Abstract
Nearly complete sequences of simian immunodeficiency viruses (SIVs) infecting 18 different nonhuman primate species in sub-Saharan Africa have now been reported; yet, our understanding of the origins, evolutionary history, and geographic distribution of these viruses still remains fragmentary. Here, we report the molecular characterization of a lentivirus (SIVdeb) naturally infecting De Brazza's monkeys (Cercopithecus neglectus). Complete SIVdeb genomes (9,158 and 9,227 bp in length) were amplified from uncultured blood mononuclear cell DNA of two wild-caught De Brazza's monkeys from Cameroon. In addition, partial pol sequences (650 bp) were amplified from four offspring of De Brazza's monkeys originally caught in the wild in Uganda. Full-length (9,068 bp) and partial pol (650 bp) SIVsyk sequences were also amplified from Sykes's monkeys (Cercopithecus albogularis) from Kenya. Analysis of these sequences identified a new SIV clade (SIVdeb), which differed from previously characterized SIVs at 40 to 50% of sites in Pol protein sequences. The viruses most closely related to SIVdeb were SIVsyk and members of the SIVgsn/SIVmus/SIVmon group of viruses infecting greater spot-nosed monkeys (Cercopithecus nictitans), mustached monkeys (Cercopithecus cephus), and mona monkeys (Cercopithecus mona), respectively. In phylogenetic trees of concatenated protein sequences, SIVdeb, SIVsyk, and SIVgsn/SIVmus/SIVmon clustered together, and this relationship was highly significant in all major coding regions. Members of this virus group also shared the same number of cysteine residues in their extracellular envelope glycoprotein and a high-affinity AIP1 binding site (YPD/SL) in their p6 Gag protein, as well as a unique transactivation response element in their viral long terminal repeat; however, SIVdeb and SIVsyk, unlike SIVgsn, SIVmon, and SIVmus, did not encode a vpu gene. These data indicate that De Brazza's monkeys are naturally infected with SIVdeb, that this infection is prevalent in different areas of the species' habitat, and that geographically diverse SIVdeb strains cluster in a single virus group. The consistent clustering of SIVdeb with SIVsyk and the SIVmon/SIVmus/SIVgsn group also suggests that these viruses have evolved from a common ancestor that likely infected a Cercopithecus host in the distant past. The vpu gene appears to have been acquired by a subset of these Cercopithecus viruses after the divergence of SIVdeb and SIVsyk.
Simian immunodeficiency viruses (SIVs) are primate lentiviruses that infect a wide variety of nonhuman primate species in sub-Saharan Africa (2, 59). Two of these, SIVcpz from chimpanzees (Pan troglodytes) and SIVsmm from sooty mangabeys (Cercocebus atys), have been transmitted to humans on multiple occasions and generated human immunodeficiency virus type 1 (HIV-1) and HIV-2 (26, 27, 31). Although the conditions and circumstances of these transfers remain unknown, human exposure to primate blood resulting from bushmeat hunting represents the most plausible source of infection. Indeed, a recent survey of bushmeat markets in Cameroon provided compelling evidence that humans engaged in the hunting and handling of primates are routinely exposed to genetically highly divergent SIVs from a multitude of different species (60). This has raised the concern that other viruses, in addition to those from chimpanzees and sooty mangabeys, could be transmitted to humans, causing yet other disease outbreaks.
To date, SIV cross-reactive antibodies in members of 36 different primate species have been identified, and SIV infection has been confirmed by sequence analysis in 29 of these (Table 1 and references therein). Phylogenetic analysis of these sequences has shown that all SIVs cluster as a single group in the evolutionary trees of mammalian lentiviruses, indicating that they are derived from a single ancestor (2). This ancestor had its origin in sub-Saharan Africa, since only Old World monkey and ape species native to sub-Saharan Africa are naturally infected with SIV. Although it remains unknown when and how primate lentiviruses first emerged, the current SIVs, which are nonpathogenic for their natural hosts, are likely to have evolved over an extended period of time.
TABLE 1.
SIV infections of Old World monkeys (Cercopithecidae) and ape (Hominidae) species in sub-Saharan Africa
| Genusa | Group | Species | Common name | SIV-HIV cross-reactive antibodiesb | SIV strain designationc | Available sequencesf | Widespread infection | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| Allenopithecus | A. nigroviridis | Allen's swamp monkey | + | NA | ? | 49 | ||
| Miopithecus | M. talapoin | Angolan Talapoin | + | SIVtal | SS-P | ? | 58 | |
| M. ogouensis | Gabon Talapoin | + | SIVtal | MS-P, SL | ? | 60 | ||
| Erythrocebus | E. patas | Patas monkey | [+]d | [SIVsab]d | No | 10 | ||
| Chlorocebus | C. sabaeus | Green monkey | + | SIVsab | SS-FL + MS-P, ML | Yes | 1, 9, 40, 55 | |
| C. aethiops | Grivet | + | SIVgri | SS-FL + MS-P, ML | Yes | 22, 40, 42 | ||
| C. tantalus | Tantalus monkey | + | SIVtan | SS-FL + MS-P, ML | Yes | 34, 40, 55, 69 | ||
| C. pygerythrus | Vervet monkey | + | SIVver | MS-FL + MS-P, ML | Yes | 24, 40, 41, 42, 78 | ||
| Cercopithecus | Dryas | C. dryas | Dryas monkey | NT | ||||
| Neglectus | C. neglectus | De Brazza's monkey | + | SIVdeb | MS-P, SL | ? | 49, 60 | |
| Diana | C. diana | Diana monkey | + | NA | ? | 49 | ||
| C. roloway | Roloway monkey | NT | ||||||
| Lhoesti | C. lhoesti | L'Hoest's monkey | + | SIVlho | MS-FL + MS-P, ML | Yes | 5, 32, 66 | |
| C. preussi | Preuss's monkey | −e | 60 | |||||
| C. solatus | Sun-tailed monkey | + | SIVsun | SS-FL | ? | 6 | ||
| Cephus | C. petaurista | Lesser Spot-nosed monkey | NT | |||||
| C. erythrogaster | White throated guenon | NT | ||||||
| C. sclateri | Sclater's guenon | NT | ||||||
| C. erythrotis | Red-eared guenon | + | SIVery | SS-P | ? | —g | ||
| C. cephus | Mustached guenon | + | SIVmus | SS-FL + MS-P, SL | ? | 16, 60 | ||
| C. ascanius | Red-tailed monkey | + | SIVasc | SS-FL + SS-P, ML | ? | 79, —h | ||
| Mona | C. mona | Mona monkey | + | SIVmon | MS-FL + MS-P, ML | Yes | 4, 16, 60 | |
| C. campbelli | Campbell's mona | + | NA | ? | 6 | |||
| C. lowei | Lowe's mona | NT | ||||||
| C. pogonias | Crested mona | + | NA | ? | 60 | |||
| C. wolfi | Wolf's mona | NT | ||||||
| C. denti | Dent's mona | + | SIVden | SS-FL | ? | —h | ||
| Mitis | C. nictitans | Greater spot-nosed monkey | + | SIVgsn | MS-FL + MS-P, SL | ? | 19, 60 | |
| C. mitis | Blue monkey | + | SIVblu | SS-FL + MS-P, SL | ? | —i | ||
| C. doggetti | Silver monkey | NT | ||||||
| C. kandti | Golden monkey | NT | ||||||
| C. albogularis | Sykes's monkey | + | SIVsyk | SS-FL | ? | 21, 33 | ||
| Hamlyni | C. hamlyni | Hamlyn's monkey | + | NA | ? | 56 | ||
| Lophocebus | L. albigena | Gray-cheeked mangabey | + | NA | ? | 60 | ||
| L. aterrimus | Black crested mangabey | NT | ||||||
| L. opdenboschi | Opdenbosch's mangabey | NT | ||||||
| Papio | P. hamadryas | Hamadryas baboon | NT | |||||
| P. papio | Guinea baboon | NT | ||||||
| P. anubis | Olive baboon | + | NA | ? | 60 | |||
| P. cynocephalus | Yellow baboon | [+]d | [SIVver]d | No | 41, 44 | |||
| P. ursinus | Chacma baboon | [+]d | [SIVver]d | No | 78 | |||
| Theropithecus | T. gelada | Gelada | NT | |||||
| Cercocebus | C. atys | Sooty mangabey | + | SIVsmm | MS-FL + MS-P, ML | Yes | 12, 13, 35, 48, 51, 61, 63, —j | |
| C. torquatus | Red-capped mangabey | + | SIVrcm | MS-FL + MS-P, ML | Yes | 3, 7 | ||
| C. agilis | Agile mangabey | + | SIVagi | SS-P | ? | —k | ||
| C. chrysogaster | Golden-bellied mangabey | NT | ||||||
| C. galeritus | Tana river mangabey | NT | ||||||
| C. sanjei | Sanje mangabey | NT | ||||||
| Mandrillus | M. sphinx | Mandrill | + | SIVmnd-1 | SS-FL + MS-P, ML | Yes | 70, 75 | |
| SIVmnd-2 | MS-FL + MS-P, ML | Yes | 37, 60, 70, 72 | |||||
| M. leucophaeus | Drill | + | SIVdrl | SS-FL + MS-P, ML | Yes | 14, 37 | ||
| Colobus | C. satanas | Black colobus | NT | |||||
| C. angolensis | Angola colobus | NT | ||||||
| C. polykomos | King colobus | NT | ||||||
| C. veflerosus | Ursine colobus | NT | ||||||
| C. guereza | Mantled guereza | + | SIVcol | SS-FL + MS-P, SL | ? | 18, 60 | ||
| Piliocolobus | P. badius | Western red colobus | + | SIVwrc | MS-P, SL | ? | 17 | |
| P. pennantii | Pennant's colobus | NT | ||||||
| P. preussi | Preuss's red colobus | NT | ||||||
| P. tholloni | Thollon's red colobus | NT | ||||||
| P. foai | Central African red colobus | NT | ||||||
| P. tephrosceles | Ugandan red colobus | NT | ||||||
| P. gordonorum | Uzungwa red colobus | NT | ||||||
| P. kirkii | Zanzibar red colobus | NT | ||||||
| P. rufomitratus | Tana river red colobus | NT | ||||||
| Procolobus | P. verus | Olive colobus | + | SIVolc | SS-P | ? | 17 | |
| Pan | P. troglodytes | Common chimpanzee | + | SIVcpz | MS-FL + MP-P, ML | Yes | 15, 26, 39, 65, 67, 76 | |
| P. paniscus | Bonobo | −e | 77 | |||||
| Gorilla | G. gorilla | Western gorilla | −e | 49, 57 | ||||
| G. beringei | Eastern gorilla | NT |
The classification of African primate species in this table is derived from Colin Groves's Primate Taxonomy (30). Colin Groves lists two other Chlorocebus species with very restricted distribution: C. djamdjamensis (Bale Mountains Vervet) and C. cynosorus (Malbrouck).
