modulated units were found 4.6 to 4.9 mm below the cerebellar surface and 1.1 to 1.3 mm lateral to the midline. A glass electrode (~3 µm inner tip diameter; filled with aCSF containing (in mM) 124 NaCl, 3 KCl, 1.5 CaCl2, 1 MgSO4, 25 NaHCO3, 0.5 NaH2PO4, and 30 d-glucose) was connected to a headstage (Siskiyou) and the signal amplified (Grass Model P511; Grass Instruments) and sampled at 20 kHz (PowerLab 16SP; ADInstruments). Due to a laser artifact apparent at the start and end of laser pulses, we used a single 1 s pulse for photostimulation to reduce noise. Most neural recordings were stable for 5 – 10 min.
Histology
After the completion of the experiment, mice were deeply anesthetized with pentobarbital and perfused transcardially with saline followed by 4% paraformaldehyde in phosphate-buffered saline (PBS). Brains were removed, postfixed overnight at 4°C, and cryoprotected in 30% sucrose in PBS for 24–48 h before sectioning. Free-floating coronal or sagittal sections (40 µm) were cut using a cryostat (CryoStar NX70, Leica Microsystems) in two series and stored at 4°C until further processing. Sections were incubated with primary antibodies in PBS containing 0.3% Triton X-100 (PBT) overnight at room temperature. After 3 washes, sections were incubated in species-appropriate secondary antibodies in PBS for 4h at room temperature. After 3 washes, sections were mounted onto gelatin-coated glass slides, dehydrated in graded alcohols, cleared in Xylenes, and coverslipped. Fluorescence was visualized with a confocal laser scanning microscope (LSM710; Carl Zeiss). Images were acquired with Zen software (Carl Zeiss), exported as TIFF files, and arranged to prepare figures in Photoshop and Illustrator (Adobe).
We used the following primary antibodies: rabbit polyclonal anti-somatostatin-14 (1:500; Peninsula Laboratories; T4103), mouse monoclonal anti-NeuN (1:500; Millipore; MAB377), chicken polyclonal anti-GFP (1:500; Aves Labs; GFP-1020), and rabbit anti-glycine (1:500; Millipore; AB5020). Rhodamine Red-X donkey anti-rabbit, DyLight488 or Cy2 donkey anti-chicken, and Cy5 donkey anti-mouse conjugated secondary antibodies (1:250; Jackson ImmunoResearch) were used to detect primary antibodies. These four antibodies are frequently used in mice and have been well validated. The validity of the SST-14 antibody has been shown in both rat1, 38 and mice5, 25. The GFP antibody has used successfully in mice as well5. In addition, the glycine antibody has been extensively used and validated as a marker of glycinergic neurons in mice and rats39, 40. The NeuN antibody has been extensively used in mice throughout the field of neuroscience research.
Data analysis and statistics
Traces were recorded on a 64-bit computer using LabChart 7 Pro (ADInstruments). The airflow signal was high-pass filtered (>0.1 Hz) to eliminate DC shifts and slow drifts, and used to calculate respiratory frequency, period, inspiratory (TI) and expiratory (TE) durations. The airflow signal was integrated to compute tidal volume (VT).
Cell counts were performed on 3 sequential sagittal sections as defined by the localization of Sst-immunoreactive neurons and soma distances relative to the caudal boundary of the facial motor nucleus were calculated. Counts were performed with ImageJ (http://rsb.info.nih.gov/ij/) and exported to Igor (Wavemetrics) for analysis with custom software.
All statistics were performed in Igor (Wavemetrics). Statistical significance was set at p < 0.05. One way ANOVAs and pairwise comparisons using Tukey’s HSD test were performed for all experiments except in Figure 5b, where an unpaired t-test was used. All values are presented as mean ± s.e.m. No statistical methods were used a priori to pre-determine sample sizes but our sample sizes are similar to those reported in previous publications1, 41. Data distribution was assumed to be normal but this was not formally tested.
