Abstract
Background & Aims
The rising incidence of hepatocellular carcinoma (HCC) in the United States has significant health and economic consequences. Ultrasound (US) surveillance is recommended for patients with cirrhosis because of their high risk of HCC and improving treatment outcomes for small tumors. We assessed the costs, clinical benefits, and cost-effectiveness of US surveillance and alternative strategies for HCC in cirrhosis using a computer-based state transition model with parameters derived from available literature.
Methods
Our model compared a policy of no surveillance to six surveillance strategies in cirrhotic patients ages 50 years and older in the United States: 1) annual US; 2) semiannual US; 3) semiannual US with alphafetoprotein; 4) annual computed tomography (CT); 5) semiannual CT; and 6) annual magnetic resonance imaging. The number of screening tests needed to detect one small HCC, cost per treated HCC, lifetime costs, quality-adjusted life expectancy, and incremental cost-effectiveness ratios were calculated.
Results
Semiannual US surveillance for HCC in cirrhosis increased quality-adjusted life expectancy by 8.6 months on average, but extended it nearly 3.5 years in patients with small treated tumors. Semiannual US surveillance had an incremental cost-effectiveness ratio of $30,700 per quality-adjusted life year (QALY) gained, and was more cost-effective than the alternative surveillance strategies using a threshold of $50,000 per QALY gained. The incremental cost-effectiveness ratios for the combined alphafetoprotein/US and annual CT strategies exceeded $50,000/QALY unless the sensitivity and specificity of US fell below 65% and 60%, respectively.
Conclusion
Semiannual US surveillance for HCC in cirrhotic patients improves clinical outcomes at a reasonable cost.
The incidence of hepatocellular carcinoma (HCC) in the U.S. has doubled over the last two decades, resulting in an estimated 1 billion dollars in direct and indirect health care costs.1–3 Because the prognosis of advanced HCC is poor, surveillance for the detection of small tumors in high-risk populations has become common practice. The majority of gastroenterologists use semiannual ultrasound and the serum tumor marker alphafetoprotein (AFP) to detect HCC in cirrhotic patients 4, of whom 2–8% develop the tumor annually.5–7 Studies suggest that surveillance detects tumors at earlier stages and improves survival, but no randomized, controlled trial has been performed in cirrhotics.8–11 Recent guidelines from the American Association for the Study of Liver Diseases (AASLD) advocate annual or semiannual surveillance with ultrasonography (US) in cirrhosis.12 Because its sensitivity and specificity are limited, use of AFP is recommended only when ultrasound is unavailable.12, 13
The cost-effectiveness of HCC surveillance with US alone in a general population of cirrhotics has not been evaluated in the United States. Surveillance with AFP and US, or with computed tomography (CT), has been demonstrated to be cost-effective in select populations such as liver transplant candidates, Child’s class A cirrhotics with long life expectancy, and those with hepatitis B or C cirrhosis.14–19 Historically, the utility of surveillance has been limited by poor treatment outcomes even in early stage disease and suboptimal screening test characteristics. With improvements in treatment options for small tumors and the diagnostic accuracy of radiologic imaging, routine surveillance for HCC in cirrhosis is more likely to provide measurable clinical benefits.
In recent years local ablative therapies such as radiofrequency ablation (RFA) have permitted patients with advanced liver disease or comorbidities that preclude resection or transplantation to be treated successfully.20 Liver transplantation is not only a viable treatment for HCC, but changes to the MELD (Model for End-Stage Liver Disease) scoring system, a numerical scale used for liver allocation, now give patients with small tumors priority in the transplant process. For patients meeting the Milan transplant criteria, those with a single HCC less than 5 centimeters or no more than three tumors each smaller than 3 centimeters, long-term survival post-transplant approaches that observed for nonmalignant indications.21 As survival for limited stage disease improves, the consequences of missing small HCC grow.
In addition to more promising treatment outcomes, technological advances have also expanded the range of available screening modalities for HCC. While US is recommended for surveillance, CT or magnetic resonance imaging (MRI) may be more sensitive in detecting small HCC.22, 23 Triple-phase CT, in which portal venous phase, arterial phase and noncontrast imaging highlight different tumor characteristics, is more sensitive than US and is used increasingly frequently for surveillance by some clinicians.4 The relative performance and costs of these tests in the context of a surveillance program have not been formally evaluated.
With the imminent peak in chronic hepatitis C cirrhosis and the rising incidence of nonalcoholic fatty liver disease, the burden of HCC is expected to grow in the coming years.2 In light of potentially more effective, yet expensive, diagnostic tests and treatments, we used the best available data to estimate the benefits, costs and cost-effectiveness of US alone compared to alternative surveillance strategies for HCC in cirrhosis.
METHODS
Analytic Overview
We developed a computer-based Markov model (TreeAge Pro software 2005) to simulate disease progression, surveillance, diagnosis, and treatment of HCC in a cohort of patients with compensated cirrhosis. In comparison to no surveillance, we assessed 1) annual US; 2) semiannual US; 3) semiannual US with AFP; 4) annual CT; 5) semiannual CT; and 6) annual MRI surveillance. Model outcomes included the number of screening tests needed to diagnose one small HCC, cost per treated HCC, lifetime costs, quality-adjusted life expectancy, and incremental cost-effectiveness ratios (2004 U.S. $ per quality-adjusted life year gained). We adopted a health system perspective and followed the recommendations of the Panel on Cost-Effectiveness in Health and Medicine.24 One-way and two-way sensitivity analyses were performed to identify individual parameters with the greatest influence on our results.
