Abstract
Herein we will review the role of glycans in determining the functionality and specificity of various components of the immune system. Specific topics covered include: the specific glycosylation sites of IgE, IgM, IgD, IgE, IgA, and IgG; how glycans can encode “self” identity by functioning as either danger associated molecular patterns (DAMPs) or self-associated molecular patterns (SAMPs); the role of glycans as markers of protein integrity and age; how the glycocalyx can dictate the migration pattern of immune cells; and how the combination of Fc N-glycans and Ig isotype dictate the effector function of immunoglobulins. We speculate that the latter may be responsible for the well-documented association between alterations of the serum glycome and autoimmunity. Due to technological limitations, the extent of these autoimmune-associated glycan alterations and their role in disease pathophysiology has not been fully elucidated to date. Thus, we also review the current technologies available for glycan analysis, placing an emphasis on Multiple Reaction Monitoring (MRM), a rapid high-throughput technology that has great potential for glycan biomarker research. Finally, we put forth The Altered Glycan Theory of Autoimmunity, which states that each autoimmune disease will have a unique glycan signature characterized by the site-specific relative abundances of individual glycan structures on immune cells and serum proteins, especially the site-specific glycosylation patterns of specific antibody classes and subclasses.
Keywords: Glycan, glycome, glycosylation, immunoglobulin, autoimmunity
Introduction and The Altered Glycan Theory of Autoimmunity
Since the discovery of altered IgG glycosylation in patients with rheumatoid arthritis [1], there has been mounting evidence favoring the role of glycans in the pathophysiology of autoimmunity. For example, it is now well established that the type of glycan present at residue Asn-180 of IgG1 helps dictate the effector function of the antibody, with some glycans being pro-inflammatory while others possessing anti-inflammatory properties [2, 3]. As is the case for the Asn-180 glycan, the antibody isotype also has a strong influence over its functionality [4]. In fact, some autoimmune diseases are strongly linked to a particular Ig class or subclass. Prototypic examples include the IgG4-mediated diseases, pemphigus foliaceus and autoimmune pancreatitis [5, 6]. Thus, in antibody-mediated autoimmunity, antigen specificity will determine the site of attack whereas the glycan/Ig isotype combination will dictate the physical nature of the attack. Of note, the glycosylation patterns of immunologically relevant cells and serum proteins are not solely dictated by gene expression profiles; environmental factors, and the age of the glycans, are equally important. Based on these fundamentals we put forth The Altered Glycan Theory of Autoimmunity, which states that each autoimmune disease will have a unique glycan signature characterized by the site-specific relative abundances of individual glycan structures present on immune cells and serum proteins, especially the site-specific glycosylation patterns of the different Ig classes and subclasses. Herein we discuss the role of glycans in the immune system and how novel Mass Spectrometry (MS) technologies, specifically Multiple Reaction Monitoring (MRM), can be used to rapidly identify the glycan signatures of the different autoimmune diseases. [Asn-180 of IgG1 corresponds to Asn-176 of IgG2, Asn-227 of IgG3, and Asn-177 of IgG4. Using the International Immunogenetics Information System (IMGT) numbering protocol the position for each of these conserved glycosylations is the same, CH2-84.4, regardless of IgG subclass. An addition point of potential confusion is that Asn-180 is also sometimes referred to as Asn-297, if one attempts to sequentially number all amino acids within the IgG molecule. For simplicity purposes this review will refer to conserved IgG glycosylation site as CH2-84.4].
A brief overview of antibody structure
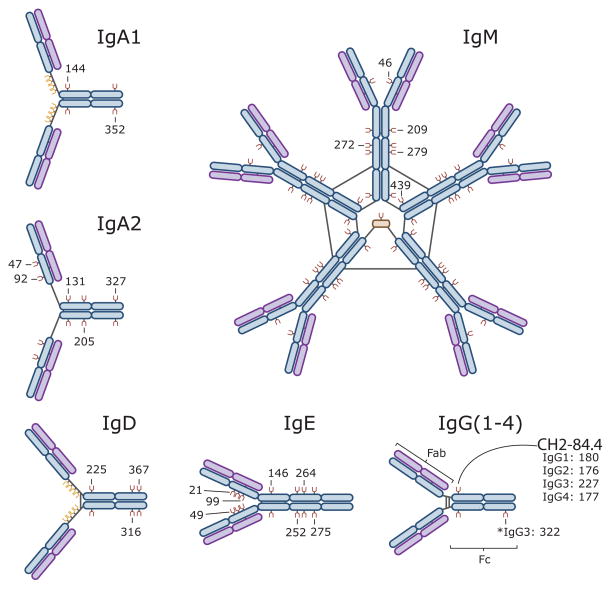
Humans have five distinct classes of immunoglobulins (Igs): IgG, IgM, IgA, IgE, and IgD (Figure 1). IgA and IgG can be further divided into two (IgA1-2) and four (IgG1-4) subclasses, respectively. All Igs are comprised of two 50–77 kDa class-specific heavy chains (γ, μ, α, ε, and δ) that are joined together by one or more disulfide bonds (Figure 1). Each heavy chain is also joined by a disulfide bond to a 25 kDa light chain, which can be one of two different isoforms (κ and λ). For IgM and IgA, disulfide bonds can further connect individual Igs (an their associated J chains) to form pentamer and dimer structures, respectively (Figure 1). The antigen-recognition region of an Ig is referred to as its Fab fragment. In contrast, the Fc fragment is comprised of the heavy chain region that interacts with the Fc receptors on immune cells. In the IgA, IgD, and IgG isoforms, a flexible linker containing N and O glycans separates the Fab and Fc regions. IgM and IgE lack this hinge region and are thus more rigid in structure. IgG1,2,4 have a single conserved N glycosylation site at residue CH2-84.4 where large (2 KDa) flexible glycans attach. The other Igs are more heavily glycosylated (Figure 1). As will be discussed in later sections, these glycan modifications are critical for the appropriate function of all Igs.
Figure 1. Immunoglobulin Isotypes and their sites of glycosylation.
Depicted here are the antibody structures including their sites of glycosylation: IgM [(N-glycans at Asn-46, 209, 272, 279, and 439 (UNIPROT), or CH1-45, CH2-120, CH3-81, CH3-84.4, and CHS-7 (IMGT)]; IgA1 [(N-glycans at Asn-144 and 352 (UNIPROT) or CH2-20, CHS-7 (IMGT)]; IgA2 [(N-glycans at Asn-47, 92, 131, 205, 327 (UNIPROT) or CH1-45.2, CH1-114, CH2-20, CH2-120 and CHS-7 (IMGT)]; IgG [(N-glycans at Asn-180 (IgG1), Asn-176 (IgG2), Asn-227 (IgG3), Asn-177 (IgG4), and Asn-322 (IgG3) (UNIPROT) or CH2-84.4 (IgG1-4) and CH3- 79 (IgG3) (IMGT)]; IgD [(N-glycans at Asn-225, 316, and 367 (UNIPROT) or CH2-84.4, CH3-45.4, CH3- 116 (IMGT)], and IgE [(N-glycans at Asn-21, 49, 99, 146, 252, 264, 275 (UNIPROT) or CH1-15.2, CH1-45.2, CH1-118, CH2-38, CH3-38, CH3-77, and CH3-84.4 (IMGT)]. Each immunoglobulin is comprised of two heavy chains (blue) and two light chains (purple) that are linked together by disulfide bonds (black lines). IgA, IgD, and IgG have a flexible hinge region that link the Igs’ antigen-binding Fab region to their Fc receptor-binding region. O-glycosylation sites are depicted in yellow and N-glycosylation sites are depicted in brown. The depicted glycans are important for the structural integrity of the antibodies and their effector function [2].
A vast amount of information is stored in a cell’s glycocalyx
Along with nucleic acids, proteins, and lipids, glycans are one of the four fundamental classes of molecules that make up all living systems [7]. However, in comparison to the advances made in the fields of genomics and proteomics, glycoscience remains relatively understudied, a disturbing fact given that glycans play a major role in the etiology of all human diseases [8]. Traditionally, the information stream of a cell is viewed as starting in the genome and ending with a set of expressed proteins, representing the cell’s phenotype. Once synthesized, proteins can interact with other proteins to form networks. However in order for a protein to function appropriately, it often requires a post-translational modification and glycans are one of the most commonly added modifiers (Figure 2). Thus, they can be considered the final step in the cell’s information stream. Following this logic, it has been suggested that the proteome predicts the phenotype but the glycome actually is the phenotype [8]. Supporting this view is the fact that glycans can function as protein “on and off” switches or as “analog regulators” to fine-tune protein function [8]. But how is information stored within the glycome?