+, positive by ELISA and/or Western blot analysis; −, negative by ELISA and/or Western blot analysis; NT, not tested.
SIV strain designations use a three-letter code that indicates their species of origin, in accordance with SIV nomenclature rules implemented by the Los Alamos HIV/SIV Sequence Database (SIVlho, SIVsmm, SIVasc, SIVver, SIVgri, SIVtan, and SIVsab have previously been designated SIVlhoest, SIVsm, SIVschm, SIVagmVer, SIVagmGri, SIVagmTan, and SIVagmSab, respectively).
Infections resulting from cross-species transmissions of SIVs infecting local African green monkey species.
Only a few animals were tested.
SS, single strain; MS, multiple strains, FL, full-length sequence; P, partial sequence, SL, same locale; ML, multiple locales.
P. Reed, S. Souquiere, M. Makuwa, P. Telfer, M. A. Ela-Mba, P. Roques, and P. A. Marx, Abstr. 2nd IAS Conf. HIV Pathog. Treatment, abstr. 224, 2003.
S. Saragosti, M. Ekwalanga, M. C. Dazza, M. Mende, P. Bitchou, and K. Bin Shamara, Abstr. 2nd IAS Conf. HIV Pathog. Treatment, abstr. 285, 2003.
F. Bibollet-Ruche, V. Courgnaud, X. Pourrut, E. N. Mpoudi, J. Mwenda, V. M. Hirsch, E. Delaporte, M. Peeters, F. Gao, G. M. Shaw, and B. H. Hahn, Abstr. 7th Conf. Retrovir. Opportun. Infect., abstr. 223, 2000.
B. Ling, P. Telfer, P. Reed, C. Apetrei, and P. A. Marx, Abstr. 2nd IAS Conf. HIV Pathog. Treatment, abstr. 286, 2003.
E. Nerrienet, C. Apetrei, Y. Foupouapouognigni, B. Ling, A. Luckay, L. Chakrabarti, A. Ayouba, and P. Marx, Abstr. 14th Int. AIDS Conf., abstr. TuPe 4405, 2002.
The known SIVs are highly divergent, and this diversity is generally species specific; that is, viruses from the same species cluster together in phylogenetic trees. This has been used to classify the various SIVs by adding a three-letter code indicating their species of origin (e.g., SIVlho from L'Hoest's monkey). In addition, several major SIV lineages representing groups of SIVs from different primate species that are more closely related to one another than they are to other SIVs have been identified. Some of these closely related viruses have been isolated from closely related host species, suggesting host-virus cospeciation (2); examples include the SIVs infecting Chlorocebus species (1, 40) and those from Cercopithecus lhoesti and Cercopithecus solatus (6). However, there are also numerous examples of cross-species transmission and recombination, indicating that many SIVs have spread among different primate species (2, 40, 70, 72). A case in point is SIVcpz, which is a hybrid virus that was acquired by chimpanzees through cross-species transmission of SIVs infecting monkeys on which chimpanzees prey (3). These data indicate that the evolutionary history of primate lentiviruses is complex and likely involved a series of consecutive interspecies transmissions, the timelines and directions of which remain to be deciphered.
Although many strains of SIV have been characterized, the current compilation of naturally occurring infections is far from complete: only 39 of the 69 recognized Old World monkey and ape species in sub-Saharan Africa have thus far been tested for SIV infection (Table 1). Since more than 90% of these species were antibody positive, many of the remaining 30 species can be expected to harbor additional SIV infections. Moreover, for many species only very few animals have been screened, and SIV infections may have gone undetected. For example, whereas one form of SIV infecting mandrills (SIVmnd-1) was discovered 15 years ago (75), it was not until more than 10 years later that screening of larger numbers of animals in different parts of the species' habitat (70, 72) revealed a second, highly divergent type (SIVmnd-2). Very limited sampling is likely also the reason why only two of the three species within the Cercopithecus lhoesti group, L'Hoest's monkeys and sun-tailed monkeys, have each been found to harbor their own form of SIV (Table 1), whereas the third species, Preuss's monkeys, has so far been negative for SIV infection (60). For three other species, i.e., patas monkeys (10), olive baboons (41), and chacma baboons (78), surveys have thus far revealed instances of cross-species transmission of SIV only from the local species of African green monkey (Table 1). The apparent absence of patas monkey- or baboon-specific SIVs suggests that these species are free of infection; however, screening has been too limited to allow definitive conclusions. Overall, only 12 of 36 species known or suspected to harbor SIV have been characterized in detail (Table 1). This has precluded a comprehensive analysis of the spectrum of the SIV diversity that exists naturally in African primates living in the wild.
Old Word monkeys (Cercopithecidae) are divided into two subfamilies, the Cercopithecinae and the Colobinae (30). The Cercopithecinae are further subdivided into two tribes: the Papionini, which include the genera Lophocebus, Papio, Theropithecus, Cercocebus, and Mandrillus; and the Cercopithecini, which include the genera Allenopithecus, Miopithecus, Erythrocebus, Chlorocebus, and Cercopithecus (Table 1). Among the Cercopithecini, the Cercopithecus genus, whose members are also termed guenons (30), comprises the largest number of species known to harbor SIV (Table 1). Of 16 species tested, 15 have been reported to harbor HIV cross-reactive antibodies and at least partial viral sequences have been derived from 11 of these (Table 1). The observation that SIVs are so widespread among members of the Cercopithecus genus has raised the possibility that these infections are ancient and, further, that these viruses may have been cospeciating with their hosts (2, 6, 59). However, phylogenetic analyses of available SIV sequences have yielded equivocal results. Three of the Cercopithecus monkey viruses, which include SIVgsn from greater spot-nosed monkeys, SIVmon from mona monkeys, and SIVmus from mustached monkeys, are closely related to one another (4, 16, 19). However, SIVsyk from Sykes' monkeys seems quite divergent from the SIVgsn/SIVmon/SIVmus clade, even though Sykes's and greater spot-nosed monkeys are both classified within the Cercopithecus mitis group. Moreover, both SIVlho from L'Hoest's and SIVsun from sun-tailed monkeys cluster with SIVmnd-1 from mandrills rather than with other Cercopithecus monkey viruses (5, 6, 32, 33). Thus, current phylogenetic data point to cross-species transmission rather than virus-host coevolution as an explanation for the phylogenetic relationships of SIVs infecting members of the Cercopithecus genus.
To gain further insights into the evolutionary history of SIVs from Cercopithecus monkeys, we characterized SIV strains naturally infecting De Brazza's monkeys (C. neglectus). This guenon has a wide distribution across equatorial Africa, ranging from southern Cameroon and the Central African Republic to the Congo River in the eastern Democratic Republic of Congo (Fig. 1), but it is described as monotypic (i.e., it is not divided into subspecies) (30). Full-length SIVdeb sequences were derived from two wild-caught De Brazza's monkeys identified as SIV infected during a bushmeat survey in Cameroon (60). In addition, partial sequences were amplified from four captive-born seropositive De Brazza's monkeys housed at safari parks in the United Kingdom. Since these were the progeny of De Brazza's monkeys originally imported from Uganda, they represent animals from the easternmost part of the De Brazza's monkey range. Finally, to ensure that the single, currently available SIVsyk sequence is indeed representative of viruses infecting wild Sykes's monkeys, we molecularly characterized additional SIVsyk strains from Kenya. Our results reveal a new primate lentivirus infecting De Brazza's monkeys and provide evidence for a Cercopithecus genus-specific clade of SIVs.
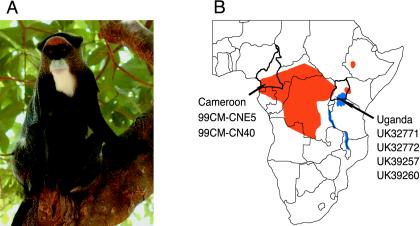
FIG. 1.
Geographical distribution of De Brazza's monkeys (Cercopithecus neglectus) in sub-Saharan Africa. (A) Female De Brazza's monkey from Cameroon. (B) Geographic origin of the De Brazza's monkeys analyzed in this study. Animals 99CM-CNE5 and 99CM-CN40 were wild caught in Cameroon (60). UK32771, UK32772, UK3925, and UK39260 were captive born in safari parks in the United Kingdom, but are progeny of De Brazza's monkeys originally imported from Uganda. Cameroon and Uganda are shown in relation to the species' natural range (orange) in sub-Saharan Africa (country borders are shown in black).
MATERIALS AND METHODS
Primate specimens.
Peripheral blood mononuclear cell (PBMC) DNA was available from two wild-caught De Brazza's monkeys (C. neglectus) previously reported to be SIV infected in a survey of primate bushmeat (99CM-CN40) and pet monkeys (99CM-CNE5) in Cameroon (60). In addition, plasma samples were available from four captive-born De Brazza's monkeys housed at safari parks in the United Kingdom (UK32771, UK32772, UK39257, UK39260), who were the progeny of animals originally imported from Uganda. PBMC DNA was also available from three SIV-infected Sykes's monkeys (Cercopithecus albogularis) from Kenya. KE51 was captive born and housed at the Institute for Primate Research in Nairobi, Kenya. The other two, KE13 and KE20, were trapped in the southern coastal region of Kenya and the vicinity of Nairobi, respectively, but released back into the wild after blood samples were taken. Finally, a spleen sample was available from a Sykes's monkey (Cm195) who had died at the Yerkes Primate Research Center of natural causes after many years in captivity. This animal had been imported from the Kenyan Institute for Primate Research at the same time as Cm173, the Sykes's monkey from which the full-length SIVsyk173 sequence is derived (21). All studies were carried out in strict accordance with international guidelines for the ethical and scientific use and humane care of primates in research (the Yerkes National Primate Research Center is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International).
Diagnostic PCR amplification.
DNA was extracted from PBMC and spleen by using the QIAamp blood kit (QIAGEN, Valencia, Calif.) and the QIAamp DNA Minikit, respectively. Viral RNA was extracted from plasma by using a modified guanidinium isothiocyanate-silica method and amplified as previously described (4, 11). Diagnostic PCR and reverse transcription (RT)-PCR amplifications were performed by using consensus primers designed according to regions of high SIV sequence conservation (primers DR1/polOR and polis4/UNI-2 [Table 2]). The resulting amplification products spanned the 3′ reverse transcriptase/integrase region of pol and were ∼650 bp in length.
TABLE 2.