Model
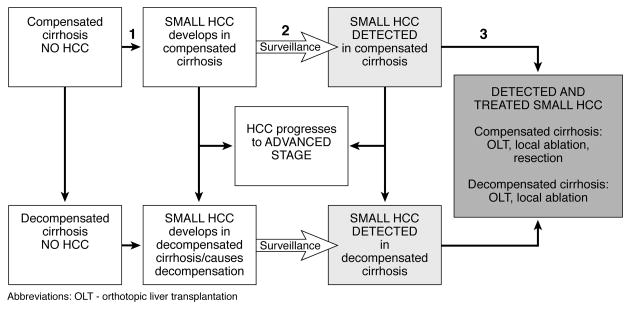
A cohort of 50 year-old individuals representative of patients with compensated cirrhosis in the U.S. enter the state transition model and move through a series of health states designed to reflect important stages in the natural history of cirrhosis, tumor progression and treatment (Figure 1).
Figure 1.
Simplified schematic of Markov health states. In each cycle, individuals may remain in the same health state, transition to the following state, or die from liver disease or unrelated causes. For example, transition 1 reflects cancer incidence; transition 2 is determined by the sensitivity of the surveillance modality; and transition 3 is the proportion of treatment-eligible tumors. Best available data were used to determine transitions. The grey boxes represent the possible benefit of surveillance: detection and treatment of small HCC.
Surveillance Algorithms and Treatment
To estimate the effect of surveillance, the model distinguishes between detected and undetected HCC. Depending on the accuracy and timing of surveillance, HCC might be detected early via surveillance or remain undetected until an advanced stage. Compensated and decompensated cirrhotics were eligible for surveillance with any of the six strategies. Tumor stage at diagnosis depended on the tumor growth rate and likelihood of detection at any surveillance interval. Patients with compensated cirrhosis in whom “small HCC” was detected (defined as tumors meeting the Milan criteria) were eligible for liver transplantation, resection or local ablative therapies; while decompensated cirrhotics were treated with transplant or local ablation only. Patients with advanced tumors received only palliative care.
A number of assumptions were made in the base case including (1) a positive surveillance US was evaluated with a dynamic imaging study, either triple-phase CT or MRI, as recommended by AASLD guidelines; (2) serum AFP was considered positive above a 20 ng/mL cut-off, and was evaluated with a dynamic imaging study even when the corresponding US was negative; (3) all suspicious lesions found on imaging were biopsied, and biopsy was assumed to be perfect, without mortality risk; (4) no additional imaging was performed prior to biopsy in the CT or MRI strategies; and (5) patients with false-positive screening tests returned to their previous surveillance test and schedule once biopsy confirmed their true disease status.
Data
Table 1 summarizes selected model parameters used in the model. A base-case value and plausible range was established for each model input based on published literature and expert assumptions. When several estimates were available, we prioritized estimates from larger, well-controlled, prospective studies in Western populations.
Table 1.
Model Variables: Baseline Values and Ranges Used in Sensitivity Analyses
| Natural history and treatment-related prognosis | ||
| Compensated cirrhosis-related prognosis19 | ||
| 10-year survival | 60% | 36%–80% |
| Adjusted annual excess mortality | 0.04 | 0.016–0.08 |
| Decompensated cirrhosis-related prognosis19–21 | ||
| 2-year survival | 50% | 47%–63% |
| Adjusted annual excess mortality | 0.28 | 0.18–0.30 |
| Annual transition of compensated to decompensated cirrhosis19 | 5% | 3%–8% |
| Annual incidence of HCC4–6,23 | 5% | 2%–6% |
| Annual transition from small to large HCC25, 28 | 40% | 20%–70% |
| Annual mortality from large untreated HCC49, 50 | 75% | 35%–95% |
| Liver transplant prognosis16, 41, 42 | ||
| Perioperative mortality | 4.3% | 2.3%–6.3% |
| 5-year survival | 70% | 65%–80% |
| Hepatic resection prognosis41,43–45 | ||
| Perioperative mortality | 3.9% | 3.7%–4.5% |
| 5-year survival | 44% | 38%–51% |
| Local ablative therapy (radiofrequency ablation) prognosis15, 46 | ||
| Perioperative mortality | 0.3% | 0%–1.8% |
| 5-year survival in compensated cirrhosis | 46% | 32%–68% |
| 5-year survival in decompensated cirrhosis | 31% | 27%–40% |
| Surveillance test characteristics, % | ||
| AFP sensitivity37, 38 | 60 | 40–65 |
| AFP specificity | 87 | 63–94 |
| US sensitivity29–33 | 75 | 40–81 |
| US specificity | 95 | 80–100 |
| Triple-phase CT scan sensitivity17,29,30,33–36 | 85 | 50–94 |
| Triple-phase CT scan specificity | 90 | 80–96 |
| MRI sensitivity33, 39, 40 | 88 | 54–90 |
| MRI specificity | 87 | 82–88 |
| Costs, US$ (Medicare, National Impatient Sample)10, 52, 53 | ||
| AFP | $31 | 20–40 |
| US | 185 | 120–300 |
| Abdominal CT scan | 640 | 390–900 |
| Abdominal MRI | 1400 | 900–1700 |
| Imaging-guided liver biopsy | 614 | 300–1400 |
| Follow-up evaluation for false-positive tests | 1200 | 600–2400 |
| Orthotopic liver transplant | 145,000 | 50,000–200,000 |
| Annual cost after liver transplant | 12,000 | 8000–20,000 |
| Hepatic resection | 35,000 | 15,000–70,000 |
| Outpatient percutaneous radiofrequency ablation | 3000 | 2500–10,000 |
| Annual cost for compensated cirrhosis | 1000 | 200–2000 |
| Annual cost for decompensated cirrhosis | 15,000 | 5000–30,000 |
| Annual cost of advanced HCC | 35,000 | 15,000–70,000 |
| Quality-of-life weights52–54 | ||
| Compensated cirrhosis | 0.8 | 0.6–1.0 |
| Decompensated cirrhosis | 0.65 | 0.5–0.8 |
| Advanced HCC | 0.25 | 0.1–0.4 |
Natural history and progression of cirrhosis
Based on large epidemiologic studies, the average life expectancy of compensated cirrhosis is between 10 and 13 years.25–28 Between 5–7% of compensated cirrhotics progress to decompensated disease each year, developing ascites, variceal bleeding or encephalopathy.28 The median life expectancy of decompensated disease is 2 years.28–30 Because 20% of the annual excess mortality from compensated and decompensated cirrhosis is attributable to HCC, we adjusted the mortality rates accordingly to avoid double-counting HCC deaths.25–30 With this adjustment, predicted life expectancy for compensated cirrhosis in the model approximated that reported in the literature.