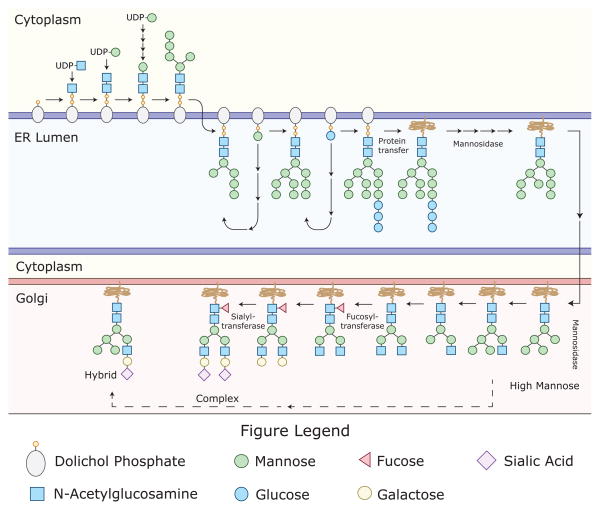
Figure 2. A limited number of sugar monomers can create thousands of complex glycans.
Post-translational glycan modifications are generally thought to be important for protein folding; steric protection from proteolytic degradation; and regulation of protein-protein interactions. It is estimated that up to 70% of mammalian proteins are glycosylated. The glycans are attached to proteins via “N” or “O” linkages, with N-glycosylations being more common. N-glycans are attached to asparagine (Asn) residues, whereas O-glycans are attached to amino acids serine (Ser) or threonine (Thr). Depicted here is the process of N-glycosylation, which begins in the endoplasmic reticulum (ER) and ends in the Golgi. N-glycans are attached to proteins at specific motifs; Asparagine-X-Serine or Asparagine-X-threonine, where X can be any amino acid except proline. During the process of N-glycosylation, monosaccharides (often donated by UDP or GDP-sugars) are sequentially added to the glycan structure. Initially, two N-Acetylglucosamine residues are added consecutively to Dol in the cytosol. This is followed by the addition of several mannose (Man) residues. After formation of the intermediate (Man5HexNAc2-PP-Dol), the complex is flipped into the ER-lumen. Then, four additional Man residues are added. This is followed by the addition of 3 glucose (Glc) residues, donated by Glc-P-dolichol, to form the Glc3Man9GlcNAc2-PP-dolichol precursor glycan, which is then transferred to an Asn residue on a newly synthesized protein. Glycosidases and glycosyltransferases then modify the precursor glycan to potentially generate over 10 thousand unique structures, which can be separated into three very broad structural categories (High Mannose, Hybrid, and Complex).
First, let us establish that glycan structures are sufficiently complex for information storage. A cell is able to synthesize thousands of unique glycan structures by linking together a finite set of sugar monomers [9] (Figure 2). Unlike DNA, RNA and proteins, glycan synthesis is not a template driven linear process. The specific glycans found at a particular site along a protein can be very heterogeneous, reflecting the cell’s narration including its history of expressed genes and its environmental encounters. Ultimately, each glycan structure will contain some information about the cell. This information is different from, but complementary to, the genetic information stored in the cell’s nucleus [8]. When one considers the massive 3-dimensional structural diversity of glycans combined with the variation in their attachment sites, the potential for information to be stored within the glycocalyx parallels that of the genome. But in contrast to a cell’s genetic information, we are just beginning to understand the information stored within the glycome. In this regard, glycoscience is similar to where the field of genetics was during the initial stages of the genome project [8].
If the glycan code has yet to be deciphered, and for the most part the exact structures and attachment sites of human glycans are largely unknown, how can we be certain that information is actually stored within the glycome? The answer is simple. Although science has yet to develop the tools needed to understand the glycome, nature has already done so. Lectins are carbohydrate-binding proteins that are used by cells and microbial pathogens to interpret the glycome [10]. They have complex specificities that not only incorporate select sugar monomers such as galactose, mannose, and fucose but also carbohydrate branching, spacing, and multivalency. To highlight how lectins can harvest the information stored within the glycome, we provide the following three examples.
Example 1: Self Identity is displayed by the glycocalyx
The role that glycans play in the pathophysiology of disease is not surprising considering every cell in the human body is decorated with a thick layer of glycans, the glycocalyx. Because the immune system is tasked with surveying the body for “danger”, the glycocalyx will be routinely engaged when an immune cell contacts another cell or for that matter any component of its environment [11]. In such interactions, glycans help dictate the behavior of immune cells. Although the exact molecular structures and attachment sites of the glycan components of the human immune system are poorly characterized, it is clear that they play a major role in all of the fundamental functions of the immune system, the most basic of which is “self/non-self” discrimination, as described below.
In order for the immune system to respond to an invading pathogen or other imminent threat, it needs to first identify the threat as “non-self”. Since the late 1990’s several seminal discoveries have demonstrated how the immune system can recognize and respond to foreign patterns [12–14]. As it turns out many of the “pathogen-associated molecular patterns” (PAMPs) and “danger associated molecular patterns” (DAMPs) are glycoconjugates, and their immune receptors are lectins. Examples include the soluble mannose-binding lectins (MBLs), which recognize foreign glycan patterns present on microbes and injured host cells. MBLs form complexes with MBL-associated serine proteases (MASPs), which in turn activate complement to destroy the microbial pathogen or potentially dangerous host cell [15] (Later in this review we will provide a specific example of how glycan alterations in the setting of autoimmunity can result in MBL-mediated tissue injury). Many other pattern recognition receptors, including most of the α-defensins, have similar carbohydrate-binding properties [16].
Not as well understood, but of equal importance, is how lectins within the immune system recognize “self-associated molecular patterns” (SAMPs) to prevent robust responses to non-pathogenic stimuli [17]. Sialic acid-containing glycans likely function as SAMPs and Siglecs (e.g., CD33), Factor H, and CD24 have all been identified as potential SAMP-recognizing receptors that can repress immune responses [18]. There are at least 16 sialic acid-binding Ig-like lectins (Siglecs) expressed by different leukocyte populations [19]. Some of these interpret non-pathogen glycans as “self” and deliver inhibitory signals to immune cells to prevent them from becoming over stimulated. Certain C-type lectin receptors (CLRs) on the surface of dendritic cells likely also function as SAMP receptors. CLRs help instruct dendritic cells as to when it is appropriate to induce immune tolerance rather than lymphocyte activation [20, 21]. Examples of CLRs expressed by immature monocyte derived dendritic cells include CD206, DEC-205, DC-SIGN, BCDA-2, Dectin-1, DCIR, DCAL-1, C-LEC, and DC-ASGPR. As a testament to how important the above interactions are, “self” glycans and their receptors are rapidly evolving to foil attempts by pathogenic microbes to mimic SAMPs in an effort to avoid immune recognition [22, 23]. Similarly, “self” glycans also evolve to hide from viruses and other microbes that use host glycans as microbial-binding sites to establish infections [17].
With respect to autoimmunity, a deficiency in SAMP-mediated signaling might predispose an individual to develop an autoreactive immune response. As described later, depending on their structure, the glycans present on IgG can function as either DAMPs or SAMPs, with the ability to potentiate or suppress an autoreactive immune response, respectively. Careful characterization of these glycans will likely yield new biomarkers of autoimmunity. Ultimately the identification of novel serum SAMPs will enable investigators to design ‘glycomimetics’ as immunomodulatory drugs for the treatment of autoimmune diseases. .
Example 2: Information on “Age” is stored within the glycocalyx
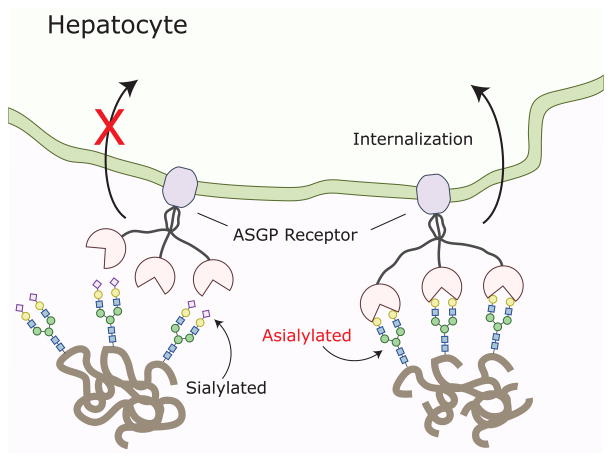
A classic example of this is the clearance of old erythrocytes and glycoproteins from the blood [24]. As an erythrocyte ages, it becomes progressively de-sialylated, which in turn increases the density of exposed galactose moieties on its surface. This allows for asialoglycoprotein receptors (ASGP-R) in the liver to identify the old erythrocytes and signal for their destruction [25].
Within the immune system sialic acid moieties sometimes identify the maturation state on immune cells. For example, the density of sialic acid on the T cell surface also changes over time. Naive T cells express CD45 that is modified with α2,6-linked sialic acid. The amount of α2,6-linked sialic acid is significantly reduced following T cell activation. This decrease in α2,6-linked sialic acid renders the activated T cells more susceptible to galectin-1 mediated apoptosis [26]. Interestingly, CD4+ Th2 cells are resistant to galectin-1 mediated apoptosis because their expression of α2,6-linked sialic acid is not decreased [27]. The exact structure and location of the sialic acid containing glycans have not been well established but since these moieties are differentially expressed on activated versus naive T cells, they might contribute to a unique autoimmune glycan signature.