Oligonucleotide primers used to amplify full-length genomes of SIVdeb and SIVsyk
| Virus strain and round of nested PCR | Fragmenta | Primer | Region | Size (kb) | Sequenceb | Reference |
|---|---|---|---|---|---|---|
| SIVdeb | ||||||
| 1 | DR1 | pol | 2.7 | TRCAYACAGGRGCWGAYGA | 14 | |
| polOR | ACBACYGCNCCTTCHCCTTTC | 18 | ||||
| 2 | A | polis4 | pol | 0.65 | CCAGCNCACAAAGGNATAGGAGG | 18 |
| UNI-2 | CCCCTATTCCTCCCCTTCTTTTAAAA | 53 | ||||
| 2 | B | DR1-YAC | pol | 0.7 | TRGAYACAGGRGCWGAYGAYAC | |
| DR5 | GGIGAYCCYTTCCAYCCYTGHGG | 14 | ||||
| 2 | C | deb-1F | pol | 2.2 | CAGTTACATCAACCTTGGACCCC | |
| deb-1R | CATATCCCCTATTCCTCCCCTTC | |||||
| 1 | deb-2F | circle | ∼8.5 | GTTACCTACCTTAATAGCCAAGCAG | ||
| deb-2R | CCTGGGTAGCCTTATTAAGGGCTCTC | |||||
| 2 | D | gag-F445 | gag/pol | ∼2.3 | CGGGCGCCCGAACAGGGACTTG | 33 |
| deb-3R | GCTATATGGGTTAGTGGGCTCTGTGG | |||||
| 2 | E | deb-3F | pol/env | ∼3.5 | CATGTAGCTAGTGGATTTATGGAGAC | |
| EnvR | CTNTCCCAYTCYTGCCANGTC | |||||
| 2 | F | deb-4F | env/gag | ∼2.5 | CAAGAGCTGTTGAAGGCTGTGGAGGC | |
| deb-4R | CACAAACGTCCCTAGTGGACCAGG | |||||
| SIVsyk | ||||||
| 1 | DR1 | pol | 2.7 | TRCAYACAGGRGCWGAYGA | 14 | |
| polOR | ACBACYGCNCCTTCHCCTTTC | 18 | ||||
| 2 | A | polis4 | pol | 0.65 | CCAGCNCACAAAGGNATAGGAGG | 18 |
| UNI-2 | CCCCTATTCCTCCCCTTCTTTTAAAA | 53 | ||||
| 2 | B | DR1-YAC | pol | 0.7 | TRGAYACAGGRGCWGAYGAYAC | |
| DR5 | GGIGAYCCYTTCCAYCCYTGHGG | 14 | ||||
| 2 | C | syk-1F | pol | 2.2 | GCAAAGGCATCACTGATGAAGTTTGG | |
| syk-1R | GACATCCAGTTACATCAACCTTGGAC | |||||
| 1 | syk-2F | circle | ∼8.5 | CCAAACTTCATCAGTGATGCCTTTGC | ||
| syk-2R | GTCCAAGGTTGATGTAACTGGATGTC | |||||
| 2 | D | gag-F445 | gag/pol | ∼2.3 | CGGGCGCCCGAACAGGGACTTG | 33 |
| syk-3R | GGTTGATGTAACTGGATGTCTTG | |||||
| 2 | E | syk-3F | pol/env | ∼3.3 | GGCAGGAGTCGAACACACCACAGG | |
| EnvR | CTNTCCCAYTCYTGCCANGTC | |||||
| 2 | F | syk-4F | env/gag | ∼2.5 | CAAGCTTTCTACCTGTTAGGATCGCC | |
| syk-4R | CCTACAAGTCAAAGGCGCTATGAGC |
See Fig. 2 for details on amplification strategy.
Y = C or T; W = A or T; R = A or G; H = A or C or T; B = C or G or T; N = A or C or T or G; I = inosine.
Amplification of complete SIVdeb and SIVsyk genomes.
Primers used to obtain full-length SIVdeb and SIVsyk sequences are listed in Table 2, and the corresponding amplification strategy is depicted in Fig. 2. All amplifications were performed by using the Expand High Fidelity PCR kit (Roche Applied Science, Indianapolis, Ind.) according to the manufacturer's instructions. Briefly, 500 ng of genomic DNA was used for first-round PCR amplifications (Table 2). For second-round PCR amplification, 1 μl from the first round was used. Each amplification reaction included a manual hot-start and 35 cycles. Annealing temperatures were 50°C except for the amplification of fragment B, for which 45°C was used. Extension times varied depending on the size of the expected fragment and were typically set at 1 min/kb. For the first-round amplification of unintegrated circular forms, a 9-min extension time was used for the first 10 cycles and extended for 5 s for each subsequent cycle. Amplified fragments were agarose gel purified, cloned by the TA cloning approach into pGEM-T-easy (Promega, Madison, Wis.), and sequenced by using a primer walking approach and the GPS-1 Genome Priming system (New England Biolab, Beverly, Mass.) using an automated sequencer (ABI3100; Applied Biosystems, Foster City, Calif.).
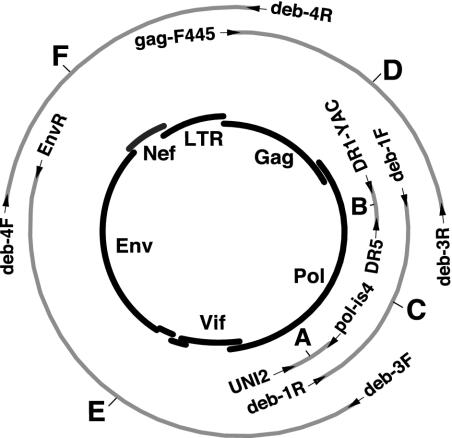
FIG. 2.
Amplification of full-length SIVdeb and SIVsyk sequences from uncultured monkey PBMC DNA. The positions of the various amplification products are shown in relation to an unintegrated (single-LTR) circular intermediate of SIV (depicted in the center) with major genomic regions indicated. Primer sets (indicated by arrows) and fragment designations (A to F) are identical to those in Table 2.
Diversity plots and phylogenetic analyses.
Nucleotide and protein sequences were aligned by using Clustal W (73), and minor adjustments were made by using Seaview (25). Sites that could not be unambiguously aligned and sites with a gap in any sequence were excluded. Proteome sequences were generated by joining deduced Gag, Pol, Vif, Env, and Nef amino acid sequences; the carboxy-terminal Gag, Pol, and Env amino acid sequences that overlapped with Pol, Vif, and Nef amino acid sequences, respectively, were excluded. Diversity plots were generated for windows of 300 residues moved by 20-residue increments as described below. Maximum likelihood trees were inferred by Bayesian estimation of phylogeny, based upon the posterior probability distribution of trees. The method was implemented in MrBayesv3.0 (38) by using the Jones Taylor and Thornton model of protein evolution (43) with gamma-distributed rates at sites (84). The program was run for 100,000 generations, including a “burn in” of 10,000 generations. The trees shown are majority rule consensus trees.
For phylogenetic analyses, sequences for the following virus strains were obtained from the GenBank database (GenBank accession numbers are given in parentheses): SIVgsnCM71 (AF468658), SIVgsnCM166 (AF468659), SIVgsnCM7 (AF478589), SIVmonCML1 (AY340701), SIVmonNG1 (AJ549283), SIVmusCMS1085 (AY340700), SIVmusCMS1239 (AF478592), SIVascSCHM1 (AJ551401), SIVdebCM1 (AF478605), SIVdebCMS1014 (AF478602), SIVdebCM1083 (AF478600), SIVdebCM1161 (AF478604), SIVsyk173 (L06042), HIV-2/D205 (X61240), SIVsm543 (U72748), SIVgri677 (M58410), SIVver155 (M29975), SIVtan1 (U58991), SIVrcmNG411 (AF349680), SIVrcmGB1 (AF382829), SIVcpzUS (AF103818), SIVcpzTAN1 (AF447763), SIVsunL14 (AF131870), SIVlho7 (AF075269), SIVmnd-1/GB1 (M27470), and SIVcolCGU1 (AF301156).
Secondary structure predictions.
The transactivation response (TAR) RNA secondary structure was predicted by using online mfold version 3.1 (http:www.bioinfo.rpi.edu/applications/mfold/) (52, 85), and secondary structures were drawn by using the LoopDLoop program (available at ftp://ftp.bio.indiana.edu/molbio/loopdloop/mac-app/).
Nucleotide sequence accession numbers.
The virus strains whose sequences were determined in this study and their GenBank accession numbers (in parentheses) are as follows: SIVdebUK39257 (AY523858), SIVdebUK32772 (AY523859), SIVdebUK32771 (AY523860), SIVdebUK39260 (AY523861), SIVsykKE20 (AY523862), SIVsykKE13 (AY523863), SIVsyk195 (AY523864), SIVdebCM40 (AY523865), SIVdebCM5 (AY523866), and SIVsykKE51 (AY523867).
RESULTS
Amplification and sequence analysis of two complete SIVdeb genomes.
The first evidence that DeBrazza's monkeys are naturally infected with SIV came from a study of primate bushmeat and pet monkeys in Cameroon (60). Serological analysis of blood samples from 34 wild-caught De Brazza's monkeys identified 10 samples harboring antibodies that were strongly cross-reactive with HIV-2 antigens, and to a lesser extent with HIV-1 antigens. Five sera (including 99CM-CNE5) reacted exclusively with the HIV-2 transmembrane envelope glycoprotein (gp36) by the INNO-LIA assay (Innogenetics, Ghent, Belgium), while the remaining five sera (including 99CM-CN40) exhibited a broader cross-reactivity that also included HIV-1 antigens (60). Subsequent PCR amplification of a 650-bp subgenomic pol fragment confirmed infection in six of these animals, and phylogenetic analysis of the corresponding sequences revealed a new group of viruses that was highly divergent from previously characterized SIVs (60).
To characterize this virus group in greater detail, we amplified complete genomic equivalents of SIVdeb (SIVdebCM5 and SIVdebCM40) from uncultured PBMC DNA from two infected animals. This was done by using nested-PCR approaches and primer combinations that targeted circular unintegrated viral DNA intermediates (Fig. 2). Because sequence analysis of the initial 650-bp pol fragment (fragment A in Fig. 2) indicated a high degree of genetic diversity, we used SIV consensus primers to target a second diagnostic fragment (699 bp) in the 5′ pol region (fragment B). The sequences of fragments A and B were then used to design strain-specific primers to amplify the remainder of the SIVdeb genome. First, the region between fragments A and B was amplified, yielding a 2.2-kb pol fragment (fragment C). The pol primers were then reversed to target the remaining 8.5 kb of the unintegrated viral DNA circle. Although this region could not be derived as a single amplicon, we were able to amplify three partially overlapping fragments by using a combination of SIVdeb-specific and consensus primers. These included a 2.3-kb gag-pol (fragment D) amplicon, a 3.5-kb pol-env (fragment E) amplicon, and a 2.5-kb env-nef-long terminal repeat (LTR)-gag (fragment F) amplicon, which were gel purified, subcloned into the pGEM-T easy vector, and sequenced. Viral sequences were assembled by using the Sequencher Software (Gene Codes Corporation, Ann Arbor, Mich.). In regions of overlap, the 5′ sequence was arbitrarily selected for compilation of the final genomic sequence. The concatenated SIVdebCM5 and SIVdebCM40 sequences (R-U5-gag-pol-env-U3) are 9,158 and 9,227 bp in length, respectively; the length differences reflect numerous scattered small insertions and deletions.
Genomic organization of SIVdeb.