Incidence and natural history of HCC
The annual incidence of HCC is approximately 5%, with a range of 2–8% in cohort studies of cirrhotics undergoing surveillance.5–8, 10, 31–33 Median survival for patients with small or advanced HCC is two years or six months, respectively.34–39 Tumor growth was assumed to be linear, with a doubling time between 117 and 195 days.37, 40, 41 Based on this doubling time, approximately 40% of tumors progress from limited to advanced stage each year. Because systemic chemotherapy and other treatments for advanced disease have not significantly improved survival 42, 43, advanced cancer was not treated in the model.
Surveillance test characteristics
The sensitivity of US for HCC surveillance ranges from 40–81% and its specificity from 80–100%.44–49 The sensitivity of triple-phase CT is higher at 85–90%, but its specificity similar at 80–96%.22, 44, 45, 48–52 Serum AFP is far less sensitive (40–65%) and specific (63–94%) at the 20 ng/mL cut-off.53–57 The sensitivity and specificity of MRI approach 90%.49, 58, 59 Test characteristics were assumed to be invariant across the spectrum of liver disease. Because test characteristics vary widely in the literature, preference was given to studies using a screening framework, a pathologic gold standard and those reporting per-patient rather than per-lesion sensitivity and specificity. Similarly, because CT and MRI studies tend to use the exacting gold standard of liver explant and include missed subcentimeter lesions in the sensitivity calculation, these lesions were excluded from the analysis when possible to permit comparability to US studies.
Risks and prognosis of HCC treatment
For compensated cirrhotics with HCC meeting Milan criteria, treatment options with curative intent include transplantation, resection or local ablative therapy. Decompensated cirrhotics with HCC are candidates for transplant or local therapy only.60 RFA was evaluated as the sole local therapy because its efficacy is similar to chemoembolization and ethanol injection61, and it is widely available in the U.S. The perioperative mortality of liver transplant, resection and RFA are 4.3%62, 3.9%63, and 0.3%64, respectively. Following successful transplantation for small HCC, five-year survival approaches 70%.21, 62, 63, 65, 66 Five-year survival after resection is less than 50%, due to tumor recurrence.63, 67–70 Five-year survival with RFA ranges from 31–46% depending on the underlying liver disease.20, 71–74 Overall survival, rather than recurrence-free survival, was used for prognosis. We assumed that recurrent HCC was not treated and that HCC was treated with a single modality only, not sequential therapy such as RFA followed by transplant.
We consulted four expert hepatologists to estimate the proportion of small HCC eligible for each of the curative therapies, since published data on this parameter are sparse. By conservative estimates, approximately 70% of compensated and 30% of decompensated cirrhotics with small HCC are treatment-eligible, with the remainder excluded due to poor tumor location, advanced liver disease, advanced age or comorbid illness. Based on a Medicare population with HCC, in which treatment rates are lower than in our model’s younger cohort, we assumed that the proportion of treatment-eligible compensated cirrhotics undergoing resection, local ablation and transplant was 40%, 40%, and 20%, respectively.75 We assumed that 40% of treatment-eligible decompensated cirrhotics were transplant-eligible. We varied these estimates widely in sensitivity analyses.
Costs and quality of life
Direct medical costs were derived from 2004 Medicare CPT reimbursement, the 2003 Nationwide Inpatient Sample, and published literature.15, 76, 77 The annual costs of cirrhosis disease management included office visits, blood work, and occasional complications of decompensated disease, but not orthotopic liver transplantation or surveillance for HCC or esophageal varices. Literature-based estimates were used for the quality of life weights.76–78 We did not consider temporary reduction in quality of life due to surveillance or treatment since these are small compared to the underlying disease.
RESULTS
Life Expectancy and HCC Outcomes
For compensated cirrhotics ages 50 and older, the average per-person quality-adjusted life expectancy (QALE) is 7.3 years in the absence of surveillance. The undiscounted gain in QALE was 6.8 months with annual US. Increasing the US frequency to twice yearly provides an additional 1.8 months of QALE. Incremental gains from the other strategies were smaller; for example, adding serum AFP improves the effectiveness of semiannual US surveillance by only 0.6 months (Table 2). Although semiannual US increases QALE by 8.6 months on average, it extends it nearly 3.5 years in patients with diagnosed and treated small HCC.
Table 2.