As is the case with old erythrocytes, the liver also uses de-sialylation to purge non-functional proteins from the circulation (Figure 3). Clearance of IgA is mediated at least in part by ASGP-Rs, which recognizes galactose-terminating IgA N-glycans [28, 29]. Differing from IgG, IgA1 and IgA2 have two conserved N-liked glycosylation sites, one on the CH2 domain and the other on their CHS tailpiece [2]. Apart from these, IgA2 has three additional N-linked glycosylation sites and IgA1 has several additional O-glycosylation sites. As a result of their increased accessibility to glycosyltransferases, IgA glycans are more likely to be sialylated, which allows IgA clearance to be regulated by ASGP-Rs. In the setting of IgA-mediated nephropathy, alterations in IgA1 glycans have been well documented [30]. Decreased sialylation and galactosylation results in altered IgA1 aggregation and impaired ASGP-R-mediated clearance. Together these contribute to the onset of nephropathy [31]. Theoretically, these isotype-specific glycan alterations are part of a unique glycan signature indicative of IgA-mediated autoimmunity. (Of note, the liver does not target de-sialylated IgG for clearance and thus the CH2-84.4-attached glycans do not regulate IgG half-life. This task seems to be carried out by the neonatal Fc receptor, which keeps IgG levels constant [32, 33]).
Figure 3. Asialoglycoprotein receptors.
Sialylated serum proteins and cells are not recognized by asialoglycoprotein receptors in the liver and are thus protected from uptake and degradation. However, as an initially sialylated molecule ages it progressively loses its sialic acid moieties making it a target for asialoglycoprotein receptors. In the liver these receptors identify desialylated proteins, targeting them for uptake and degradation.
Example 3: The glycocalyx dictates lymphocyte migration
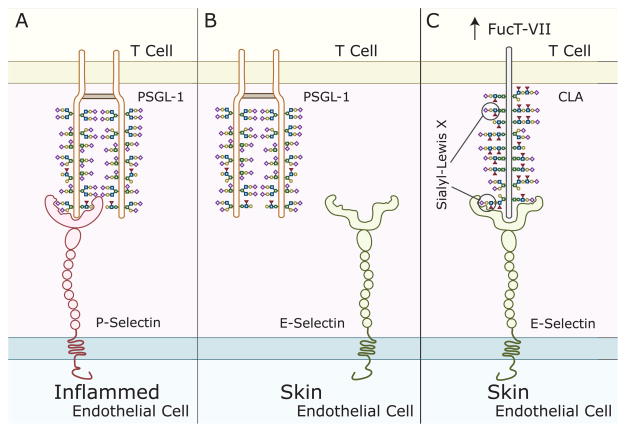
In order for a leukocyte to extravasate into a tissue, it must first slow down and roll along the endothelial surface. Leukocyte rolling is initiated by selectins, which are highly regulated glycan-binding glycoproteins. Selectins are exquisitely specific at binding to certain glycan structures. For example, the high endothelial venules of the secondary lymphoid tissues express the ligand for L-selectin, the glycosylation-dependent cell adhesion molecule-1 (GlyCAM-1). Since L-selectin is expressed by naive and central memory T cells, binding of L-selectin to GlyCAM-1 homes these T cells to the lymph nodes. In contrast, effector memory T cells lack L-selectin and thus stay in the periphery [34]. In the above example, the lack of a glycoconjugate prevents the effector memory T cells from homing to the lymph nodes. However, sometimes the difference is even more subtle. For example, skin-infiltrating T cells express the cutaneous lymphocyte-associated antigen (CLA), which contains a carbohydrate epitope recognized by the monoclonal antibody, HECA-452. CLA mediates lymphocyte migration to the skin through its interaction with E-selectin. Remarkably, it was discovered that CLA is actually an inducible carbohydrate modification of P-selectin glycoprotein ligand-1 (PSGL-1), a glycoprotein expressed by all human T cells [35]. This is an excellent example of a glycan functioning as an “on-off” switch. In the case of CLA, the CLA-specific glycans “turn on” PSGL-1’s skin-homing function (Figure 4). Other lectins such as DC-SIGN might also participate as rolling receptors. Thus, the process is regulated by a repertoire of glycans present on both the effector and target cells (e.g. lymphocytes and endothelial cells, respectively) [36]. Given that autoimmunity is often organ-specific it is likely that the autoreactive cells will have unique glycan tissue-homing signatures, which could potentially serve as novel glycan biomarkers of autoimmunity. With a better understanding of the glycan signatures that home lymphocytes to different anatomical sites, exquisitely specific therapeutics can be developed to inhibit inflammation in a tissue-specific manner.
Figure 4. Cutaneous Leukocyte Antigen (CLA) is a glycovariant of P-selectin glycoprotein ligand-1 (PSGL-1).
A) PSGL-1, present on the surface of T cells, binds to P-selectin, which is upregulated on endothelial cells in the setting of inflammation. PSGL-1 binding to P-selectin helps initiate leukocyte rolling. B) In contrast, PSGL-1 does not bind to E-selectin, which is present on endothelial cells within the skin. C) Skin-homing T cells up-regulate the glycosylation enzyme FucT-VII, which leads to an increase in the number of sialyl-Lewis X moieties on PSGL-1, bestowing it with the capacity to bind to E-selectin. The sialyl-Lewis X-decorated E-selectin-binding glycoform of PSGL-1 is called CLA, which also differs from PSGL-1 in that it occurs as a monomer.
The impact of Ig isotype on immunoglobulin effector function
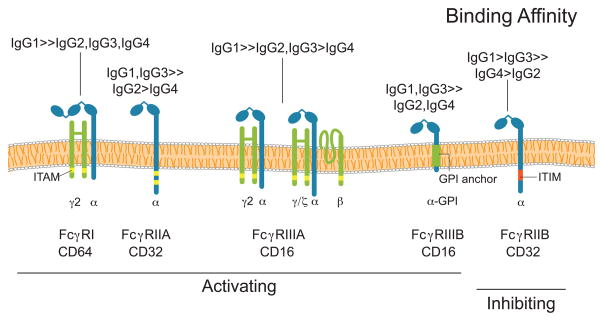
Although an immunoglobulin’s Fab region dictates its antigen-specificity (i.e. the site of attack for autoreactive antibodies), effector function is determined by the Fc region [4]. For example, FcR-bearing innate immune cells can initiate pro-inflammatory or cytotoxic pathways following engagement of their FcRs with antigen-bound multimeric Ig complexes [37–39]; the particular type of response induced by this interaction will depend on the FcRs that are engaged. Fc receptors are a family of glycoproteins that are comprised of an IgG-binding subunit that, depending on the receptor, may pair with accessory γ, ζ,βor β subunits, which are important for receptor signaling [40]. In humans there are three classes of FcRs: FcγRI, FcγRII, and FcγRIII (Figure 5). These can be broadly categorized as either inhibitory FcRs (FcγRIIB) or activating FcRs (FcγRI, FcγRIIA, and FcγRIIIA) [4]. With the exception of FcγRI, which has a high affinity for monomeric IgG, all other FcRs bind to multimeric antigen-antibody complexes [4]. The affinity of the FcR for these complexes depends on the subclass of the complexed IgGs (Figure 5).
Figure 5. Fc receptors can have unique Ig specificities.
FcγRI, FcγRIIA, FcγRIIIA are activating Fc receptors that differ in their affinities for the individual IgG subclasses; the only commonality being that each of them binds best to IgG1. Of the activating receptors, FcγRI is unique in that it can bind with high affinity to monomeric IgG antibodies. In contrast, FcγRIIA and FcγRIIIA are low affinity Fc receptors that bind only to antigen-antibody complexes. Activating signals originating from FcR-Ig interactions can be initiated by intracellular immunoreceptor tyrosine-based activation motifs (ITAMs), either within the Fc receptor or as part of the Fc receptor complex. In contrast, FcγRIIB is a low affinity inhibitory Fc receptor, which has an immunoreceptor tyrosine-based inhibition motif (ITIM) as part of its cytoplasmic domain. FcγRIIIB, found exclusively on neutrophils, is an activating glycosylphosphatidylinositol (GPI)-linked receptor.
With respect to the different activating FcRs, the consequences of their engagement will depend on the type of cell that expresses them (Figure 5), as different cell types have markedly different effector responses. Additionally, the same FcR can be linked to different signaling molecules when expressed by different cell types [4]. Common effector functions initiated by activating FcRs include: phagocytosis, antigen processing, cytokine release, degranulation and antibody-dependent cellular cytotoxicity (ADCC) [41, 42]. However, individual activating FcRs can initiate different cellular responses even when expressed by the same cell. For example, human neutrophils express two activating FcRs, FcγRIIA and FcγRIIIB. When engaged, FcγRIIA increases L-selectin expression [43] and promotes phagocytosis [44]. In contrast, engagement of FcγRIIIB results in robust phosphorylation of ERK and the transcription factor ELK-1 and increased β1 integrin activation [45, 46].