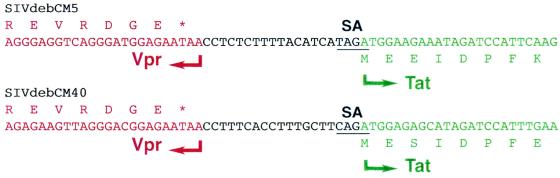
Inspection of the deduced amino acid sequences of SIVdebCM5 and SIVdebCM40 revealed the expected reading frames for gag, pol, vif, vpr, tat, rev, env, and nef, none of which contained inactivating mutations. Like most other SIVs, the two SIVdeb genomes did not encode a vpu gene homologue. Major regulatory sequences, including promoter and enhancer elements in the LTR, the primer binding site, and major splice sites all appeared to be intact. However, inspection of the relative positions of SIVdeb structural and accessory genes revealed an unusual arrangement of the vpr gene and the first exon of tat. In all SIVs characterized to date, these genes overlap. By contrast, in both SIVdeb strains these genes were separated by 19 bp of noncoding sequence (Fig. 3). Given the known propensity of primate lentiviruses for genetic change, it is highly unlikely that a nonessential spacer sequence of identical length is conserved in two different SIV strains. Rather, this spacer sequence is likely to have an as-yet-unknown function unique to the SIVdeb group of viruses.
FIG. 3.
Vpr-tat intergenic region in SIVdeb. SIVdeb vpr (red) and first exon of tat (green) genes are separated by a 19-bp noncoding sequence (black) in two different SIVdeb strains (CM5 and CM40). The deduced amino acid sequences of the C terminus of Vpr and the N terminus of Tat are indicated. SA indicates the splice acceptor site for the tat mRNA.
Distance and phylogenetic analysis of SIVdeb.
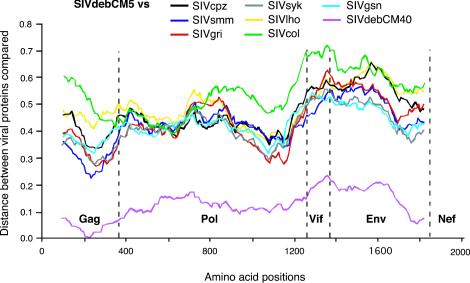
To compare the two SIVdeb sequences to previously characterized SIV strains, we performed diversity plot analyses of concatenated protein sequences. Pairwise sequence distances were plotted for windows of 300 amino acids, which were moved in steps of 20 amino acids along the alignment. Figure 4 depicts the proportion of amino acid sequence differences between SIVdebCM5 and SIVdebCM40 as well as between SIVdebCM5 and other primate lentiviruses. As expected, the two SIVdeb strains were quite similar, differing by 16% of the amino acids over the entire length of their proteome. All other SIV strains were considerably more distant, with values ranging from 30% sequence diversity in Gag to 70% in Vif. Finally, distance analysis revealed no evidence of recombination between SIVdeb and any of the other SIV strains.
FIG. 4.
Diversity plots of concatenated protein sequences illustrating the extent of genetic difference between SIVdebCM5 and other primate lentiviruses. The proportion of amino acid sequence differences between SIVdebCM5 and other SIV strains are shown in different colors. Values are plotted at the midpoint of the sequence window on the x axis, with the N termini of Gag, Pol, Vif, Env, and Nef indicated. The y axis indicates the distance between the viral proteins (0.1 = 10% difference).
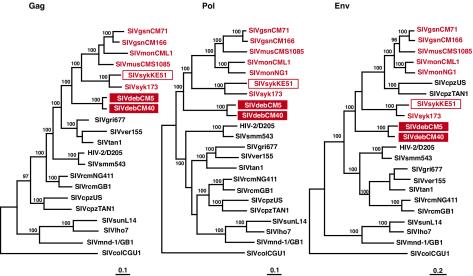
To estimate the phylogenetic relationships of the new SIVdeb strains to other primate lentiviruses, we constructed evolutionary trees from Gag, Pol, and Env amino acid sequences (Fig. 5). These analyses included all available full-length guenon virus sequences, including those derived most recently from mona monkeys (C. mona) and mustached guenons (C. cephus) (4, 16). Moreover, to ensure that the single available SIVsyk sequence was indeed representative of viruses infecting Sykes's monkeys, we amplified, cloned, and sequenced a second full-length SIVsyk strain (KE51). This strain was amplified from uncultured PBMC DNA from an animal housed at the Kenyan Primate Research Center by using nested-PCR approaches identical to the ones described for SIVdeb (Table 2). Using neighbor-joining and maximum likelihood approaches, we found that SIVsykKE51 and SIVsyk173 indeed clustered together, thus confirming the existence of a species-specific SIVsyk clade. We also found that the two new SIVdeb viruses clustered together but that they were quite divergent from previously characterized SIVs. The closest relatives of both SIVdeb and SIVsyk were members of the SIVgsn/SIVmon/SIVmus clade. All of these SIVs grouped together in all major coding regions, forming a distinct cluster that was statistically highly significant. The trees in Fig. 5 are midpoint rooted, and the true position of the root is difficult to establish because there are no known close outgroups for the primate lentiviruses. However, it seems very unlikely that the root falls within the SIVdeb/SIVsyk/SIVgsn/SIVmon/SIVmus cluster, suggesting that the viruses infecting De Brazza's, Sykes's, greater spot-nosed, mona, and mustached monkeys all share a common ancestry that is distinct from that of other SIVs.
FIG. 5.
Phylogenetic relationship of SIVdeb strains to other primate lentiviruses. Midpoint-rooted maximum-likelihood trees were inferred from protein sequence alignments. The numbers on internal branches indicate estimated posterior probabilities (only values of 95% or greater are shown); the scale bars indicate 0.1 (Gag and Pol) and 0.2 (Env) substitutions per site. SIVs from Cercopithecus species (excluding the C. lhoesti group, whose taxonomic classification has been disputed [74]) are shown in red, including three new sequences obtained in this study (boxed).
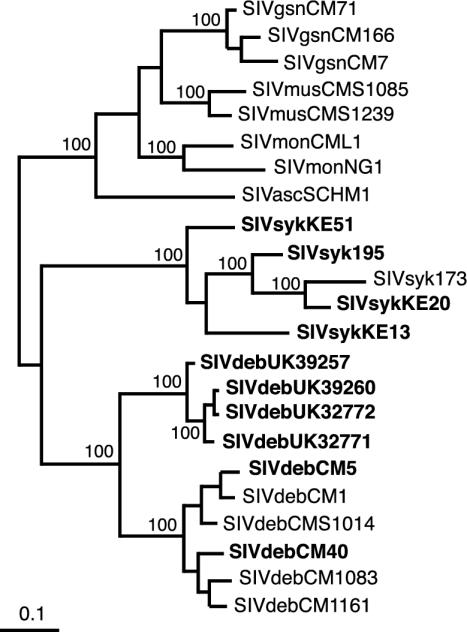
Molecular characterization of SIVdeb strains from East Africa.
To investigate whether De Brazza's monkeys are infected with SIVdeb-like viruses throughout the species' range, we obtained additional SIV sequences from four captive-born De Brazza's monkeys living in safari parks in the United Kingdom. Records showed that these animals were the progeny of De Brazza's monkeys originally imported from Uganda. Using nested reverse transcription-PCR approaches, we amplified partial pol sequences (650 bp) from plasma samples of all four animals (fragment A in Fig. 2). Phylogenetic analyses of these sequences indicated that they were very closely related to one another (Fig. 6). Moreover, the four East African SIVdeb strains clustered with six Cameroonian SIVdeb strains, forming a single species-specific clade that was supported with high confidence. As expected, the Cameroonian and Ugandan SIVdeb strains were more divergent, although not substantially more so than what was seen for other SIVs. In the amplified pol region, Cameroonian and Ugandan SIVdeb strains differed by 19.4% of the deduced protein sequence. We also obtained partial pol sequences from three additional SIVsyk strains from both wild and captive Sykes's monkeys in Kenya. Analysis of these sequences yielded an intraclade diversity of 17.5%, whereas SIVmon strains from Nigeria and Cameroon differed by 17.2%. These data indicate that De Brazza's monkeys are naturally infected with a single type of SIV that seems prevalent throughout the species' habitat.
FIG. 6.
Phylogenetic relationships of SIVdeb from Cameroon and Uganda and SIVsyk from Kenya to SIVs from other Cercopithecus species (excluding the C. lhoesti group). Maximum-likelihood trees were inferred from a partial (170-amino-acid) Pol protein sequence alignment and rooted as for Fig. 5. The numbers on internal branches indicate estimated posterior probabilities (only values of 95% or greater are shown); the scale bar indicates 0.1 substitution per site. Sequences obtained in this study are shown in bold.
Very recently, partial sequence information has become available (79) for an SIV from another Cercopithecus species, the red-tailed guenon (C. ascanius schmidti). The reported pol sequences overlap the region studied here, and so this SIVasc was included in the phylogenetic analysis. As shown in Fig. 6, this new virus also fell within the SIVdeb/SIVsyk/SIVgsn/SIVmon/SIVmus cluster referred to in the previous section.
Functional motifs shared by Cercopithecus monkey viruses.
SIVsyk, SIVgsn, SIVmus, SIVmon, and SIVdeb are all derived from Old World monkey species belonging to the genus Cercopithecus (Table 1). The close phylogenetic relationships of these viruses suggested that they might constitute a genus-specific SIV lineage. However, the independent grouping of SIVlho from L'Hoest's monkeys and SIVsun from sun-tailed monkeys, two species also classified within the Cercopithecus genus (30), seemed to argue against this possibility. This prompted us to examine the literature for recent molecular studies of guenon evolution and taxonomy. Indeed, comparing Y chromosome-specific (TSPY) sequences of representatives from 10 different Old World monkey genera, Tosi and colleagues found that members of the lhoesti group (including L'Hoest's and sun-tailed monkeys) were significantly more closely related to African green monkeys (Chlorocebus) and patas monkeys (Erythrocebus) than they were to other Cercopithecus species (74). These authors thus proposed a new guenon taxonomy, which placed the lhoesti group outside the Cercopithecus genus. In light of these data, we inspected available SIVgsn, SIVmus, SIVmon, SIVsyk, and SIVdeb sequences for common regulatory and/or protein motifs that would suggest a Cercopithecus host-specific function.