Costs, QALE, and ICERs for Alternative HCC Surveillance Strategies
| Surveillance strategy | QALE, y (undiscounted) | QALE, y (discounted) | Costs, US$ (discounted) | ICER |
|---|---|---|---|---|
| No surveillance | 7.28 | 5.97 | 26,170 | |
| Annual US | 7.84 | 6.35 | 34,161 | 21,200 |
| Annual CT scan | 7.94 | 6.41 | 39,087 | a |
| Annual MRI | 7.95 | 6.42 | 45,830 | a |
| Semiannual US | 7.99 | 6.45 | 37,272 | 30,700 |
| Semiannual AFP/US | 8.04 | 6.48 | 39,552 | 73,500 |
| Semiannual CT scan | 8.06 | 6.50 | 45,185 | 331,800 |
Annual CT and MRI are dominated by semiannual ultrasound, which is both a more effective and less costly strategy.
Lifetime Costs and Cost-Effectiveness Analysis
The average discounted lifetime cost in the absence of surveillance for 50 year-old cirrhotic patients is $26,200. Discounted lifetime per-person cost increased by $8,000 with annual US and by $19,700 with MRI. (Table 2) The incremental cost-effectiveness ratio for annual US was $21,200 per quality-adjusted life year (QALY) gained. In comparison, semiannual US provided nearly two months of additional quality-adjusted life expectancy for $30,700 per QALY. Adding serum AFP yielded smaller gains in QALE and had an incremental cost-effectiveness ratio of $73,500 per QALY. Semiannual CT, the most effective strategy, had an incremental cost-effectiveness ratio exceeding $300,000 per QALY gained. Annual MRI was more costly and less effective than both of these strategies.
Clinical Impact and Resource Use of Semiannual Surveillance
The clinical impact and resource use of semiannual US surveillance in a model cohort of 10,000 cirrhotics age 50 and over are reported in Table 3. Over a thirty-year period, more than 180,000 surveillance ultrasounds would be performed. Semiannual US surveillance would detect an estimated 3,000 small tumors in the population. Therefore, approximately 60 surveillance ultrasounds are required to detect one small HCC. The average costs per detected and treated small HCC were $117,200 and $179,900, respectively.
Table 3.
Clinical Impact and Resource Use of Semiannual Ultrasound Surveillance in a Cohort of 10,000 Cirrhotic Patients Over 30 Years
| Outcomes | Value |
|---|---|
| Number of surveillance tests completed | 184,354 |
| Number of small HCC detected | 3179 |
| Number of US needed to detect one small HCC | 58 |
| Cost per small HCC detected (US$) | 117,247 |
| Cost per small HCC detected and treated (US$) | 179,865 |
| QALE gained per treated small HCC (Y) | 3.45 |
Sensitivity Analysis
Sensitivity analyses were performed on all model parameters to identify variables with the greatest influence on (1) the cost-effectiveness of HCC surveillance overall and (2) the performance of semiannual US compared to the alternative strategies. The cost-effectiveness of surveillance in general was most sensitive to alternative assumptions about the population age at the onset of surveillance, the mortality of HCC and the rate of progression. The cost of surveillance exceeds $50,000 per QALY gained if the annual incidence of HCC is less than 0.8%, fewer than 14% of tumors progress from limited to advanced stage each year, patient age is greater than 83 years, or if the likelihood of incidental diagnosis of HCC in patients not undergoing surveillance exceeds 60%. On the other hand, surveillance remains cost-effective even when compliance is imperfect or when non-diagnostic or false negative biopsies occur.
The effect of varying selected parameters on the incremental cost-effectiveness of semiannual US surveillance is demonstrated in Figure 2. If the annual incidence of HCC falls below 2.2%, annual US is preferred to semiannual US based on a $50,000/QALY threshold. When US sensitivity is lower than 65% or the specificity of AFP exceeds 95%, combined AFP/US surveillance is preferred. Annual CT becomes the preferred strategy when US specificity is less than 60%. The incremental cost-effectiveness ratios of semiannual CT and annual MRI consistently exceed $100,000 per QALY.
Figure 2.
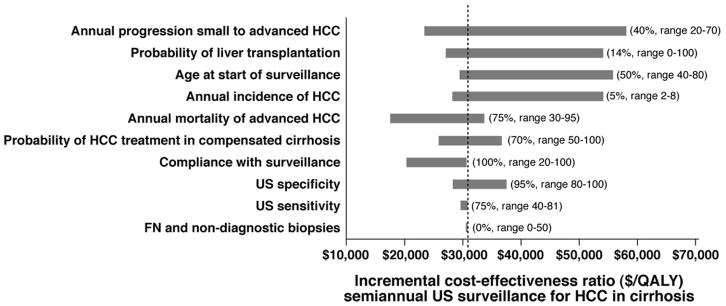
Sensitivity analysis. The effect of varying selected model variables on the ICER of semiannual US surveillance. The values in parentheses are the baseline values for each variable and the plausible range tested in sensitivity analyses. The dashed line indicates the ICER in the base case. The bars show the variability of the ICER caused by changes in the indicated variable, all other variables held constant.
In 2-way sensitivity analysis, we simultaneously varied the test characteristics of US, CT and MRI. When US sensitivity is poor (0.5), combined AFP/US surveillance is preferred for all tested sensitivities of CT and MRI. Otherwise, semiannual US surveillance is cost-effective in all conditions tested.
DISCUSSION
Our analysis demonstrates that surveillance for HCC in patients with cirrhosis provides substantial life-expectancy gains. Semiannual US surveillance extends per-person QALE by 8.6 months on average compared to no surveillance, a benefit that compares favorably to screening programs for colon or breast cancer.79–81 The QALE gain for cirrhotics with detected and treated small HCC exceeds three years. The utility of surveillance in cirrhosis is unsurprising because the incidence of HCC is high in this population; the tumor is asymptomatic in its early phases and unlikely to be detected without surveillance; and treatments are effective for early HCC, while the prognosis for advanced disease is poor. With continued improvement in the life expectancy of cirrhosis and the treatment modalities for HCC, the benefit of surveillance is likely to increase.