Signals originating from the activating FcRs are balanced by those from the inhibitory FcR, FcγRIIB, which helps to regulate the antigen-IgG-induced activation threshold of FcR-expressing cells, maintain peripheral tolerance, and ultimately terminate IgG mediated inflammatory effector responses. FcγRIIB is expressed by a variety of innate immune effector cells (except for NK cells) but unlike other FcRs, FcγRIIB is unique in that it is also expressed by B cells. The expression pattern of the different FcRs is regulated by a variety of environmental factors including the presence of: LPS, interleukins, TNF, complement proteins, and TGF-β[38]. Aberrant increases of a particular FcR will result in an imbalanced immune response that can lead to either a pro- or anti-inflammatory state depending on the integration of FcR-originating activating and inhibitory signals. For example, without FcγRIIB, mice develop autoimmunity in a B cell-autonomous manner, demonstrating FcγRIIB’s direct role in maintaining self-tolerance [47]. Clearly, maintaining an appropriate balance between activating and inhibitory FcRs can help prevent the development of autoimmunity.
IgGs of different subclasses preferentially interact with different FcRs. It is therefore not surprising that several antibody-mediated autoimmune diseases have been associated with a particular Ig isotype (Table 1). IgG4-mediated diseases include pemphigus vulgaris and autoimmune pancreatitis. In such cases the pathophysiology of the autoimmune disease is likely linked either to the particular Fcbinding profile of the autoreactive Igs, or to some other immunologically relevant lectin receptor that can interact with the Igs’ Fc glycan(s) (Figure 1). As described later, detection of biologically relevant perturbations of serum IgG subclass concentrations can be problematic given the large variation in normal IgG concentrations. In the future, the relationship between antibody-mediated autoimmunity and Ig isotype will become increasingly apparent as investigators focus more on the relative abundance of the different isotypes rather than their absolute concentrations. By determining the relative abundance of the different Ig class and subclasses and integrating this with the site-specific glycosylation profiles we predict that disease-specific glycan signatures will emerge for most autoimmune diseases.
Table 1.
Disease Specific Antigens and Immunoglobulin Subclasses
| Autoimmune Disease | Known Antigens | Ig Subclass | Reference |
|---|---|---|---|
| Autoimmune Pancreatitis | Unspecified | IgG4 | [6] |
| Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP) | Neurofascin 155 | IgG4 | [101] |
| Hashimoto’s Thyroiditis (HT) | Thyroid Peroxidase | IgG4 | [102] |
| Thyroglobulin | IgG4 | ||
| Pemphigus Foliaceus | Desmosome-associated glycoprotein | IgG4 | [5] |
| Pemphigus Vulgaris | Desmoglein 3 | IgG1, IgG4 | [103] |
| Rheumatoid Arthritis | Cyclic citrullinated proteins | IgM, IgG, IgA | [104] |
| IgA Nephropathy | Unspecified | IgA1/IgA | [105] |
| Thyroid Eye Disease (TED) | Calcium binding protein (calsequestrin) | IgG1, IgG3 | [106] |
| Grave’s Disease | Thyroid Stimulating Hormone Receptor | IgG3/IgG | [107] |
| Primary Biliary Cirrhosis (PBC) | Antimitochondrial antibodies (AMAs) | IgM | [108–109] |
| Antinuclear antibodies (ANA) | IgG3 | ||
| Rim-like/membrane (RL/M) | IgG1 | ||
| Multiple nuclear dot (MND) | IgG3 |
Serum glycopeptides as biomarkers of autoimmunity?
With a glycoprotein concentration of 40 grams/L, serum is an excellent source of glycans to search for novel biomarkers of human disease. Unlike RNA and protein, there is no template for glycan synthesis. Glycosylation is a process influenced by a variety of factors including: the type of cell and its activation state; environmental factors, such as the presence of available metabolites; the age of the cell, as glycan moieties can be lost over time; and inflammatory mediators, such as cytokines and chemokines. All of these factors may be altered in the setting of autoimmunity. For example, some autoimmune diseases have a predominant cytokine signature. These cytokines will profoundly influence the expression of glycosidases, sialidases and glycosyltransferases, which are known to impact glycan structure [48, 49]. Thus, a particular cytokine signature should theoretically be reflected in the serum glycome. Alterations in the serum glycome can also be due to other factors. In some cases, cells will only synthesize a particular glycoprotein under extreme conditions, such as within an inflamed joint of a rheumatoid arthritis (RA) patient. Indeed, during episodes of inflammation there are large fluctuations in serum glycoproteins, including the acute phase proteins [50]. Another factor to consider is that Ig glycosylation can be antigen-dependent. When the glycosylation patterns of anti-HIV antibodies were characterized in HIV-infected patients, researchers discovered that anti-gp120-specific antibodies tended to be less sialylated, and more likely to be of the G0 type when compared to bulk anti-HIV antibodies from the same patients [51]. Since different autoantigens are dominant in patients with various autoimmune diseases, it is possible that this will drive unique disease-specific glycosylation profiles. In summary, the resulting serum glycome is an expression of the overall state of the individual, making it possible for unique autoimmune signatures to be detectable in the serum. Our specific hypothesis is that each particular autoimmune disease will have a unique glycan profile. To identify these disease-specific signatures, we have begun to characterize the composition and relative abundance of the different glycan structures at specific glycosylation sites in patients with autoimmunity.
The impact of glycosylation on immunoglobulin effector function
The idea that glycans will be differentially expressed in the setting of autoimmunity is supported by decades-old research. Early insightful but technologically limited studies revealed alterations of haptoglobin glycosylation in diseases such as rheumatoid arthritis and Crohn’s disease [52, 53]. Other studies focused on characterizing the IgG and IgA-linked glycans. Patients with rheumatoid arthritis, Lambert-Eaton myasthenic syndrome, myasthenia gravis, Crohn’s disease, juvenile arthritis, systemic lupus erythematosus, IgA nephropathy and systemic vasculitis have all been shown to have altered Ig glycosylation [1, 54–64] (Table 2). The observed changes in Ig glycosylation are of immense biological significance because the N-glycans present within the Fc region of Ig help to dictate the antibody’s effector function. The Fc region CH2-84.4 glycosylation site is conserved in all IgG subclasses (IgG1-4) and over 30 different glycans have been shown to attach there [54]. Other classes of immunoglobulins also have N-glycans within their Fc regions. These occur at asparagine residues 263 and 459 for IgA1; 263, 337, and 459 for IgA2; 265, 371, 383, and 394 for IgE; and 332, 395, 402, and 563 for IgM (Figure 1). However, CH2-84.4 of IgG remains the most well-characterized glycosylation site.
Table 2.
Variances in Antibody Glycan Composition
| Glycan Modification | Reference | |
|---|---|---|
| Rheumatoid Arthritis | Decreased N-linked galactosylation and sialylation of IgG | [111,112] |
| Decreased N-linked galactosylation and terminal sialylation of ACPA IgG1 | ||
| Increased fucosylation of ACPA-IgG1 | ||
| IgA Nephropathy | Decreased terminal O-linked galactosylation on the hinge region of IgA1 heavy chains | [113–116] |
| Gastric Cancer | Decreased galactosylation and sialylation of IgG | [60] |
| Henoch-Schonlein Purpura | Decreased galactosylation and sialylation of IgA1 | [113] |
| HIV | Decreased galactosylation, sialylation, and fucosylation in HIV-specific antibodies associated with enhanced Fc-mediated reduction of viral replication and enhanced Fc receptor binding | [51] |
| Influenza and Tetanus Vaccination | Increased galactosylation and sialylation of anti-vaccine IgG | [61] |
| Increased number of sialic acid per glucose during vaccination | ||
| Decreased IgG1 bisecting N-acetylglucosamine | ||
| Lambert-Eaton Syndrome | Decreased galactosylation of IgG1 and IgG2 | [59] |
| Increased levels of bisecting N-acetylglucosamine on IgG1 and IgG2 in patients less than 50 years old | ||
| MPO-ANCA Vasculitis | Decreased galactosylation of IgG | [56,117] |
| Decreased sialylation of IgG Fab′2 | ||
| Myasthenia Gravis | Decreased galactosylation of IgG2 | [59] |
| Ovarian Cancer | Increased agalactosylated biantennary glycanson IgG heavy chains | [58] |
| PR3-ANCA Vasculitis | Decreased N-glycan 2,6-linked sialylation | [56,117,118] |
| Decreased galactosylation of IgG | ||
| Sjogren’s Syndrome | Decreased sialylation of IgG | [119] |
ACPA: anti-citrullinated protein antibodies; Ig: immunoglobulin; MPO: myeloperoxidase; PR3: anti-proteinase 3
To accommodate its CH2-84.4-linked glycan, IgG has a hydrophobic patch that utilizes more than 70 non-covalent bonds to configure the glycan within the interstitial space between its CH2 domains. These non-covalent interactions help maintain the quaternary structure and thermodynamic stability of the Ig Fc region [65]. Thus, the CH2-84.4 glycan is critical for normal Fc function [66]. Because of their location, CH2-84.4 glycans are difficult substrates for glycosyltransferases. This is of importance because the type of glycan present at CH2-84.4 will directly impact Ig effector function. For example, fucose containing CH2-84.4 glycans reduce Ig affinity for FcγRIIIa [67]. Specifically, fucose containing glycans at IgG CH2-84.4 create steric hindrance and thereby prevent FcγRIIIa’s Asn-162 glycan from interacting with the Ig Fc region. Evidently, the FcγRIIIa Asn-162 glycan is required for high avidity interactions with the Fc region of IgG and fucose containing glycans at IgG CH2-84.4 prevent these interactions from occurring [68].