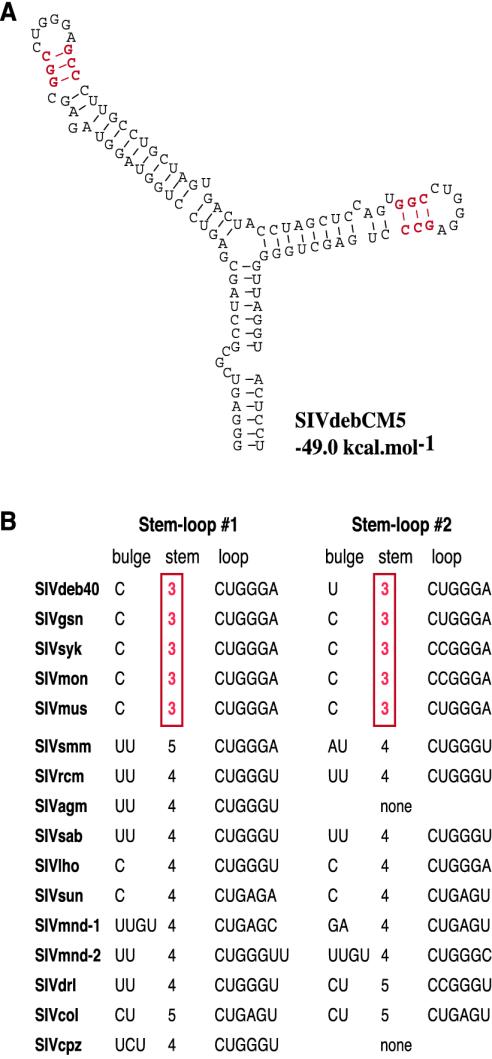
We first inspected the predicted secondary structure of the Tat-TAR element because this regulatory region had previously been described as exhibiting SIV lineage-specific differences (40). Indeed, secondary structure predictions for both SIVdeb strains indicated a duplicated stem-loop structure consisting of a one-base bulge (C or U), a 3-bp stem, and a six-base terminal loop with the sequence 5′-CUGGGA-3′ (Fig. 7). Interestingly, the predicted TAR secondary structures for SIVsyk, SIVgsn, SIVmon, and SIVmus also exhibited duplicated TAR elements containing a 3-bp stem between the bulge and the terminal loop (Fig. 7). By contrast, all other primate lentiviruses contained a 4-bp stem, with the exception of SIVsmm and SIVcol strains, which encoded a 5-bp stem between the bulge and the loop (Fig. 7). For the HIV-1 TAR element, the spacing between the bulge and the loop is known to be critical for efficient Tat-mediated transactivation, possibly by optimizing the structural interactions of the Tat protein which binds the bulge and cellular TAR binding proteins such as cyclinT1, which bind the terminal loop (8, 20, 81). It will be interesting to determine whether there are genus-specific differences in interactions between TAR and TAR binding proteins that favor a shorter stem in the SIVsyk, SIVgsn, SIVmus, SIVmon, and SIVdeb TAR structures.
FIG. 7.
Secondary structure predictions of SIV TAR elements. (A) The secondary structure for the SIVdebCM5 TAR with the lowest free energy value (−49.0 kcal/mol) is shown. The predicted SIVdebCM40 TAR secondary structure was almost identical, with a free energy value of −47.6 kcal/mol (not shown). (B) Comparison of TAR elements from other primate lentiviruses. The five Cercopithecus SIVs listed at the top (SIVdeb, SIVgsn, SIVsyk, SIVmon, and SIVmus) share a unique 3-bp stem separating the bulge and the terminal loop (highlighted in red).
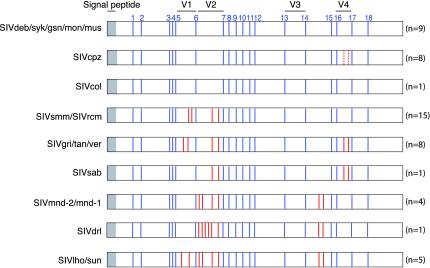
Cysteine residues in the HIV or SIV envelope glycoprotein surface subunit are known to form paired disulfide bonds that determine the tertiary structure and folding of the gp120 subunit and are essential for envelope function (46, 47). To determine whether the number and position of cysteine residues varied among SIVs from different monkey species, we aligned available SIV gp120 protein sequences and examined the alignments for differences in cysteine spacing. This analysis revealed that all Cercopithecus monkey viruses, including SIVdeb (two strains), SIVgsn (n = 2), SIVmon (n = 2), SIVmus (n = 1), and SIVsyk (n = 2) contained 18 cysteine residues in their extracellular envelope domain (Fig. 8). These same cysteine residues were also conserved in all other primate lentiviruses; however, most of the other SIVs encoded two, three, or four additional pairs of cysteine residues, which typically fell within variable domains of the gp120 glycoprotein. In HIV-1, variable loops are not directly involved in CD4 and CCR5 binding but are believed to influence receptor and coreceptor binding through conformational masking and steric occlusion of the binding sites (54, 82, 83). Thus, the function of the additional cysteine pairs is likely to stabilize and diversify loop folding, thereby resulting in a greater complexity of envelope glycoprotein surface structures. Apart from the Cercopithecus viruses referred to above, the only other SIVs lacking additional cysteine residues were SIVcpz and SIVcol. Since the 3′ half of the SIVcpz genome is derived from a member of the Cercopithecus virus group (3), such that in an Env tree SIVcpz falls within this clade (Fig. 5), it is not necessarily surprising that SIVcpz lacks additional cysteine residues. However, the absence of additional cysteine pairs in SIVcol, the most divergent member of the primate lentiviruses (Fig. 5), is not immediately obvious. The conservation of the 18 cysteine residues across all SIVs suggests that this “18 Cys state” was the situation in the common ancestor of the primate lentiviruses. Eventually there were additions of particular pairs of cysteines in different SIV lineages, but the 18 Cys state has been conserved independently in SIVcol and in the Cercopithecus SIVs.
FIG. 8.
Position of cysteine residues in the Env glycoprotein surface subunit of primate lentiviruses. The gp120 domain is depicted, with signal peptide and variable regions (V1-V4) indicated. Blue bars indicate the relative location of the 18 cysteine residues (numbered 1 to 18 at the top) that are conserved among all primate lentiviruses; red bars depict additional cysteine residues that are present in a subset of SIV strains (broken lines indicate one cysteine pair unique to SIVcpzCAM5). The number of strains per SIV group is indicated in parentheses.
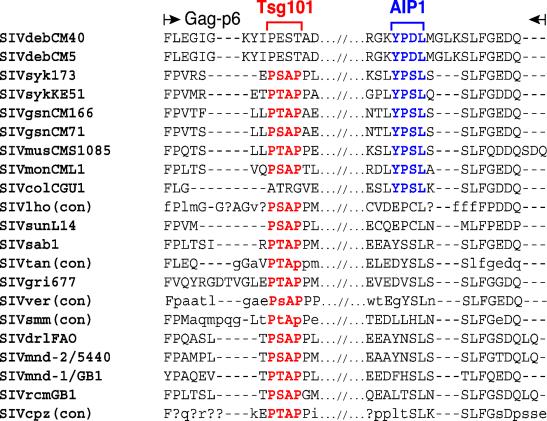
Finally, alignments of SIV Gag p6 protein sequences revealed an unusual arrangement of late (L) domain sequences known to be critical for primate lentivirus budding (Fig. 9). Two different lentiviral L domains with the sequences PT/SAP and YPXL have been identified (23, 71, 80). All lentiviruses, except for EIAV and SIVcol, have a PT/SAP motif, and several reports have now shown that the functionally relevant binding partner of this motif is Tsg101, a cellular protein that facilitates the budding of vesicles into late endosomes as part of a larger complex. EIAV contains a YPDL motif that compensates for the absence of the PT/SAP motif because it binds with high affinity to AIP1, a second host protein involved in endosomal sorting and retroviral budding (50, 62, 71). Interestingly, a similar YPSL motif is also found in SIVcol (Fig. 9). Inspection of the alignment in Fig. 9 now shows that SIVdeb strains, like SIVcol and EIAV, lack a PT/SAP site but contain a YPDL site at the C terminus of the p6 protein. This YPDL site should mediate high-affinity binding of the SIVdeb p6 Gag protein to AIP1, as has been shown for EIAV (62), and its presence is thus not unexpected. However, the finding of a similar YPSL domain in all other Cercopithecus viruses is surprising, since all of these also encode an N-terminal PT/SAP Tsg101 binding motif. While the functional significance of the presence of both PT/SAP and YPS/DL motifs in the same SIV Gag p6 protein remains to be elucidated, the conservation of the YPD/SL motif in SIVdeb, SIVsyk, SIVgsn, SIVmus, and SIVmon strains represents another functional motif shared by SIVs infecting Cercopithecus monkey species.
FIG. 9.
Alignment of primate lentiviral Gag p6 protein sequences. Only N- and C-terminal sequences are shown, with strain designations indicated on the left. For SIVlho, SIVtan, SIVver, SIVsmm, and SIVcpz, consensus sequences (con) are shown (45). Uppercase letters indicate amino acids that are conserved in all sequences. Lowercase letters (or a question mark [?]) indicate amino acids conserved in more (or fewer) than 50% of sequences used to generate the consensus sequence. Dashes indicate spaces introduced to optimize the alignment. Tsg101 (PT/SAP) and AIP1 (YPD/SL) binding motifs are shown in red and blue, respectively.
DISCUSSION
De Brazza's monkeys (Cercopithecus neglectus) inhabit a large area of sub-Saharan Africa that stretches from southern Cameroon, northern Gabon, and southern Central African Republic through the Zaire River complex and eastward to Uganda, with isolated communities in western Kenya and southwestern Ethiopia (Fig. 1B). In this paper, we report that De Brazza's monkeys are naturally infected with a primate lentivirus, termed SIVdeb, which is only distantly related to previously characterized SIVs. Analyzing strains from Cameroon and Uganda, we found that geographically diverse SIVdeb strains cluster in a single species-specific virus group. Together with previous prevalence estimates (60), these data suggest that SIVdeb infection is frequent among wild De Brazza's monkeys and widespread throughout the species' habitat. While SIVdeb appears to represent the only SIV type commonly infecting De Brazza's monkeys, the distinct clustering of Cameroonian and Ugandan strains in Fig. 6 may reflect the existence of geographic sublineages.
Evidence for a Cercopithecus monkey virus lineage.
Until recently, the known strains of SIV were classified into six major viral lineages (2). These lineages were readily identified in phylogenetic trees because they represented clusters that were separated from one another by deep branches. Moreover, these lineages were assumed to represent nonrecombinant viruses. However, as new SIVs from additional primate species are discovered and molecularly characterized, the identification of specific SIV lineages has become increasingly arbitrary. This is because the deep branches that separated the six major SIV lineages are now broken up by the joining of new branches. Thus, phylogenetic trees like those in Fig. 5 no longer reveal obvious places at which to draw the line for lineage designation. Moreover, an increasing number of viruses exhibit discordant branching orders in different parts of their genome (3, 40, 69, 70). For example, it has been known for some time that SIVcpz, SIVrcm, and SIVgsn are connected by ancestral recombination events. For historic reasons, SIVcpz has always been regarded as a distinct lineage, although it was difficult to determine which of the three viruses represented the recombinants and which represented the parental lineages (7, 19). More recently, it has become apparent that it is SIVcpz that is the recombinant virus (3), suggesting that it should no longer be termed a lineage. However, there is no a priori reason why an extensive clade of hybrids derived from a single recombination event, such as the SIVcpz group, should not be regarded as a lineage. These observations clearly indicate that the SIV lineage nomenclature requires revision.