While there is no consensus on a cost-effectiveness threshold below which an intervention would be considered unequivocally cost-effective, the value of $50,000 per QALY gained is often cited as a reasonable benchmark for the United States. 24 At this threshold, semiannual US ($30,700 per QALY) is attractive. While one could argue that society has been willing to pay up to $100,000 per QALY gained for certain health interventions, which would imply that combined AFP/US surveillance confers reasonable value for the money ($73,500 per QALY), CT and MRI surveillance would not be regarded as cost-effective under widely varying assumptions.
We found that only when US sensitivity and specificity fall below 65% and 60% respectively, do the AFP/US or annual CT strategies become preferred at the $50,000/QALY benchmark. Based on the results of published studies, test characteristics are unlikely to fall into this range. However, in specific circumstances in which US sensitivity or specificity might be poor, such as in patients with ascites, individual decision-making should prevail. As single tests, CT and MRI are each more sensitive than US for detecting early HCC; however, when performed annually these tests are less effective than semiannual US. The rapid doubling time of HCC favors a semiannual surveillance schedule. When compared to semiannual US, semiannual CT or MRI provides a small incremental benefit at a much higher cost. US surveillance also improves as the characteristics of CT and MRI improve because the latter are used as confirmatory tests for a positive US. Our analysis did not consider the risks of radiation or contrast with CT or the additional time needed for MRI, factors that may further favor US surveillance in practice. We also did not consider strategies combining AFP with CT or MRI. Although combining frequent AFP measurements with annual CT or MRI may be more effective, this is unlikely given the limited sensitivity and specificity of AFP compared to advanced imaging.
The addition of AFP to US increases detection of HCC somewhat, but is more costly because falsely positive AFP elevations prompt additional imaging studies. This cost rises further if AFP levels lower than the 20 ng/mL cut-off were to trigger further testing. Despite using favorable test characteristics for AFP, the cost of the combined strategy exceeds $50,000 per QALY. Newer serum tumor markers such as AFP-L3 were not included in the analysis because they are not yet validated for use in screening.82 If their test characteristics prove to be significantly better than standard AFP, then the combined strategy may become more favorable.
Our results are reasonably consistent with prior cost-effectiveness analyses of HCC surveillance, although no study has evaluated surveillance with US alone in a general population of cirrhotics in the United States. Sarasin and colleagues evaluated semiannual AFP/US surveillance in compensated cirrhotics.15 Their model considered surgical resection as the sole treatment modality. Under a best-case scenario, average life expectancy gain from surveillance in this study was 9 months. The cost-effectiveness of surveillance ranged from $26,000 to $284,000 per life year gained, depending on patient age, the incidence of HCC, and prognosis following surgery. Three other studies of surveillance in hepatitis C cirrhosis reported similar cost-effectiveness estimates but did not consider the use of US alone for surveillance.16–18 A recent study of HCC surveillance in the United Kingdom comparing periodic surveillance with combined AFP/US to strategies using AFP or US alone in alcoholic, HBV-related and HCV-related cirrhosis found that semiannual AFP/US surveillance was most effective but exceeded the typical willingness-to-pay threshold in the U.K. (£30,000/QALY).19 In this population, a novel “AFP triage” strategy, in which elevated AFP (>20 ng/ml) triggered a diagnostic US, captured much of the clinical benefit of surveillance at a reasonable cost. We did not consider this strategy because an elevated AFP is typically evaluated with CT or MRI in the United States. However, our results suggest that US alone would be a similarly efficient strategy in the United States.
This analysis has several limitations. Because the performance of imaging tests under specific conditions is not described in published studies, we assumed that test characteristics are independent of patient characteristics and tumor size. The preferred strategy may differ if a test consistently performs poorly in certain subgroups, such as in patients with obesity. Similarly, because of methodological differences in published imaging studies, the sensitivities and specificities of US, CT, and MRI utilized in this study may refer to tumors of different sizes. Whereas MRI studies typically incorporate missed subcentimeter lesions into the sensitivity calculations, no lesion this small is detected by US in any study. However, our findings did not differ when each test was assigned perfect test characteristics in sensitivity analyses. Our model was limited by the lack of published data on certain parameters, such as the proportion of small HCCs treated with curative intent, but surveillance remained cost-effective even when these parameters were varied widely in sensitivity analyses. The actual costs of surveillance may be underestimated if clinical practice deviates significantly from our model assumptions.
The risk of HCC varies among the different etiologies of cirrhosis, although the precise risk for some etiologies is not well defined. Surveillance of cirrhotic populations with a relatively low risk of HCC, such as patients with primary biliary cirrhosis, appears justified by our finding that surveillance remains cost-effective even with an annual incidence of cancer as low as 0.8%. However, if the estimated incidence of HCC in these populations is less than 2.2%, annual US surveillance rather than semiannual may be preferable. Because our natural history parameters derive from Western populations with mixed etiologies of cirrhosis and our cost data is solely U.S. based, the cost-effectiveness estimates may differ in specific cirrhotic populations and in other countries.
The results of our study predict that surveillance for early HCC will improve clinical outcomes. Our findings support recent AASLD guidelines recommending surveillance with US alone. While the preferred strategy may vary in specific situations, we found that semiannual US is both effective and cost-effective for routine surveillance for HCC in cirrhosis.
Acknowledgments
Financial support: K24DK078772 and T32DK007191
Abbreviations
- HCC
hepatocellular carcinoma
- US
ultrasound
- CT
computed tomography
- QALY
quality-adjusted life year
- AFP
alphafetoprotein
- MELD
Model for End-Stage Liver Disease
- MRI
magnetic resonance imaging
- QALE
quality-adjusted life expectancy
- ICER
incremental cost-effectiveness ratio
Footnotes
Conflict of interest: No author has a conflict of interest to disclose.