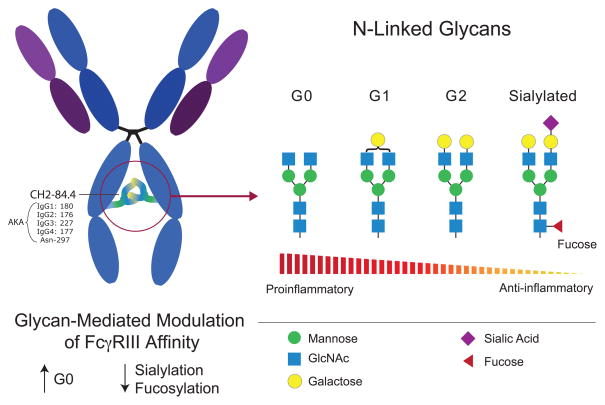
Irrespective of their fucosylation status, IgG CH2-84.4 glycans can be rudimentarily classified as belonging to one of three glycoforms: G0, G1, or G2 (Figure 6), each having different FcR affinities. G0 glycans lack galactose and terminate instead with GlcNAc moieties. In contrast, G1 and G2 glycans contain one or two galactose residues, respectively. In patients with RA, juvenile RA, Crohn’s disease, and some forms of lupus, the glycans at CH2-84.4 were found to often lack galactose, terminating instead with GlcNAc moieties, the so-called G0 glycans [1, 63] (Figure 6). Increased levels of G0 glycans also correlate with RA disease activity [69], but the cause for their increase is not well understood. Some reports have demonstrated an association between increases in G0 glycans and decreased galactosyltransferase (GTase) activity [70, 71], which may be one mechanism. Regardless of how they are created, the proinflammatory nature of G0 glycans is becoming increasingly evident. Investigators have demonstrated that IgGs bearing G0 glycans at CH2-84.4 have increased affinity for FcγRIII, an activating FcR. They also serve as epitopes for lectin binding, including the complement-activating mannose-binding lectins (MBLs) [72]. MBLs interact most efficiently with IgG-G0 clusters, an interaction that has been linked to rapidly progressive RA [73]. In addition to activation of the lectin pathway of complement, G0-decorated IgGs can also activate both the classical and alternative complement pathways making them especially problematic in the setting of autoimmunity [74]. Another interesting finding is that elevations in G0 glycans correlate with the onset of autoimmunity, as they appear with increasing frequency after the age of 25 [75]. Lastly, matching the epidemiology of autoimmunity, is the finding that glycosylation profiles are altered in males and pregnant females. The latter produce antibodies with increased galactose and sialic acid moieties, which would fit an anti-inflammatory profile [69].
Figure 6. IgG Glycoforms and their inflammatory properties.
Ig heavy chain residue CH2-84.4 is post-translationally modified with the addition of an N-glycan, depicted here as a blue, green, and yellow Y-shaped structure between the two IgG heavy chains. This glycosylation site is conserved in all IgG subclasses (IgG1-4). To accommodate its CH2-84.4-linked glycan, IgG has a hydrophobic patch (not depicted). The CH2-84.4-linked glycan can be classified broadly as being either G0, G1, or G2. G0 glycans have a higher affinity for FcγRIII and are associated with a variety of autoimmune diseases. G0 glycans terminate with GlcNac residues and thus have zero galactose residues, hence their name. In contrast, G2 glycans terminate with two galactose residues. CH2-84.4 glycans can also be sialylated or fucosylated, which can bestow the antibody with anti-inflammatory properties because these modifications decrease Ig affinity for FcγRIII and also allow the antibody to interact with endogenous lectins on antigen presenting cells, e.g. sialylated antibodies likely bind to DC-SIGN.
Similar to the effect observed with fucose, sialic acid containing glycans at CH2-84.4 reduce IgG affinity for FcγRIII, bestowing it with anti-inflammatory properties [76]. Correlating with this finding is the fact that sialylated IgGs are often decreased in the setting of autoimmunity, promoting a pro-inflammatory state. There is also a link between the therapeutic effect of intravenous immunoglobulin (IVIG) and sialylation, which epitomizes the importance of glycans containing sialic acid at CH2-84.4 [77]. IVIG is a pooled preparation of immunoglobulin made from thousands of donors and its antiinflammatory properties have been known for more than three decades [78, 79]. IVIG’s mechanism of action is multifactorial but there is good evidence that it can function as a SAMP. By binding to SAMP receptors on antigen presenting cells IVIG can increase the expression of the inhibitory FcR, FcγRIIB, and shorten the half-life of auto-reactive antibodies [80–82]. With respect to sialylation, the antiinflammatory properties of IVIG have been mapped to the CH2-84.4 glycan within the Fc region of IgG. Specifically, de-sialylated preparations of IVIG lose their therapeutic activity and the anti-inflammatory effects of IVIG can be recapitulated with administration of recombinant sialylated IgG1 Fc [76]. From these findings, it is likely that in healthy individuals sialylated antibodies may function as endogenous SAMPs, providing immune-modulatory effects by binding to SAMP receptors. To date, SAMP receptors for sialylated IgG have not been fully characterized but some evidence points to known immunologically relevant lectins such as DC-SIGN, which are thought to be required for the anti-inflammatory properties of IVIG [77].
The importance of CH2-84.4 glycans has also been extensively demonstrated for commercial monoclonal antibodies [92–94]. For example, the efficacy of rituximab, an anti-CD20 monoclonal used to treat lymphoma, appears to be linked to its ability to bind FcγRIIIa [83, 84]. By producing anti-cancer monoclonal antibodies in a variety of cell lines that differ in their glycosylation machinery it was discovered that the presence of a bisecting GlcNAc correlated with superior anti-cancer properties [85]. Subsequently, it was demonstrated that ADCC activity could be increased by producing monoclonal antibodies in CHO cells engineered to over express GnTIII, an enzyme that adds bisecting GlcNac residues [86, 87]. An alternative explanatory model for these results is that bisecting glycans often lack fucose, the absence of which would increase IgG affinity for FcγRIIIa and subsequently promote ADCC [88–90]. Indeed the activity of rituximab is also increased when it is produced in CHO cells that lack α(1–6) fucosyl transferase [88–90].
Although glycosylation of IgG has been extensively studied in the setting of autoimmunity, less is known about the glycosylation patterns of other immunoglobulins such as IgA and IgM in patients with autoimmune diseases. This is due in part to our current limitations in technology and the complexity of these glycosylated proteins. IgA has two subclasses: IgA1 and IgA2, where IgA1 is decorated with 5 O-glycans and 2 N-glycans and IgA2 is decorated with 5 N-glycans. IgM, which typically exists as a homopentamer in blood, also has 5 N-glycosylation sites on each of its heavy chains (Figure 1). It is likely that glycan alterations at these sites will have an impact on how the immunoglobulins interact with other components of the immune system and the relative abundances of these glycans may also be indicative of specific autoimmune disease states, which is currently under investigation.
Peptide and glycopeptide-specific technological advancements in mass spectrometery
The majority of the early glycan profiling research used matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (MS) [91–95] and generally focused on enzymatically released glycans. Analyzing released N-glycans by MALDI MS yields composition profiles that can be converted to putative structures based on known biology, which is highly conserved. (N-Glycans contain a common core composed of a chitobiose and a trimannosyl moiety) (Figure 2). Perhaps for this reason, the majority of glycan profiling studies have characterized N-glycosylations. However, MALDI MS profiling is limited because it does not provide information on isomers and focusing on released glycans provides no information about the originating sites of glycosylation. Such information is important in order to develop a full understanding of how glycans are related to the pathophysiology of autoimmunity. Details on glycosylation sites are also important for biomarker discovery research. We believe that accurately measuring the relative abundance of individual site-specific glycan structures within the immune system is the key to identifying glycan biomarkers of autoimmunity.