One group of SIVs that clusters consistently in phylogenetic trees is the group comprised of SIVsyk, SIVdeb, SIVgsn, SIVmus, and SIVmon (Fig. 5). In Gag, Pol, and Env trees, these viruses group together, and the cluster is supported with high confidence. Moreover, these same viruses share functional motifs that distinguish them from other primate lentiviruses. These include a unique predicted TAR structure with an unusually short (3-bp) stem, an identical number of cysteine residues in their extracellular envelope glycoprotein (gp120), and a high-affinity AIP1 binding site (YPS/DL) in their p6 Gag protein. Although the last two features are not unique to SIVsyk, SIVdeb, SIVgsn, SIVmus, and SIVmon, their conservation among otherwise highly divergent viruses further supports a common evolutionary history. Finally, recent studies of Old World monkey genetics and evolution indicate that SIVlho and SIVsun should no longer be viewed as Cercopithecus monkey viruses but should be considered a separate group of SIVs (74). Thus, the clustering of SIVsyk, SIVdeb, SIVgsn, SIVmus, and SIVmon points to the existence of an ancient SIV lineage that infected members of the Cercopithecus genus in the distant past. Analysis of partial pol sequences for SIVasc, from Cercopithecus ascanius (79), is consistent with this (Fig. 6), and preliminary sequence data for SIVs from C. erythrotis (P. Reed, S. Souquiere, M. Makuwa, P. Telfer, M. A. Ela-Mba, P. Roques, and P. A. Marx, Abstr. 2nd IAS Conf. HIV Pathog. Treatment, abstr. 224, 2003) C. denti (S. Saragosti, S., M. Ekwalanga, M. C. Dazza, M. Mende, P. Bitchou, and K. Bin Shamara, Abstr. 2nd IAS Conf. HIV Pathog. Treatment abstr. 285, 2003), and C. mitis (F. Bibollet-Ruche, V. Courgnaud, X. Pourrut, E. N. Mpoudi, J. Mwenda, V. M. Hirsch, E. Delaporte, M. Peeters, F. Gao, G. M. Shaw, and B. H. Hahn, Abstr. 7th Conf. Retrovir. Opportun. Infect., abstr. 223, 2000) suggest that all of these viruses also fall within the same phylogenetic cluster, further supporting this hypothesis.
Have Cercopithecus monkey viruses coevolved with their primate hosts?
To understand the origin of SIV in the various species, a better understanding of the timescale of primate lentiviral evolution is needed. Virus-host coevolution over hundreds of thousands or even millions of years is consistent with a nonpathogenic phenotype. However, such a timescale is not supported by current molecular clock estimates (36, 68). A way to address this is to characterize SIV infections for monkey species for which information concerning evolutionary history is available. If clear-cut examples of matching virus and host phylogenies could be identified, this would strengthen the argument of an ancient evolutionary history for at least some SIVs. One attractive group in which to look for such relationships is that of Cercopithecus monkeys and their viruses.
If SIVs from Cercopithecus monkey species had coevolved with their primate hosts, then virus and host phylogenies would be very similar. At first glance, this does not seem to be the case. Figure 5 shows that SIVsyk and SIVgsn are as divergent from each other as they are from SIVdeb, but this does not seem to be true for their hosts. Current taxonomy (Table 1) classifies Sykes’s and greater spot-nosed monkeys together in the mitis group, suggesting that these two species are more closely related to one another than either one is to De Brazza's monkeys. Taken at face value, this would argue against virus-host coevolution; however, there are two important caveats. First, existing Cercopithecus monkey phylogenies are based on very limited sequence information and thus may not be entirely accurate. Second, it is quite possible that some of the currently known Cercopithecus SIVs are not the only SIVs infecting these particular species: we have recently developed strain-specific antibody detection assays for SIVgsn and SIVmus by expressing their respective gp41 ectodomains and using the recombinant proteins as enzyme-linked immunosorbent assay (ELISA) antigens. Employing these ELISAs to test 149 wild-caught greater spot-nosed monkeys and 137 mustached guenons, we found SIVgsn and SIVmus infection rates of 5 and 4%, respectively (W.-M. Liu, A. Aghokeng, F. Bibollet-Ruche, M. Peeters, and B. H. Hahn, unpublished). Compared to the SIV infection rates of other primate species, which frequently exceed 50% in adult animals, these prevalence rates are rather low (1, 5, 10, 13, 17). Moreover, many of the greater spot-nosed and mustached monkey sera that were SIVgsn and SIVmus ELISA negative cross-reacted strongly with HIV-1 and HIV-2 antigens in the INNO-LIA assay (60), suggesting that the strain-specific assays detected only a fraction of the SIV infections in these monkeys. Thus, the viruses currently identified as SIVgsn or SIVmus may not represent the only or even the true greater spot-nosed or mustached monkey SIVs. Indeed, greater spot-nosed monkeys and mustached guenons are known to form polyspecific groups with each other, and both species are also known to associate with crested mona monkeys (C. pogonias) and red-eared guenons (C. erythrotis), thus providing opportunity for interaction and cross-species transfers (28, 29). By contrast, De Brazza's monkeys avoid contact with all other monkey species (64). Taken together, these data suggest that not all the SIVs infecting the various Cercopithecus monkey species have yet been determined, thus leaving the issue of virus host coevolution within this genus open to question.
Origin of the vpu gene.
Until recently, only SIVcpz and HIV-1 strains were known to encode a vpu gene. vpu has now also been identified in the genomes of SIVgsn, SIVmus, and SIVmon (4, 17, 19), consistent with the finding that this part of the SIVcpz genome was derived from an ancestor of this group of viruses (Fig. 5C). Since SIVsyk and SIVdeb, the other members of the Cercopithecus virus group, do not encode a vpu, it is most likely that this gene originated in the common ancestor of SIVgsn, SIVmus, and SIVmon, after the divergence of SIVsyk and SIVdeb.
Future studies.
Table 1 summarizes the current state of knowledge concerning naturally occurring primate lentiviruses. Although many new SIVs have been discovered and characterized in recent years, the task of characterizing the extent of diversity, geographic spread, and prevalence of naturally occurring SIV infections is far from complete. A case in point is SIVsyk, for which only a single strain has been available for over 10 years. Although our present data confirm that this sequence is indeed representative of the viruses infecting Sykes's monkeys, this could not have been assumed a priori. Further studies are thus needed to determine which and how many different viruses are circulating in any one species, to what extent each SIV is distributed across any one species' habitat, and whether infection rates fluctuate among and within different species. Although labor-intensive and time-consuming, such information will be important to gain further insight into the origins and evolution of this medically important group of viruses.
Acknowledgments
We thank Maria Salazar for technical assistance, the Central Public Health HIV-1 Reference Laboratory staff for serological testing of UK32771, UK32772, UK39257, and UK39260, Heinrich Gottlinger and Peter Kwong for helpful discussions, Bernadette Abela for the photograph of a pet De Brazza's monkey, and W. J. Abbott for artwork and manuscript preparation.
This work was supported in part by grants from the Agence Nationale de Recherche sur le SIDA, France, the National Institutes of Health (R01 AI 55380, R01 AI 44596, R01 AI 50529, and P30 AI 27767), and the Howard Hughes Medical Institute.
REFERENCES
- 1.Allan, J. S., M. Short, M. E. Taylor, S. Su, V. M. Hirsch, P. R. Johnson, G. M. Shaw, and B. H. Hahn. 1991. Species-specific diversity among simian immunodeficiency viruses from African green monkeys. J. Virol. 65:2816-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailes, E., R. R. Chaudhuri, M. L. Santiago, F. Bibollet-Ruche, B. H. Hahn, and P. M. Sharp. 2002. The evolution of primate lentiviruses and the origins of AIDS, p. 65-96. In T. A. Leitner (ed.), The molecular epidemiology of human viruses. Kluwer Academic Publishers, Boston, Mass.