Contributor Information
Karin L. Andersson, Email: kandersson@partners.org, Gastrointestinal Unit, Massachusetts General Hospital.
Joshua A. Salomon, Email: Joshua.Salomon@harvard.edu, Program in Health Decision Science, Harvard School of Public Health.
Raymond T. Chung, Email: rtchung@partners.org, Harvard Medical School and GI Unit, Massachusetts General Hospital.
Sue J. Goldie, Email: sgoldie@hsph.harvard.edu, Program in Health Decision Science, Harvard School of Public Health.
References
- 1.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5 Suppl 1):S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139(10):817–23. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 3.Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122(5):1500–11. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 4.Chalasani N, Said A, Ness R, et al. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224–9. doi: 10.1111/j.1572-0241.1999.01297.x. [DOI] [PubMed] [Google Scholar]
- 5.Colombo M, Mannucci PM, Brettler DB, et al. Hepatocellular carcinoma in hemophilia. Am J Hematol. 1991;37(4):243–6. doi: 10.1002/ajh.2830370406. [DOI] [PubMed] [Google Scholar]
- 6.Cottone M, Turri M, Caltagirone M, et al. Screening for hepatocellular carcinoma in patients with Child’s A cirrhosis: an 8-year prospective study by ultrasound and alphafetoprotein. J Hepatol. 1994;21(6):1029–34. doi: 10.1016/s0168-8278(05)80613-0. [DOI] [PubMed] [Google Scholar]
- 7.Pateron D, Ganne N, Trinchet JC, et al. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol. 1994;20(1):65–71. doi: 10.1016/s0168-8278(05)80468-4. [DOI] [PubMed] [Google Scholar]
- 8.Sangiovanni A, Del Ninno E, Fasani P, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126(4):1005–14. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 9.Solmi L, Primerano AM, Gandolfi L. Ultrasound follow-up of patients at risk for hepatocellular carcinoma: results of a prospective study on 360 cases. Am J Gastroenterol. 1996;91(6):1189–94. [PubMed] [Google Scholar]
- 10.Oka H, Kurioka N, Kim K, et al. Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. Hepatology. 1990;12(4 Pt 1):680–7. doi: 10.1002/hep.1840120411. [DOI] [PubMed] [Google Scholar]
- 11.Toyoda H, Kumada T, Kiriyama S, et al. Impact of surveillance on survival of patients with initial hepatocellular carcinoma: a study from Japan. Clin Gastroenterol Hepatol. 2006;4(9):1170–6. doi: 10.1016/j.cgh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 13.Sherman M. Alphafetoprotein: an obituary. J Hepatol. 2001;34(4):603–5. doi: 10.1016/s0168-8278(01)00025-3. [DOI] [PubMed] [Google Scholar]
- 14.Saab S, Ly D, Nieto J, et al. Hepatocellular carcinoma screening in patients waiting for liver transplantation: a decision analytic model. Liver Transpl. 2003;9(7):672–81. doi: 10.1053/jlts.2003.50120. [DOI] [PubMed] [Google Scholar]
- 15.Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101(4):422–34. doi: 10.1016/S0002-9343(96)00197-0. [DOI] [PubMed] [Google Scholar]
- 16.Arguedas MR, Chen VK, Eloubeidi MA, et al. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol. 2003;98(3):679–90. doi: 10.1111/j.1572-0241.2003.07327.x. [DOI] [PubMed] [Google Scholar]
- 17.Lin OS, Keeffe EB, Sanders GD, et al. Cost-effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther. 2004;19(11):1159–72. doi: 10.1111/j.1365-2036.2004.01963.x. [DOI] [PubMed] [Google Scholar]
- 18.Patel D, Terrault NA, Yao FY, et al. Cost-effectiveness of hepatocellular carcinoma surveillance in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2005;3(1):75–84. doi: 10.1016/s1542-3565(04)00443-4. [DOI] [PubMed] [Google Scholar]
- 19.Thompson Coon J, Rogers G, Hewson P, et al. Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. Health Technol Assess. 2007;11(34):1–206. doi: 10.3310/hta11340. [DOI] [PubMed] [Google Scholar]
- 20.Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234(3):961–7. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 21.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 22.Miller WJ, Baron RL, Dodd GD, et al. Malignancies in patients with cirrhosis: CT sensitivity and specificity in 200 consecutive transplant patients. Radiology. 1994;193(3):645–50. doi: 10.1148/radiology.193.3.7972802. [DOI] [PubMed] [Google Scholar]
- 23.de Ledinghen V, Laharie D, Lecesne R, et al. Detection of nodules in liver cirrhosis: spiral computed tomography or magnetic resonance imaging? A prospective study of 88 nodules in 34 patients. Eur J Gastroenterol Hepatol. 2002;14(2):159–65. doi: 10.1097/00042737-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Gold MR, SJ, Russell LB, Weistein MC, editors. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 25.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112(2):463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 26.Gines P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7(1):122–8. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 27.Realdi G, Fattovich G, Hadziyannis S, et al. Survival and prognostic factors in 366 patients with compensated cirrhosis type B: a multicenter study. The Investigators of the European Concerted Action on Viral Hepatitis (EUROHEP) J Hepatol. 1994;21(4):656–66. doi: 10.1016/s0168-8278(94)80115-0. [DOI] [PubMed] [Google Scholar]