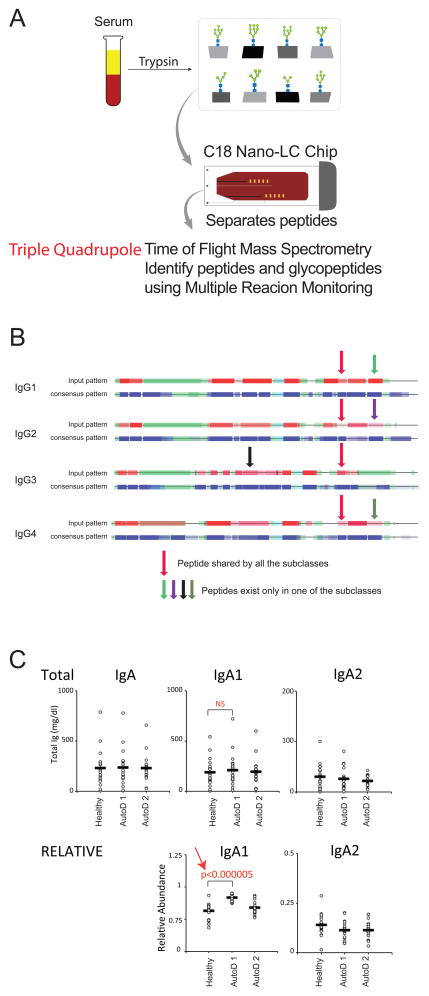
One of the major goals set forth by the National Research Council of the National Academies is for the development of novel technology for the characterization of glycan structures [11]. To rapidly identify autoimmune glycan signatures a technology that provides absolute quantitation of all major serum proteins and their individual glycoforms would be ideal. This would require, for example, determination of the relative and absolute abundance of each IgG subclass including their site-specific glycosylations. With this goal in mind, our laboratory (CL) has used multiple reaction monitoring (MRM) to reliably quantify the absolute and relative Ig glycoforms directly from serum or plasma without the need for additional enrichment procedures [96]. Although MRM has been used extensively in metabolomics and proteomics [97–100], its high sensitivity and linear response over a wide dynamic range make it especially suited for glycan biomarker research and discovery. MRM is performed on a triple quadrupole (QqQ) instrument, which is set to detect a predetermined precursor ion in the first quadrupole, a fragmented in the collision quadrupole, and a predetermined fragment ion in the third quadrupole. It is a non-scanning technique, wherein each transition is detected individually and the detection of multiple transitions occurs concurrently in duty cycles. Altering the cycle time (time spent monitoring all transitions in one duty cycle) affects sampling efficiency and therefore data quality while changes in dwell time (time spent acquiring a specific transition) affects the signal-to-noise ratio. To reduce the number of concurrent transitions the number of monitored transitions per glycopeptide can be decreased to a single transition. Single transition monitoring is possible because the typical fragment ions of a glycan, the so-called “oxonium ions”, can easily identify the compound as a glycopeptide and provide good quantitation.
The main advantage of MRM is that it allows the site-specific glycosylation profile to be normalized to the absolute protein concentration. For IgG quantitation, a tryptic peptide common to the Fc region of all four subclasses of IgG is used for total IgG quantification (Figure 7). Simultaneously, subclass-specific peptides are used to determine the absolute and relative quantities of all four Ig subclasses (Figure 7). Similarly, to increase sensitivity, relative IgM can be calculated using values for the high affinity IgGs. By focusing on relative abundance instead of total Ig concentration, significant disease-associated elevations of an Ig subclass become easy to identify (Figure 7). Importantly, this method does not require special immunoglobulin enrichment procedures that may create bias for specific structures and specific glycoforms. It is also rapid, allowing for high throughput site-specific analyses, ideal for autoimmune biomarker discovery. Combining MRM with ultrahigh pressure liquid chromatography (UHPLC), utilizing a C18 nano-LC column, provides excellent separation of glycopeptides and nonglycosylated peptides at great speed (10–15 minutes versus 50–90 minutes in HPLC) prior to mass spectrometry (Figure 7). UHPLC also reduces charge competition during electrospray ionization, making the technique more sensitive for glycoconjugate detection. Together this approach will allow us to accurately determine an individual’s serum glycome, which will ultimately lead to the identification of novel glycan signatures of autoimmunity.
Figure 7. Multiple Reaction Monitoring (MRM) to identify glycopeptide biomarkers of autoimmunity.
A) Without the need for additional purification, serum or plasma from peripheral blood is digested with trypsin to yield peptide fragments, including glycopeptides. A C18 Nano-LC Chip is then employed to separate the peptides and glycopeptides form one another utilizing Ultra High-Pressure Liquid Chromatography. The separated sample is then ionized using electron spray ionization and analyzed using a triple quadrupole time of flight mass spectrometry (QqQ-MS) using multiple reaction monitoring (MRM). B) MRM requires prior knowledge of the collision-induced dissociation (CID) behavior of the peptides and glycopeptides of interest. This knowledge allows for the appropriate MRM transitions to be developed for QqQ-MS detection. The process also requires a great deal of instrument optimization and knowledge of the peptide and glycopeptide retention times, but once established MRM can rapidly identify peptide and glycopeptides from serum samples with great sensitivity. Depicted here, a tryptic peptide common to the Fc region of all four IgG subclasses (red arrow) is used for absolute quantitation of total IgG. IgG subclass-specific peptides (light green, purple, black, and dark green arrows) are then used for comparison to the common Fc region peptide to determine the relative abundance of the individual IgG subclasses. C) Using a data set of theoretical IgA values, no significant difference between total IgA or IgA subclass-specific titers is seen between healthy controls and patients with two different autoimmune diseases (AutoD1, AutoD2). However, when the data is graphed as the relative abundance of the different IgA subclasses, it becomes clear that patients with AutoD1 have an increase in IgA1 that is highly significant.
Summary and future directions
Although the role of glycans in the immune system is too broad of a topic to successfully cover in one review, we have attempted to highlight some of their important functions, especially with respect to immune homeostasis. We predict that as we continue to develop the appropriate analysis tools, it will become increasingly apparent that a full understanding of one’s immune glycome will provide the greatest insight into their overall immune health, including their likelihood of developing autoimmunity or other immune abnormality. As described above, MRM, is one innovative method capable of quickly characterizing the relative abundance of different glycoconjugates within the serum of an individual. This technology will be the key to identifying novel glycan biomarkers of autoimmunity as well as other immunopathologies. The same technique can also be applied to other human tissues and to experimental systems, including animal models. Ultimately such research will provide additional measures of disease phenotype, help predict patients’ responsiveness to treatment, and provide new insight into the pathogenic immune response responsible for their disease. We anticipate that in the near future glycan analysis will become integral to the diagnosis and management of human disease. [51, 101–118]
Every cell in the body is decorated with a layer of glycans known as the glycocalyx
Site-specific glycosylation of proteins may confer self- or non-self identity
The Altered Glycan Theory of Autoimmunity proposes that autoimmune diseases are associated with unique glycan signatures
Acknowledgments
Funding sources: EM is an early career awardee of the Howard Hughes Medical Institute and the Burroughs Wellcome Fund. This work was supported by NIH DP2OD008752 and NIH DK 39588.