- 3.Bailes, E., F. Gao, F. Bibollet-Ruche, V. Courgnaud, M. Peeters, P. A. Marx, B. H. Hahn, and P. M. Sharp. 2003. Hybrid origin of SIV in chimpanzees. Science 300:1713.. [DOI] [PubMed] [Google Scholar]
- 4.Barlow, K. L., A. O. Ajao, and J. P. Clewley. 2003. Characterization of a novel simian immunodeficiency virus (SIVmonNG1) genome sequence from a mona monkey (Cercopithecus mona). J. Virol. 77:6879-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer, B. E., E. Bailes, G. Dapolito, B. J. Campbell, R. M. Goeken, M. K. Axthelm, P. D. Markham, J. Bernard, D. Zagury, G. Franchini, P. M. Sharp, and V. M. Hirsch. 2000. Patterns of genomic sequence diversity among their simian immunodeficiency viruses suggest that L'Hoest monkeys (Cercopithecus lhoesti) are a natural lentivirus reservoir. J. Virol. 74:3892-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beer, B. E., E. Bailes, R. Goeken, G. Dapolito, C. Coulibaly, S. G. Norley, R. Kurth, J.-P. Gautier, A. Gautier-Hion, D. Vallet, P. M. Sharp, and V. M. Hirsch. 1999. Simian immunodeficiency virus (SIV) from sun-tailed monkeys (Cercopithecus solatus): evidence for host-dependent evolution of SIV within the C. lhoesti superspecies. J. Virol. 73:7734-7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beer, B. E., B. T. Foley, C. L. Kuiken, Z. Tooze, R. M. Goeken, C. R. Brown, J. Hu, M. St Claire, B. T. Korber, and V. M. Hirsch. 2001. Characterization of novel simian immunodeficiency viruses from red-capped mangabeys from Nigeria (SIVrcmNG409 and SIVrcmNG411). J. Virol. 75:12014-12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkhout, B., and K. T. Jeang. 1991. Detailed mutational analysis of TAR RNA: critical spacing between the bulge and loop recognition domains. Nucleic Acids Res. 19:6169-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bibollet-Ruche, F., C. Brengues, A. Galat-Luong, G. Galat, X. Pourrut, N. Vidal, F. Veas, J. P. Durand, and G. Cuny. 1997. Genetic diversity of simian immunodeficiency viruses from West African green monkeys: evidence of multiple genotypes within populations from the same geographical locale. J. Virol. 71:307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bibollet-Ruche, F., A. Galat-Luong, G. Cuny, P. Sarni-Manchado, G. Galat, J.-P. Durand, X. Pourrut, and F. Veas. 1996. Simian immunodeficiency virus infection in a patas monkey (Erythrocebus patas): evidence for cross-species transmission from African green monkeys (Cercopithecus aethiops sabaeus) in the wild. J. Gen. Virol. 77:773-781. [DOI] [PubMed] [Google Scholar]
- 11.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakrabarti, L. A., A. Luckay, and P. A. Marx. 2001. A divergent simian immunodeficiency virus from sooty mangabey with an atypical Tat-TAR structure. AIDS Res. Hum. Retrovir. 17:1155-1165. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Z., P. Telfer, A. Gettie, P. Reed, L. Zhang, D. D. Ho, and P. A. Marx. 1996. Genetic characterization of New West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J. Virol. 70:3617-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clewley, J. P., J. C. M. Lewis, D. W. G. Brown, and E. L. Gadsby. 1998. Novel simian immunodeficiency virus (SIVdrl) pol sequence from the drill monkey, Mandrillus leucophaeus. J. Virol. 72:10305-10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbet, S., M. C. Muller-Trutwin, P. Versmisse, S. Delarue, A. Ayouba, J. Lewis, S. Brunak, P. Martin, F. Brun-Vezinet, F. Simon, F. Barre-Sinoussi, and P. Mauclere. 2000. Env sequences of simian immunodeficiency viruses from chimpanzees in Cameroon are strongly related to those of human immunodeficiency virus group N from the same geographic area. J. Virol. 74:529-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courgnaud, V., B. Abela, X. Pourrut, E. Mpoudi-Ngole, S. Loul, E. Delaporte, and M. Peeters. 2003. Identification of a new simian immunodeficiency virus lineage with a vpu gene present among different Cercopithecus monkeys (C. mona, C. cephus, and C. nictitans) from Cameroon. J. Virol. 77:12523-12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courgnaud, V., P. Formenty, C. Akoua-Koffi, R. Noe, C. Boesch, E. Delaporte, and M. Peeters. 2003. Partial molecular characterization of two simian immunodeficiency viruses (SIV) from African colobids: SIVwrc from Western Red colobus (Piliocolobus badius) and SIVolc from olive colobus (Procolobus verus). J. Virol. 77:744-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Courgnaud, V., X. Pourrut, F. Bibollet-Ruche, E. Mpoudi-Ngole, A. Bourgeois, E. Delaporte, and M. Peeters. 2001. Characterization of a novel simian immunodeficiency virus from Guereza Colobus (Colobus guereza) in Cameroon: a new lineage in the nonhuman primate lentivirus family. J. Virol. 75:857-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courgnaud, V., M. Salemi, X. Pourrut, E. Mpoudi-Ngole, B. Abela, P. Auzel, F. Bibollet-Ruche, B. Hahn, A. Vandamme, E. Delaport, and M. Peeters. 2002. Characterization of a novel simian immunodeficiency virus with a vpu gene from greater spot-nosed monkeys (Cercopithecus nicititans) provides new insights into simian/human immunodeficiency virus phylogeny. J. Virol. 76:8298-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delling, U., L. S. Reid, R. W. Barnett, M. Y. Ma, S. Climie, M. Sumner-Smith, and N. Sonenberg. 1992. Conserved nucleotides in the TAR RNA stem of human immunodeficiency virus type 1 are critical for Tat binding and trans activation: model for TAR RNA tertiary structure. J. Virol. 66:3018-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emau, P., H. M. McClure, M. Isahakia, J. G. Else, and P. N. Fultz. 1991. Isolation from African Sykes' monkeys (Cercopithecus mitis) of a lentivirus related to human and simian immunodeficiency viruses. J. Virol. 65:2135-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fomsgaard, A., V. M. Hirsch, J. S. Allan, and P. R. Johnson. 1991. A highly divergent proviral DNA clone of SIV from a distinct species of African green monkey. Virology 182:397-402. [DOI] [PubMed] [Google Scholar]
- 23.Freed, E. O. 2002. Viral late domains. J. Virol. 76:4679-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukasawa, M., T. Miura, A. Hasegawa, S. Morikawa, H. Tsujimoto, K. Miki, T. Kitamura, and M. Hayami. 1988. Sequence of simian immunodeficiency virus from African green monkey, a new member of the HIV/SIV group. Nature 333:457-461. [DOI] [PubMed] [Google Scholar]
- 25.Galtier, N., M. Gouy, and C. Gautier. 1996. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comp. Appl. Biosci. 12:543-548. [DOI] [PubMed] [Google Scholar]
- 26.Gao, F., E. Bailes, D. L. Robertson, Y. Chen, C. M. Rodenburg, S. F. Michael, L. B. Cummins, L. O. Arthur, M. Peeters, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397:436-441. [DOI] [PubMed] [Google Scholar]
- 27.Gao, F., L. Yue, A. T. White, P. G. Pappas, J. Barchue, A. P. Hanson, B. M. Greene, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 1992. Human infection by genetically diverse SIVSM-related HIV-2 in West Africa. Nature 358:495-499. [DOI] [PubMed] [Google Scholar]
- 28.Gautier-Hion, A. 1988. Polyspecific associations among forest guenons: ecological, behavioral, and evolutionary aspects, p. 452-476. In A. Gautier-Hion, F. Bourliere, J.-P. Gautier, and J. Kingdon (ed.), A primate radiation: evolutionary biology of the African guenons. Cambridge University Press, Cambridge, United Kingdom.
- 29.Gautier-Hion, A., R. Quris, and J. P. Gautier. 1983. Monospecific vs. polyspecific life: a comparative study of foraging and antipredatory tactics in a community of Cercopithecus monkeys. Behav. Ecol. Sociobiol. 12:325-335. [Google Scholar]
- 30.Groves, C. 2001. Primate taxonomy. Smithsonian series in comparative evolution biology. Smithsonian Institution Press, Washington, D.C.
- 31.Hahn, B. H., G. M. Shaw, K. M. De Cock, and P. M. Sharp. 2000. AIDS as a zoonosis: scientific and public health implications. Science 287:607-617. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch, V. M., B. J. Campbell, E. Bailes, R. Goeken, C. Brown, W. R. Elkins, M. Axthelm, M. Murphey-Corb, and P. M. Sharp. 1999. Characterization of a novel simian immunodeficiency virus (SIV) from L'Hoest monkeys (Cercopithecus l'hoesti): implications for the origins of SIVmnd and other primate lentiviruses. J. Virol. 73:1036-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsch, V. M., G. Dapolito, S. Goldstein, H. M. McClure, P. Emau, P. N. Fultz, M. Isahakia, R. Lenroot, G. Myers, and P. R. Johnson. 1993. A distinct African lentivirus from Sykes's monkeys. J. Virol. 67:1517-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirsch, V. M., C. McGann, G. Dapolito, S. Goldstein, A. Ogen-Odoi, B. Biryawaho, T. Lakwo, and P. R. Johnson. 1993. Identification of a new subgroup of SIVagm in tantalus monkeys. Virology 197:426-430. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch, V. M., R. A. Olmsted, M. Murphey-Corb, R. H. Purcell, and P. R. Johnson. 1989. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature 339:389-392. [DOI] [PubMed] [Google Scholar]
- 36.Holmes, E. C. 2003. Molecular clocks and the puzzle of RNA virus origins. J. Virol. 77:3893-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu, J., W. M. Switzer, B. T. Foley, D. L. Robertson, R. M. Goeken, B. T. Korber, V. M. Hirsch, and B. E. Beer. 2003. Characterization and comparison of recombinant simian immunodeficiency virus from drill (Mandrillus leucophaeus) and mandrill (Mandrillus sphinx) isolates. J. Virol. 77:4867-4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huelsenbeck, J. P., and F. Ronquist. 2001. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754-755. [DOI] [PubMed] [Google Scholar]
- 39.Huet, T., R. Cheynier, A. Meyerhans, G. Roelants, and S. Wain-Hobson. 1990. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature 345:356-359. [DOI] [PubMed] [Google Scholar]
- 40.Jin, M. J., H. Hui, D. L. Robertson, M. C. Müller, F. Barre-Sinoussi, V. M. Hirsch, J. S. Allan, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Mosaic genome structure of simian immunodeficiency virus from West African green monkeys. EMBO J. 13:2935-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin, M. J., J. Rogers, J. E. Phillips-Conroy, J. S. Allan, R. C. Desrosiers, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1994. Infection of a yellow baboon with simian immunodeficiency virus from African green monkeys: evidence for cross-species transmission in the wild. J. Virol. 68:8454-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson, P. R., A. Fomsgaard, J. Allan, M. Gravell, W. T. London, R. A. Olmsted, and V. M. Hirsch. 1990. Simian immunodeficiency viruses from African green monkeys display unusual genetic diversity. J. Virol. 64:1086-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones, D. T., W. R. Taylor, and J. M. Thornton. 1992. The rapid generation of mutation data matrices from protein sequences. Comp. Appl. Biosci. 8:275-282. [DOI] [PubMed] [Google Scholar]
- 44.Kodama, T., D. P. Silva, M. D. Daniel, J. E. Phillips-Conroy, C. J. Jolly, J. Rogers, and R. C. Desrosiers. 1989. Prevalence of antibodies to SIV in baboons in their native habitat. AIDS Res. Hum. Retrovir. 5:337-343. [DOI] [PubMed] [Google Scholar]
- 45.Kuiken, C., B. Foley, E. Freed, B. Hahn, B. Korber, P. A. Marx, F. McCutchan, J. W. Mellors, and S. Wolinsky (ed.). 2002. HIV Sequence Compendium 2002. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.M.