- 28.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–31. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Salerno F, Borroni G, Moser P, et al. Survival and prognostic factors of cirrhotic patients with ascites: a study of 134 outpatients. Am J Gastroenterol. 1993;88(4):514–9. [PubMed] [Google Scholar]
- 30.D’Amico G, Morabito A, Pagliaro L, et al. Survival and prognostic indicators in compensated and decompensated cirrhosis. Dig Dis Sci. 1986;31(5):468–75. doi: 10.1007/BF01320309. [DOI] [PubMed] [Google Scholar]
- 31.Imberti D, Fornari F, Sbolli G, et al. Hepatocellular carcinoma in liver cirrhosis. A prospective study. Scand J Gastroenterol. 1993;28(6):540–4. doi: 10.3109/00365529309098263. [DOI] [PubMed] [Google Scholar]
- 32.Zoli M, Magalotti D, Bianchi G, et al. Efficacy of a surveillance program for early detection of hepatocellular carcinoma. Cancer. 1996;78(5):977–85. doi: 10.1002/(SICI)1097-0142(19960901)78:5<977::AID-CNCR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Bolondi L, Sofia S, Siringo S, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48(2):251–9. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29(1):62–7. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 35.Villa E, Moles A, Ferretti I, et al. Natural history of inoperable hepatocellular carcinoma: estrogen receptors’ status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32(2):233–8. doi: 10.1053/jhep.2000.9603. [DOI] [PubMed] [Google Scholar]
- 36.Calvet X, Bruix J, Bru C, et al. Natural history of hepatocellular carcinoma in Spain. Five year’s experience in 249 cases. J Hepatol. 1990;10(3):311–7. doi: 10.1016/0168-8278(90)90138-h. [DOI] [PubMed] [Google Scholar]
- 37.Barbara L, Benzi G, Gaiani S, et al. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992;16(1):132–7. doi: 10.1002/hep.1840160122. [DOI] [PubMed] [Google Scholar]
- 38.Cottone M, Virdone R, Fusco G, et al. Asymptomatic hepatocellular carcinoma in Child’s A cirrhosis. A comparison of natural history and surgical treatment. Gastroenterology. 1989;96(6):1566–71. doi: 10.1016/0016-5085(89)90528-3. [DOI] [PubMed] [Google Scholar]
- 39.Yeung YP, Lo CM, Liu CL, et al. Natural history of untreated nonsurgical hepatocellular carcinoma. Am J Gastroenterol. 2005;100(9):1995–2004. doi: 10.1111/j.1572-0241.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- 40.Ebara M, Ohto M, Shinagawa T, et al. Natural history of minute hepatocellular carcinoma smaller than three centimeters complicating cirrhosis. A study in 22 patients. Gastroenterology. 1986;90(2):289–98. doi: 10.1016/0016-5085(86)90923-6. [DOI] [PubMed] [Google Scholar]
- 41.Sheu JC, Sung JL, Chen DS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985;89(2):259–66. doi: 10.1016/0016-5085(85)90324-5. [DOI] [PubMed] [Google Scholar]
- 42.Simonetti RG, Liberati A, Angiolini C, et al. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol. 1997;8(2):117–36. doi: 10.1023/a:1008285123736. [DOI] [PubMed] [Google Scholar]
- 43.Nowak AK, Chow PK, Findlay M. Systemic therapy for advanced hepatocellular carcinoma: a review. Eur J Cancer. 2004;40(10):1474–84. doi: 10.1016/j.ejca.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 44.Rizzi PM, Kane PA, Ryder SD, et al. Accuracy of radiology in detection of hepatocellular carcinoma before liver transplantation. Gastroenterology. 1994;107(5):1425–9. doi: 10.1016/0016-5085(94)90545-2. [DOI] [PubMed] [Google Scholar]
- 45.Miller WJ, Federle MP, Campbell WL. Diagnosis and staging of hepatocellular carcinoma: comparison of CT and sonography in 36 liver transplantation patients. AJR Am J Roentgenol. 1991;157(2):303–6. doi: 10.2214/ajr.157.2.1649550. [DOI] [PubMed] [Google Scholar]
- 46.Dodd GD, 3rd, Miller WJ, Baron RL, et al. Detection of malignant tumors in end-stage cirrhotic livers: efficacy of sonography as a screening technique. AJR Am J Roentgenol. 1992;159(4):727–33. doi: 10.2214/ajr.159.4.1326883. [DOI] [PubMed] [Google Scholar]
- 47.Bennett GL, Krinsky GA, Abitbol RJ, et al. Sonographic detection of hepatocellular carcinoma and dysplastic nodules in cirrhosis: correlation of pretransplantation sonography and liver explant pathology in 200 patients. AJR Am J Roentgenol. 2002;179(1):75–80. doi: 10.2214/ajr.179.1.1790075. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro RS, Katz R, Mendelson DS, et al. Detection of hepatocellular carcinoma in cirrhotic patients: sensitivity of CT and ultrasonography. J Ultrasound Med. 1996;15(7):497–502. doi: 10.7863/jum.1996.15.7.497. quiz 3–4. [DOI] [PubMed] [Google Scholar]
- 49.Libbrecht L, Bielen D, Verslype C, et al. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8(9):749–61. doi: 10.1053/jlts.2002.34922. [DOI] [PubMed] [Google Scholar]
- 50.Lim JH, Kim CK, Lee WJ, et al. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic livers: accuracy of helical CT in transplant patients. AJR Am J Roentgenol. 2000;175(3):693–8. doi: 10.2214/ajr.175.3.1750693. [DOI] [PubMed] [Google Scholar]
- 51.Peterson MS, Baron RL, Marsh JW, Jr, et al. Pretransplantation surveillance for possible hepatocellular carcinoma in patients with cirrhosis: epidemiology and CT-based tumor detection rate in 430 cases with surgical pathologic correlation. Radiology. 2000;217(3):743–9. doi: 10.1148/radiology.217.3.r00dc28743. [DOI] [PubMed] [Google Scholar]
- 52.Brancatelli G, Baron RL, Peterson MS, et al. Helical CT screening for hepatocellular carcinoma in patients with cirrhosis: frequency and causes of false-positive interpretation. AJR Am J Roentgenol. 2003;180(4):1007–14. doi: 10.2214/ajr.180.4.1801007. [DOI] [PubMed] [Google Scholar]