Footnotes
Financial disclosures: The authors have no conflict of interest to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parekh RB, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316(6027):452–7. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 2.Arnold JN, et al. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 3.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8(3):226–34. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 4.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 5.Rock B, et al. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) N Engl J Med. 1989;320(22):1463–9. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- 6.Kamisawa T, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38(10):982–4. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 7.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473(1):4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 8.Transforming Glycoscience: A Roadmap for the Future. Washington (DC): 2012. [PubMed] [Google Scholar]
- 9.Cummings RD. The repertoire of glycan determinants in the human glycome. Mol Biosyst. 2009;5(10):1087–104. doi: 10.1039/b907931a. [DOI] [PubMed] [Google Scholar]
- 10.Smith DF, Cummings RD. Application of microarrays for deciphering the structure and function of the human glycome. Mol Cell Proteomics. 2013;12(4):902–12. doi: 10.1074/mcp.R112.027110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388(6640):394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 13.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91(3):295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 14.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13(1):114–9. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 15.Banda NK, et al. Mechanisms of mannose-binding lectin-associated serine proteases-1/3 activation of the alternative pathway of complement. Mol Immunol. 2011;49(1–2):281–9. doi: 10.1016/j.molimm.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehrer RI, et al. Multivalent binding of carbohydrates by the human alpha-defensin, HD5. J Immunol. 2009;183(1):480–90. doi: 10.4049/jimmunol.0900244. [DOI] [PubMed] [Google Scholar]
- 17.Varki A, Gagneux P. Human-specific evolution of sialic acid targets: explaining the malignant malaria mystery? Proc Natl Acad Sci U S A. 2009;106(35):14739–40. doi: 10.1073/pnas.0908196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen HY, et al. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc Natl Acad Sci U S A. 2009;106(34):14496–501. doi: 10.1073/pnas.0903497106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillai S, et al. Siglecs and immune regulation. Annu Rev Immunol. 2012;30:357–92. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geijtenbeek TB, et al. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 21.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 22.Carlin AF, et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113(14):3333–6. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatua B, et al. Sialic acids acquired by Pseudomonas aeruginosa are involved in reduced complement deposition and siglec mediated host-cell recognition. FEBS Lett. 2010;584(3):555–61. doi: 10.1016/j.febslet.2009.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–54. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- 25.Aminoff D, et al. Role of sialic acid in survival of erythrocytes in the circulation: interaction of neuraminidase-treated and untreated erythrocytes with spleen and liver at the cellular level. Proc Natl Acad Sci U S A. 1977;74(4):1521–4. doi: 10.1073/pnas.74.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Earl LA, Bi S, Baum LG. N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J Biol Chem. 2010;285(4):2232–44. doi: 10.1074/jbc.M109.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toscano MA, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8(8):825–34. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 28.Basset C, et al. Glycosylation of immunoglobulin A influences its receptor binding. Scand J Immunol. 1999;50(6):572–9. doi: 10.1046/j.1365-3083.1999.00628.x. [DOI] [PubMed] [Google Scholar]
- 29.Stockert RJ. The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev. 1995;75(3):591–609. doi: 10.1152/physrev.1995.75.3.591. [DOI] [PubMed] [Google Scholar]
- 30.Hiki Y, et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59(3):1077–85. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 31.Kokubo T, et al. Protective role of IgA1 glycans against IgA1 self-aggregation and adhesion to extracellular matrix proteins. J Am Soc Nephrol. 1998;9(11):2048–54. doi: 10.1681/ASN.V9112048. [DOI] [PubMed] [Google Scholar]
- 32.Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I- related receptor FcRn. Annu Rev Immunol. 2000;18:739–66. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 33.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 34.Sallusto F, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 35.Fuhlbrigge RC, et al. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389(6654):978–81. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- 36.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79(1):181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 37.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5(10):981–6. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 38.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24(1):19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–33. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 40.Rosales CU-QE. Fc receptors: cell activators of antibody functions. Advances in Bioscience and Biotechnology. 2013;4:21–33. [Google Scholar]
- 41.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 42.Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 43.Kocher M, et al. Cross-linking of Fc gamma receptor IIa and Fc gamma receptor IIIb induces different proadhesive phenotypes on human neutrophils. J Immunol. 1997;159(8):3940–8. [PubMed] [Google Scholar]
- 44.Rivas-Fuentes S, et al. Fcgamma receptors exhibit different phagocytosis potential in human neutrophils. Cell Immunol. 2010;263(1):114–21. doi: 10.1016/j.cellimm.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Garcia-Garcia E, et al. FcgammaRIIA and FcgammaRIIIB mediate nuclear factor activation through separate signaling pathways in human neutrophils. J Immunol. 2009;182(8):4547–56. doi: 10.4049/jimmunol.0801468. [DOI] [PubMed] [Google Scholar]
- 46.Ortiz-Stern A, Rosales C. Fc gammaRIIIB stimulation promotes beta1 integrin activation in human neutrophils. J Leukoc Biol. 2005;77(5):787–99. doi: 10.1189/jlb.0504310. [DOI] [PubMed] [Google Scholar]
- 47.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13(2):277–85. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 48.De Graaf TW, et al. Inflammation-induced expression of sialyl Lewis X-containing glycan structures on alpha 1-acid glycoprotein (orosomucoid) in human sera. J Exp Med. 1993;177(3):657–66. doi: 10.1084/jem.177.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azuma Y, Murata M, Matsumoto K. Alteration of sugar chains on alpha(1)-acid glycoprotein secreted following cytokine stimulation of HuH-7 cells in vitro. Clin Chim Acta. 2000;294(1–2):93–103. doi: 10.1016/s0009-8981(99)00248-x. [DOI] [PubMed] [Google Scholar]
- 50.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 51.Ackerman ME, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123(5):2183–92. doi: 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson S, et al. The glycosylation of haptoglobin in rheumatoid arthritis. Clin Chim Acta. 1993;220(1):107–14. doi: 10.1016/0009-8981(93)90011-r. [DOI] [PubMed] [Google Scholar]
- 53.Goodarzi MT, Turner GA. Reproducible and sensitive determination of charged oligosaccharides from haptoglobin by PNGase F digestion and HPAEC/PAD analysis: glycan composition varies with disease. Glycoconj J. 1998;15(5):469–75. doi: 10.1023/a:1006930902625. [DOI] [PubMed] [Google Scholar]
- 54.Parekh RB, et al. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1988;1(8592):966–9. doi: 10.1016/s0140-6736(88)91781-3. [DOI] [PubMed] [Google Scholar]
- 55.Moore JS, et al. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS. 2005;19(4):381–9. doi: 10.1097/01.aids.0000161767.21405.68. [DOI] [PubMed] [Google Scholar]
- 56.Holland M, et al. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim Biophys Acta. 2006;1760(4):669–77. doi: 10.1016/j.bbagen.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 57.Homma H, et al. Abnormal glycosylation of serum IgG in patients with IgA nephropathy. Clin Exp Nephrol. 2006;10(3):180–5. doi: 10.1007/s10157-006-0422-y. [DOI] [PubMed] [Google Scholar]
- 58.Saldova R, et al. Ovarian cancer is associated with changes in glycosylation in both acute-phase proteins and IgG. Glycobiology. 2007;17(12):1344–56. doi: 10.1093/glycob/cwm100. [DOI] [PubMed] [Google Scholar]
- 59.Selman MH, et al. IgG fc N-glycosylation changes in Lambert-Eaton myasthenic syndrome and myasthenia gravis. J Proteome Res. 2011;10(1):143–52. doi: 10.1021/pr1004373. [DOI] [PubMed] [Google Scholar]
- 60.Kodar K, et al. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconj J. 2012;29(1):57–66. doi: 10.1007/s10719-011-9364-z. [DOI] [PubMed] [Google Scholar]
- 61.Selman MH, et al. Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol Cell Proteomics. 2012;11(4):M111014563. doi: 10.1074/mcp.M111.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruhaak LR, et al. Enrichment strategies in glycomics-based lung cancer biomarker development. Proteomics Clin Appl. 2013 doi: 10.1002/prca.201200131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parekh R, et al. A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J Autoimmun. 1989;2(2):101–14. doi: 10.1016/0896-8411(89)90148-0. [DOI] [PubMed] [Google Scholar]
- 64.Bond A, et al. A detailed lectin analysis of IgG glycosylation, demonstrating disease specific changes in terminal galactose and N-acetylglucosamine. J Autoimmun. 1997;10(1):77–85. doi: 10.1006/jaut.1996.0104. [DOI] [PubMed] [Google Scholar]
- 65.Mimura Y, et al. Role of oligosaccharide residues of IgG1-Fc in Fc gamma RIIb binding. J Biol Chem. 2001;276(49):45539–47. doi: 10.1074/jbc.M107478200. [DOI] [PubMed] [Google Scholar]
- 66.Lund J, et al. Multiple interactions of IgG with its core oligosaccharide can modulate recognition by complement and human Fc gamma receptor I and influence the synthesis of its oligosaccharide chains. J Immunol. 1996;157(11):4963–9. [PubMed] [Google Scholar]
- 67.Okazaki A, et al. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J Mol Biol. 2004;336(5):1239–49. doi: 10.1016/j.jmb.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Ferrara C, et al. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem. 2006;281(8):5032–6. doi: 10.1074/jbc.M510171200. [DOI] [PubMed] [Google Scholar]