- 46.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678-682. [DOI] [PubMed] [Google Scholar]
- 47.Leonard, C. K., M. W. Spellman, L. Riddle, R. J. Harris, J. N. Thomas, and T. J. Gregory. 1990. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 265:10373-10382. [PubMed] [Google Scholar]
- 48.Ling, B., M. L. Santiago, S. Meleth, B. Gormus, H. M. McClure, C. Apetrei, B. H. Hahn, and P. A. Marx. 2003. Noninvasive detection of new simian immunodeficiency virus lineages in captive sooty mangabeys: ability to amplify virion RNA from fecal samples correlates with viral load in plasma. J. Virol. 77:2214-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lowenstine, L. J., N. C. Pederson, J. Higgins, K. C. Pallis, A. Uyeda, P. Marx, N. W. Lerche, F. J. Munn, and M. B. Gardner. 1986. Seroepidemiologic survey of captive Old World primates for antibodies to human and simian retroviruses, and isolation of a lentivirus from sooty mangabeys (Cercocebus atys). Int. J. Cancer 38:563-574. [DOI] [PubMed] [Google Scholar]
- 50.Martin-Serrano, J., T. Zang, and P. D. Bieniasz. 2001. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 7:1313-1319. [DOI] [PubMed] [Google Scholar]
- 51.Marx, P. A., Y. Li, N. W. Lerche, S. Sutjipto, A. Gettie, J. A. Yee, B. H. Brotman, A. M. Prince, A. Hanson, R. G. Webster, and R. C. Desrosiers. 1991. Isolation of a simian immunodeficiency virus related to human immunodeficiency virus type 2 from a west African pet sooty mangabey. J. Virol. 65:4480-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathews, D. H., J. Sabina, M. Zuker, and D. H. Turner. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288:911-940. [DOI] [PubMed] [Google Scholar]
- 53.Miura, T., J. Sakuragi, M. Kawamura, M. Fukasawa, E. N. Moriyama, T. Gojobori, K. Ishikawa, J. A. Mingle, V. B. Nettey, H. Akari, M. Enami, H. Tsujimoto, and M. Hayami. 1990. Establishment of a phylogenetic survey system for AIDS-related lentiviruses and demonstration of a new HIV-2 subgroup. AIDS 4:1257-1261. [DOI] [PubMed] [Google Scholar]
- 54.Mondor, I., M. Moulard, S. Ugolini, P. J. Klasse, J. Hoxie, A. Amara, T. Delaunay, R. Wyatt, J. Sodroski, and Q. J. Sattentau. 1998. Interactions among HIV gp120, CD4, and CXCR4: dependence on CD4 expression level, gp120 viral origin, conservation of the gp120 COOH- and NH2-termini and V1/V2 and V3 loops, and sensitivity to neutralizing antibodies. Virology 248:394-405. [DOI] [PubMed] [Google Scholar]
- 55.Muller, M. C., K. Saksena, E. Nerrienet, C. Chappey, V. M. A. Herve, J.-P. Durand, P. Legal-Campodonico, M.-C. Lang, J. P. Digoutte, A. J. Georges, M.-C. Georges-Courbot, P. Sonigo, and F. Barre-Sinoussi. 1993. Simian immunodeficiency viruses from Central and Western Africa: evidence for a new species-specific lentivirus in tantalus monkeys. J. Virol. 67:1227-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicol, I., D. Messinger, P. Dubouch, J. Bernard, I. Desportes, R. Jouffre, R. Snart, P. Nara, R. C. Gallo, and D. Zagury. 1989. Use of Old World monkeys for acquired immunodeficiency syndrome research. J. Med. Primatol. 18:227-236. [PubMed] [Google Scholar]
- 57.Ohta, Y., T. Masuda, H. Tsujimoto, K. Ishikawa, T. Kodama, S. Morikawa, M. Nakai, S. Honjo, and M. Hayami. 1988. Isolation of simian immunodeficiency virus from African green monkeys and seroepidemiologic survey of the virus in various non-human primates. Int. J. Cancer 41:115-122. [DOI] [PubMed] [Google Scholar]
- 58.Osterhaus, A. D. M. E., N. Pedersen, G. van Amerongen, M. T. Frankenhuis, M. Marthas, E. Reay, T. M. Rose, J. Pamungkas, and M. L. Bosch. 1999. Isolation and partial characterization of a lentivirus from Talapoin Monkeys (Myopithecus talapoin). Virology 260:116-124. [DOI] [PubMed] [Google Scholar]
- 59.Peeters, M., and V. Courgnaud. 2002. Overview of primate lentiviruses and their evolution of non-human primates in Africa, p. 2-23. In C. Kuiken, B. Foley, E. Freed, B. Hahn, B. Korber, P. A. Marx, F. McCutchan, J. W. Mellors, and S. Wolinsky (ed.), HIV Sequence Compendium 2002. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.M.
- 60.Peeters, M., V. Courgnaud, B. Abela, P. Auzel, X. Pourrut, F. Bibollet-Ruche, S. Loul, F. Liegeois, C. Butel, D. Koulagna, E. Mpoudi-Ngole, G. M. Shaw, B. H. Hahn, and E. Delaporte. 2002. Risk to human health from a plethora of simian immunodeficiency viruses in primate bushmeat. Emerg. Infect. Dis. 8:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peeters, M., W. Janssens, K. Fransen, J. Brandful, L. Heyndrickx, K. Koffi, E. Delaporte, P. Piot, G. M. Gershy-Damet, and G. van der Groen. 1994. Isolation of simian immunodeficiency viruses from two sooty mangabeys in Cote d'Ivoire: virological and genetic characterization and relationship to other HIV type 2 and SIVsm/mac strains. AIDS Res. Hum. Retrovir. 10:1289-1294. [DOI] [PubMed] [Google Scholar]
- 62.Puffer, B. A., L. J. Parent, J. W. Wills, and R. C. Montelaro. 1997. Equine infectious anemia virus utilizes a YXXL motif within the late assembly domain of the Gag p9 protein. J. Virol. 71:6541-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rey-Cuille, M. A., J. L. Berthier, M. C. Bomsel-Demontoy, Y. Chaduc, L. Montagnier, A. G. Hovanessian, and L. A. Chakrabarti. 1998. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J. Virol. 72:3872-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roswell, T. E. 1988. The social system of guenons, compared with baboons, macaques, and mangabeys, p. 439-451. In A. Gautier-Hion, F. Bourliere, J.-P. Gautier, and J. Kingdon (ed.), A primate radiation: evolutionary biology of the African guenons. Cambridge University Press, Cambridge, United Kingdom.
- 65.Santiago, M. L., F. Bibollet-Ruche, E. Bailes, S. Kamenya, M. N. Muller, M. Lukasik, A. E. Pusey, D. A. Collins, R. W. Wrangham, J. Goodall, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 2003. Amplification of a complete simian immunodeficiency virus genome from fecal RNA of a wild chimpanzee. J. Virol. 77:2233-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santiago, M. L., F. Bibollet-Ruche, N. Gross-Camp, A. C. Majewski, M. Masozera, I. Munanura, B. A. Kaplin, G. M. Shaw, and B. H. Hahn. 2003. Non-invasive detection of simian immunodeficiency virus infection in a wild-living L'Hoest’s monkey (Cercopithecus lhoesti). AIDS Res. Hum. Retrovir. 19:1163-1166. [DOI] [PubMed] [Google Scholar]
- 67.Santiago, M. L., C. M. Rodenburg, S. Kamenya, F. Bibollet-Ruche, F. Gao, E. Bailes, S. Meleth, S. J. Soong, J. M. Kilby, Z. Moldoveanu, B. Fahey, M. N. Muller, A. Ayouba, E. Nerrienet, H. M. McClure, J. L. Heeney, A. E. Pusey, D. A. Collins, C. Boesch, R. W. Wrangham, J. Goodall, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 2002. SIVcpz in wild chimpanzees. Science 295:465.. [DOI] [PubMed] [Google Scholar]
- 68.Sharp, P. M., E. Bailes, F. Gao, B. E. Beer, V. M. Hirsch, and B. H. Hahn. 2000. Origins and evolution of AIDS viruses: estimating the time-scale. Biochem. Soc. Trans. 28:275-282. [DOI] [PubMed] [Google Scholar]
- 69.Soares, M. A., D. L. Robertson, H. Hui, J. S. Allan, G. M. Shaw, and B. H. Hahn. 1997. A full length replication-competent proviral clone of SIVAGM from tantalus monkeys. Virology 228:394-399. [DOI] [PubMed] [Google Scholar]
- 70.Souquiere, S., F. Bibollet-Ruche, D. L. Robertson, M. Makuwa, C. Apetrei, R. Onanga, C. Kornfeld, J. C. Plantier, F. Gao, K. Abernethy, L. J. White, W. Karesh, P. Telfer, E. J. Wickings, P. Mauclere, P. A. Marx, F. Barre-Sinoussi, B. H. Hahn, M. C. Muller-Trutwin, and F. Simon. 2001. Wild Mandrillus sphinx are carriers of two types of lentivirus. J. Virol. 75:7086-7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Strack, B., A. Calistri, S. Craig, E. Popova, and H. G. Gottlinger. 2003. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114:689-699. [DOI] [PubMed] [Google Scholar]
- 72.Takehisa, J., Y. Harada, N. Ndembi, I. Mboudjeka, Y. Taniguchi, C. Ngansop, S. Kuate, L. Zekeng, K. Ibuki, T. Shimada, B. Bikandou, Y. Yamaguchi-Kabata, T. Miura, M. Ikeda, H. Ichimura, L. Kaptue, and M. Hayami. 2001. Natural infection of wild-born mandrills (Mandrillus sphinx) with two different types of simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 17:1143-1154. [DOI] [PubMed] [Google Scholar]
- 73.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W—improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tosi, A. J., T. R. Disotell, J. C. Morales, and D. J. Melnick. 2003. Cercopithecine Y-chromosome data provide a test of competing morphological evolutionary hypotheses. Mol. Phylogenet. Evol. 27:510-521. [DOI] [PubMed] [Google Scholar]
- 75.Tsujimoto, H., A. Hasegawa, N. Maki, M. Fukasawa, T. Miura, S. Speidel, R. W. Cooper, E. N. Moriyama, T. Gojobori, and M. Hayami. 1989. Sequence of a novel simian immunodeficiency virus from a wild-caught African mandrill. Nature 341:539-541. [DOI] [PubMed] [Google Scholar]
- 76.Vanden Haesevelde, M. M., M. Peeters, G. Jannes, W. Janssens, G. van der Groen, P. M. Sharp, and E. Saman. 1996. Sequence analysis of a highly divergent HIV-1-related lentivirus isolated from a wild captured chimpanzee. Virology 221:346-350. [DOI] [PubMed] [Google Scholar]
- 77.Van Dooren, S., W. M. Switzer, W. Heneine, P. Goubau, E. Verschoor, B. Parekh, W. De Meurichy, C. Furley, M. Van Ranst, and A. M. Vandamme. 2002. Lack of evidence for infection with simian immunodeficiency virus in bonobos. AIDS Res. Hum. Retrovir. 18:213-216. [DOI] [PubMed] [Google Scholar]
- 78.van Rensburg, E. J., S. Engelbrecht, J. Mwenda, J. D. Laten, B. A. Robson, T. Stander, and G. K. Chege. 1998. Simian immunodeficiency viruses (SIVs) from eastern and southern Africa: detection of a SIVagm variant from a chacma baboon. J. Gen. Virol. 79:1809-1814. [DOI] [PubMed] [Google Scholar]
- 79.Verschoor, E. J., Z. Fagrouch, I. Bontjer, H. Niphuis, and J. L. Heeney. 2004. A novel simian immunodeficiency virus isolated from a Schmidt's guenon (Cercopithecus ascanius schmidti). J. Gen. Virol. 85:21-24. [DOI] [PubMed] [Google Scholar]
- 80.von Schwedler, U. K., M. Stuchell, B. Muller, D. M. Ward, H. Y. Chung, E. Morita, H. E. Wang, T. Davis, G. P. He, D. M. Cimbora, A. Scott, H. G. Krausslich, J. Kaplan, S. G. Morham, and W. I. Sundquist. 2003. The protein network of HIV budding. Cell 114:701-713. [DOI] [PubMed] [Google Scholar]
- 81.Wei, P., M. E. Garber, S. M. Fang, W. H. Fischer, and K. A. Jones. 1998. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92:451-462. [DOI] [PubMed] [Google Scholar]
- 82.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 83.Wyatt, R., J. Moore, M. Accola, E. Desjardin, J. Robinson, and J. Sodroski. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J. Virol. 69:5723-5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang, Z. 1993. Maximum likelihood estimation of phylogeny from DNA sequences when substitution rates differ over sites. Mol. Biol. Evol. 10:1396-1401. [DOI] [PubMed] [Google Scholar]
- 85.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]