- 53.Chalasani N, Horlander JC, Sr, Said A, et al. Screening for hepatocellular carcinoma in patients with advanced cirrhosis. Am J Gastroenterol. 1999;94(10):2988–93. doi: 10.1111/j.1572-0241.1999.01448.x. [DOI] [PubMed] [Google Scholar]
- 54.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139(1):46–50. doi: 10.7326/0003-4819-139-1-200307010-00012. [DOI] [PubMed] [Google Scholar]
- 55.Trevisani F, D’Intino PE, Morselli-Labate AM, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34(4):570–5. doi: 10.1016/s0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 56.Gambarin-Gelwan M, Wolf DC, Shapiro R, et al. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol. 2000;95(6):1535–8. doi: 10.1111/j.1572-0241.2000.02091.x. [DOI] [PubMed] [Google Scholar]
- 57.Di Bisceglie AM, Sterling RK, Chung RT, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43(3):434–41. doi: 10.1016/j.jhep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 58.Krinsky GA, Lee VS, Theise ND, et al. Transplantation for hepatocellular carcinoma and cirrhosis: sensitivity of magnetic resonance imaging. Liver Transpl. 2002;8(12):1156–64. doi: 10.1053/jlts.2002.35670. [DOI] [PubMed] [Google Scholar]
- 59.Krinsky GA, Lee VS, Theise ND, et al. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219(2):445–54. doi: 10.1148/radiology.219.2.r01ma40445. [DOI] [PubMed] [Google Scholar]
- 60.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 61.Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228(1):235–40. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 62.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 63.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30(6):1434–40. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 64.Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226(2):441–51. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 65.Shetty K, Timmins K, Brensinger C, et al. Liver transplantation for hepatocellular carcinoma validation of present selection criteria in predicting outcome. Liver Transpl. 2004;10(7):911–8. doi: 10.1002/lt.20140. [DOI] [PubMed] [Google Scholar]
- 66.Zavaglia C, De Carlis L, Alberti AB, et al. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol. 2005;100(12):2708–16. doi: 10.1111/j.1572-0241.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 67.Fong Y, Sun RL, Jarnagin W, et al. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229(6):790–9. doi: 10.1097/00000658-199906000-00005. discussion 9–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235(3):373–82. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wayne JD, Lauwers GY, Ikai I, et al. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235(5):722–30. doi: 10.1097/00000658-200205000-00015. discussion 30–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Franco D, Capussotti L, Smadja C, et al. Resection of hepatocellular carcinomas. Results in 72 European patients with cirrhosis. Gastroenterology. 1990;98(3):733–8. [PubMed] [Google Scholar]
- 71.Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103(6):1201–9. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 72.Machi J, Bueno RS, Wong LL. Long-term follow-up outcome of patients undergoing radiofrequency ablation for unresectable hepatocellular carcinoma. World J Surg. 2005;29(11):1364–73. doi: 10.1007/s00268-005-7829-6. [DOI] [PubMed] [Google Scholar]
- 73.Sala M, Llovet JM, Vilana R, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40(6):1352–60. doi: 10.1002/hep.20465. [DOI] [PubMed] [Google Scholar]
- 74.Camma C, Di Marco V, Orlando A, et al. Treatment of hepatocellular carcinoma in compensated cirrhosis with radio-frequency thermal ablation (RFTA): a prospective study. J Hepatol. 2005;42(4):535–40. doi: 10.1016/j.jhep.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 75.El-Serag HB, Siegel AB, Davila JA, et al. Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: a population-based study. J Hepatol. 2006;44(1):158–66. doi: 10.1016/j.jhep.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127(10):855–65. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 77.Younossi ZM, Singer ME, McHutchison JG, Shermock KM. Cost effectiveness of interferon alpha2b combined with ribavirin for the treatment of chronic hepatitis C. Hepatology. 1999;30(5):1318–24. doi: 10.1002/hep.510300518. [DOI] [PubMed] [Google Scholar]
- 78.Kim WR, Poterucha JJ, Hermans JE, et al. Cost-effectiveness of 6 and 12 months of interferon-alpha therapy for chronic hepatitis C. Ann Intern Med. 1997;127(10):866–74. doi: 10.7326/0003-4819-127-10-199711150-00002. [DOI] [PubMed] [Google Scholar]
- 79.Lindfors KKMR, John CMD. The Cost-effectiveness of Mammographic Screening Strategies. JAMA. 1995;274(11):881–4. [PubMed] [Google Scholar]
- 80.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of Screening for Colorectal Cancer in the General Population. JAMA. 2000;284(15):1954–61. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 81.Sonnenberg A, Delco F. Cost-effectiveness of a Single Colonoscopy in Screening for Colorectal Cancer. Arch Intern Med. 2002;162(2):163–8. doi: 10.1001/archinte.162.2.163. [DOI] [PubMed] [Google Scholar]
- 82.Yuen M-F, Lai C-L. Serological markers of liver cancer. Best Practice & Research Clinical Gastroenterology. 2005;19(1):91–9. doi: 10.1016/j.bpg.2004.10.003. [DOI] [PubMed] [Google Scholar]