Phase response analysis
The photostimulation-evoked reset of the respiratory rhythm was studied by applying a single 100 ms light pulse at various times during the respiratory cycle. The respiratory phase in each photostimulation or photoinhibition breath was based on the prior (control) respiratory period and defined as spanning 0° – 360°. Across experiments, inspiration (defined as the period of inward airflow) lasted from onset at 0° until 71.8° ± 11.6° (n = 5) in ChR2 experiments and until 77.9° ± 7.1° (n = 7) for Arch experiments; expiration was defined as the remainder of the cycle. Thus, the phase of any event could be calculated based on the time at which it occurred relative to the preceding inspiratory onset and referenced to the preceding control period. More specifically, phase values were obtained by calculating the ratio of the event onset time to the respiratory period and multiplying by 360°.
Stimulus phase (ϕstim), induced phase (ϕi), and expected phase (ϕe) of the respiratory cycle were computed (see Fig. 2a)38, 42 and plotted using software written in Igor (Wavemetrics). ϕstim, i.e., the time of laser onset relative to the ongoing respiratory cycle, was defined as the delay from the beginning of the previous inspiration to the time of laser onset. ϕe represents the total respiratory period, defined using the previous cycle as a control. ϕi is the respiratory period during the light-affected cycle. The difference between ϕi and ϕe was the net phase shift (ϕshift). If the light had no effect, ϕe = ϕi, and ϕshift = 0.
Supplementary Material
Acknowledgements
The authors thank Grace Li for excellent technical work and Drs. Nicholas Brecha, Thomas Otis, and Kaiwen Kam for thoughtful discussion. This work was supported by National Institutes of Health grants NS072211 and NS58280.
Footnotes
Competing Financial Interests: The authors declare no competing financial interests.
Contributions
DS and JLF conceived of the study and designed the experiments. DS performed the experiments with help from JWW and YC, and DS and JWW analyzed data. DS and JLF made the figures and wrote the manuscript with help from JWW.
A supplementary methods checklist is available.
References
- 1.Tan W, et al. Silencing preBotzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science (New York, N.Y. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winter SM, et al. Glycinergic interneurons are functionally integrated into the inspiratory network of mouse medullary slices. Pflugers Arch. 2009;458:459–469. doi: 10.1007/s00424-009-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stornetta RL, et al. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Botzinger complex. J Comp Neurol. 2003;455:499–512. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- 5.Bouvier J, et al. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci. 2010;13:1066–1074. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]
- 6.Gray PA, et al. Developmental origin of preBotzinger complex respiratory neurons. J Neurosci. 2010;30:14883–14895. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koizumi H, et al. Structural-functional properties of identified excitatory and inhibitory interneurons within pre-Botzinger complex respiratory microcircuits. J Neurosci. 2013;33:2994–3009. doi: 10.1523/JNEUROSCI.4427-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiology. 2014;29:58–71. doi: 10.1152/physiol.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballantyne D, Richter DW. Post-synaptic inhibition of bulbar inspiratory neurones in the cat. J Physiol. 1984;348:67–87. doi: 10.1113/jphysiol.1984.sp015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. J Neurosci. 2013;33:5454–5465. doi: 10.1523/JNEUROSCI.1595-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgado-Valle C, Baca SM, Feldman JL. Glycinergic pacemaker neurons in preBotzinger complex of neonatal mouse. J Neurosci. 2010;30:3634–3639. doi: 10.1523/JNEUROSCI.3040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deisseroth K, et al. Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow BY, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalphin AV, Saha MS. The specification of glycinergic neurons and the role of glycinergic transmission in development. Frontiers in molecular neuroscience. 2010;3:11. doi: 10.3389/fnmol.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. Journal of neurophysiology. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Botzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- 19.Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Botzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol. 1999;407:583–597. doi: 10.1002/(sici)1096-9861(19990517)407:4<583::aid-cne8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Botzinger complex in the cat. Exp Brain Res. 1990;81:639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- 21.Ezure K, Tanaka I, Kondo M. Glycine is used as a transmitter by decrementing expiratory neurons of the ventrolateral medulla in the rat. J Neurosci. 2003;23:8941–8948. doi: 10.1523/JNEUROSCI.23-26-08941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alheid GF, McCrimmon DR. The chemical neuroanatomy of breathing. Respiratory physiology & neurobiology. 2008;164:3–11. doi: 10.1016/j.resp.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. The Journal of comparative neurology. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- 24.Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. The Journal of comparative neurology. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- 25.Kam K, Worrell JW, Ventalon C, Emiliani V, Feldman JL. Emergence of Population Bursts from Simultaneous Activation of Small Subsets of preBötzinger Complex Inspiratory Neurons. The Journal of Neuroscience. 2013;33:3332–3338. doi: 10.1523/JNEUROSCI.4574-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns BD. The central control of respiratory movements. British medical bulletin. 1963;19:7–9. doi: 10.1093/oxfordjournals.bmb.a070010. [DOI] [PubMed] [Google Scholar]
- 27.Bradley GW, von Euler C, Marttila I, Roos B. A model of the central and reflex inhibition of inspiration in the cat. Biological cybernetics. 1975;19:105–116. doi: 10.1007/BF00364107. [DOI] [PubMed] [Google Scholar]
- 28.von Euler C. On the central pattern generator for the basic breathing rhythmicity. Journal of applied physiology: respiratory, environmental and exercise physiology. 1983;55:1647–1659. doi: 10.1152/jappl.1983.55.6.1647. [DOI] [PubMed] [Google Scholar]
- 29.Feldman JL, Cowan JD. Large-scale activity in neural nets II: A model for the brainstem respiratory oscillator. Biol Cybern. 1975;17:39–51. doi: 10.1007/BF00326708. [DOI] [PubMed] [Google Scholar]
- 30.Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol. 1982;100:93–107. doi: 10.1242/jeb.100.1.93. [DOI] [PubMed] [Google Scholar]
- 31.Schmid K, Foutz AS, Denavit-Saubie M. Inhibitions mediated by glycine and GABAA receptors shape the discharge pattern of bulbar respiratory neurons. Brain Res. 1996;710:150–160. doi: 10.1016/0006-8993(95)01380-6. [DOI] [PubMed] [Google Scholar]
- 32.Xia L, Damon T, Niblock MM, Bartlett D, Leiter JC. Unilateral microdialysis of gabazine in the dorsal medulla reverses thermal prolongation of the laryngeal chemoreflex in decerebrate piglets. Journal of Applied Physiology. 2007;103:1864–1872. doi: 10.1152/japplphysiol.00524.2007. [DOI] [PubMed] [Google Scholar]
- 33.Curran AK, Xia L, Leiter JC, Bartlett D., Jr Elevated body temperature enhances the laryngeal chemoreflex in decerebrate piglets. J Appl Physiol (1985) 2005;98:780–786. doi: 10.1152/japplphysiol.00906.2004. [DOI] [PubMed] [Google Scholar]
- 34.Heman-Ackah YD, Pernell KJ, Goding GS. The laryngeal chemoreflex: An evaluation of the normoxic response. The Laryngoscope. 2009;119:370–379. doi: 10.1002/lary.20007. [DOI] [PubMed] [Google Scholar]
- 35.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gradinaru V, et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paxinos G, Franklin KBJ, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2001. [Google Scholar]
- 38.Pagliardini S, et al. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci. 2011;31:2895–2905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poyatos I, Ponce J, Aragon C, Gimenez C, Zafra F. The glycine transporter GLYT2 is a reliable marker for glycine-immunoreactive neurons. Brain research. Molecular brain research. 1997;49:63–70. doi: 10.1016/s0169-328x(97)00124-1. [DOI] [PubMed] [Google Scholar]
- 40.Zeilhofer HU, et al. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. The Journal of comparative neurology. 2005;482:123–141. doi: 10.1002/cne.20349. [DOI] [PubMed] [Google Scholar]
- 41.McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nature neuroscience. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis J, Bachoo M, Polosa C, Glass L. The effects of superior laryngeal nerve stimulation on the respiratory rhythm: phase-resetting and aftereffects. Brain Research. 1990;517:44–50. doi: 10.1016/0006-8993(90)91005-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.