- 69.Rook GA, et al. Changes in IgG glycoform levels are associated with remission of arthritis during pregnancy. J Autoimmun. 1991;4(5):779–94. doi: 10.1016/0896-8411(91)90173-a. [DOI] [PubMed] [Google Scholar]
- 70.Axford JS, et al. Reduced B-cell galactosyltransferase activity in rheumatoid arthritis. Lancet. 1987;2(8574):1486–8. doi: 10.1016/s0140-6736(87)92621-3. [DOI] [PubMed] [Google Scholar]
- 71.Axford JS, et al. Changes in normal glycosylation mechanisms in autoimmune rheumatic disease. J Clin Invest. 1992;89(3):1021–31. doi: 10.1172/JCI115643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malhotra R, et al. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1995;1(3):237–43. doi: 10.1038/nm0395-237. [DOI] [PubMed] [Google Scholar]
- 73.Garred P, et al. Two edged role of mannose binding lectin in rheumatoid arthritis: a cross sectional study. J Rheumatol. 2000;27(1):26–34. [PubMed] [Google Scholar]
- 74.Banda NK, et al. Initiation of the alternative pathway of murine complement by immune complexes is dependent on N-glycans in IgG antibodies. Arthritis Rheum. 2008;58(10):3081–9. doi: 10.1002/art.23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Parekh R, et al. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J Exp Med. 1988;167(5):1731–6. doi: 10.1084/jem.167.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670–3. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 77.Anthony RM, et al. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008;105(50):19571–8. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fehr J, Hofmann V, Kappeler U. Transient reversal of thrombocytopenia in idiopathic thrombocytopenic purpura by high-dose intravenous gamma globulin. N Engl J Med. 1982;306(21):1254–8. doi: 10.1056/NEJM198205273062102. [DOI] [PubMed] [Google Scholar]
- 79.Imbach P, et al. High-dose intravenous gammaglobulin therapy of refractory, in particular idiopathic thrombocytopenia in childhood. Helv Paediatr Acta. 1981;36(1):81–6. [PubMed] [Google Scholar]
- 80.Bruhns P, et al. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity. 2003;18(4):573–81. doi: 10.1016/s1074-7613(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 81.Tackenberg B, et al. Impaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc Natl Acad Sci U S A. 2009;106(12):4788–92. doi: 10.1073/pnas.0807319106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hansen RJ, Balthasar JP. Effects of intravenous immunoglobulin on platelet count and antiplatelet antibody disposition in a rat model of immune thrombocytopenia. Blood. 2002;100(6):2087–93. [PubMed] [Google Scholar]
- 83.Watier H. Variability factors in the clinical response to recombinant antibodies and IgG Fccontaining fusion proteins. Expert Opin Biol Ther. 2005;5(Suppl 1):S29–36. doi: 10.1517/14712598.5.1.s29. [DOI] [PubMed] [Google Scholar]
- 84.Marcus R, Hagenbeek A. The therapeutic use of rituximab in non-Hodgkin’s lymphoma. Eur J Haematol Suppl. 2007;(67):5–14. doi: 10.1111/j.1600-0609.2006.00789.x. [DOI] [PubMed] [Google Scholar]
- 85.Lifely MR, et al. Glycosylation and biological activity of CAMPATH-1H expressed in different cell lines and grown under different culture conditions. Glycobiology. 1995;5(8):813–22. doi: 10.1093/glycob/5.8.813. [DOI] [PubMed] [Google Scholar]
- 86.Umana P, et al. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17(2):176–80. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]
- 87.Davies J, et al. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: Expression of antibodies with altered glycoforms leads to an increase in ADCC through higher affinity for FC gamma RIII. Biotechnol Bioeng. 2001;74(4):288–94. [PubMed] [Google Scholar]
- 88.Niwa R, et al. IgG subclass-independent improvement of antibody-dependent cellular cytotoxicity by fucose removal from Asn297-linked oligosaccharides. J Immunol Methods. 2005;306(1–2):151–60. doi: 10.1016/j.jim.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 89.Imai-Nishiya H, et al. Double knockdown of alpha1,6-fucosyltransferase (FUT8) and GDP-mannose 4,6-dehydratase (GMD) in antibody-producing cells: a new strategy for generating fully non-fucosylated therapeutic antibodies with enhanced ADCC. BMC Biotechnol. 2007;7:84. doi: 10.1186/1472-6750-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamane-Ohnuki N, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87(5):614–22. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- 91.An HJ, et al. Glycomics analyses of tear fluid for the diagnostic detection of ocular rosacea. J Proteome Res. 2005;4(6):1981–7. doi: 10.1021/pr0501620. [DOI] [PubMed] [Google Scholar]
- 92.An HJ, et al. Profiling of glycans in serum for the discovery of potential biomarkers for ovarian cancer. J Proteome Res. 2006;5(7):1626–35. doi: 10.1021/pr060010k. [DOI] [PubMed] [Google Scholar]
- 93.An HJ, et al. Glycomics and disease markers. Curr Opin Chem Biol. 2009;13(5–6):601–7. doi: 10.1016/j.cbpa.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kronewitter SR, et al. Human serum processing and analysis methods for rapid and reproducible N-glycan mass profiling. J Proteome Res. 2010;9(10):4952–9. doi: 10.1021/pr100202a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kronewitter SR, et al. The glycolyzer: automated glycan annotation software for high performance mass spectrometry and its application to ovarian cancer glycan biomarker discovery. Proteomics. 2012;12(15–16):2523–38. doi: 10.1002/pmic.201100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hong Q, et al. Absolute quantitation of immunoglobulin G and its glycoforms using multiple reaction monitoring. Anal Chem. 2013;85(18):8585–93. doi: 10.1021/ac4009995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li AC, et al. Simultaneously quantifying parent drugs and screening for metabolites in plasma pharmacokinetic samples using selected reaction monitoring information-dependent acquisition on a QTrap instrument. Rapid Commun Mass Spectrom. 2005;19(14):1943–50. doi: 10.1002/rcm.2008. [DOI] [PubMed] [Google Scholar]
- 98.Xiao JF, Zhou B, Ressom HW. Metabolite identification and quantitation in LC-MS/MS-based metabolomics. Trends Analyt Chem. 2012;32:1–14. doi: 10.1016/j.trac.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kitteringham NR, et al. Multiple reaction monitoring for quantitative biomarker analysis in proteomics and metabolomics. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(13):1229–39. doi: 10.1016/j.jchromb.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 100.Gallien S, Duriez E, Domon B. Selected reaction monitoring applied to proteomics. J Mass Spectrom. 2011;46(3):298–312. doi: 10.1002/jms.1895. [DOI] [PubMed] [Google Scholar]
- 101.Querol L, et al. Neurofascin IgG4 antibodies in CIDP associate with disabling tremor and poor response to IVIg. Neurology. 2014;82(10):879–86. doi: 10.1212/WNL.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang J, et al. A classification of Hashimoto’s thyroiditis based on immunohistochemistry for IgG4 and IgG. Thyroid. 2014;24(2):364–70. doi: 10.1089/thy.2013.0211. [DOI] [PubMed] [Google Scholar]
- 103.Dhandha MM, Seiffert-Sinha K, Sinha AA. Specific immunoglobulin isotypes correlate with disease activity, morphology, duration and HLA association in Pemphigus vulgaris. Autoimmunity. 2012;45(7):516–26. doi: 10.3109/08916934.2012.702811. [DOI] [PubMed] [Google Scholar]
- 104.Jaskowski TD, et al. Relationship between rheumatoid factor isotypes and IgG anti-cyclic citrullinated peptide antibodies. J Rheumatol. 2010;37(8):1582–8. doi: 10.3899/jrheum.091236. [DOI] [PubMed] [Google Scholar]
- 105.Liu H, et al. Expression of IgA class switching gene in tonsillar mononuclear cells in patients with IgA nephropathy. Inflamm Res. 2011;60(9):869–78. doi: 10.1007/s00011-011-0347-0. [DOI] [PubMed] [Google Scholar]
- 106.de Haan S, et al. Epitopes, immunoglobulin classes and immunoglobulin G subclasses of calsequestrin antibodies in patients with thyroid eye disease. Autoimmunity. 2010;43(8):698–703. doi: 10.3109/08916931003774954. [DOI] [PubMed] [Google Scholar]
- 107.Santoh T, et al. Ratio of serum IgG3 to total IgG concentration and goiter size are independent factors in intractability of Graves’ disease. Endocr J. 2007;54(6):887–94. doi: 10.1507/endocrj.k07-005. [DOI] [PubMed] [Google Scholar]
- 108.Lleo A, et al. Immunoglobulin M levels inversely correlate with CD40 ligand promoter methylation in patients with primary biliary cirrhosis. Hepatology. 2012;55(1):153–60. doi: 10.1002/hep.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rigopoulou EI, et al. Prevalence and clinical significance of isotype specific antinuclear antibodies in primary biliary cirrhosis. Gut. 2005;54(4):528–32. doi: 10.1136/gut.2003.036558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scherer HU, et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62(6):1620–9. doi: 10.1002/art.27414. [DOI] [PubMed] [Google Scholar]
- 111.Matsumoto A, et al. Autoantibody activity of IgG rheumatoid factor increases with decreasing levels of galactosylation and sialylation. J Biochem. 2000;128(4):621–8. doi: 10.1093/oxfordjournals.jbchem.a022794. [DOI] [PubMed] [Google Scholar]
- 112.Novak J, et al. IgA nephropathy and Henoch-Schoenlein purpura nephritis: aberrant glycosylation of IgA1, formation of IgA1-containing immune complexes, and activation of mesangial cells. Contrib Nephrol. 2007;157:134–8. doi: 10.1159/000102455. [DOI] [PubMed] [Google Scholar]
- 113.Mestecky J, et al. Defective galactosylation and clearance of IgA1 molecules as a possible etiopathogenic factor in IgA nephropathy. Contrib Nephrol. 1993;104:172–82. doi: 10.1159/000422410. [DOI] [PubMed] [Google Scholar]
- 114.Mestecky J, et al. Role of aberrant glycosylation of IgA1 molecules in the pathogenesis of IgA nephropathy. Kidney Blood Press Res. 2008;31(1):29–37. doi: 10.1159/000112922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol. 1995;100(3):470–4. doi: 10.1111/j.1365-2249.1995.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holland M, et al. Hypogalactosylation of serum IgG in patients with ANCA-associated systemic vasculitis. Clin Exp Immunol. 2002;129(1):183–90. doi: 10.1046/j.1365-2249.2002.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Espy C, et al. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s) Arthritis Rheum. 2011;63(7):2105–15. doi: 10.1002/art.30362. [DOI] [PubMed] [Google Scholar]
- 118.Youinou P, et al. Galactose terminating oligosaccharides of IgG in patients with primary Sjogren’s syndrome. J Autoimmun. 1992;5(3):393–400. doi: 10.1016/0896-8411(92)90151-f. [DOI] [PubMed] [Google Scholar]