Abstract
Managing oil spill residues washing onto sandy beaches is a common worldwide environmental problem. In this study, we have analyzed the first-arrival oil spill residues collected from two Gulf of Mexico (GOM) beach systems following two recent oil spills: the 2014 Galveston Bay (GB) oil spill, and the 2010 Deepwater Horizon (DWH) oil spill. This is the first study to provide field observations and chemical characterization data for the 2014 GB oil spill. Here we compare the physical and chemical characteristics of GB oil spill samples with DWH oil spill samples and present their similarities and differences. Our field observations indicate that both oil spills had similar shoreline deposition patterns; however, their physical and chemical characteristics differed considerably. We highlight these differences, discuss their implications, and interpret GB data in light of lessons learned from previously published DWH oil spill studies. These analyses are further used to assess the long-term fate of GB oil spill residues and their potential environmental impacts.
Introduction
On March 22, 2014, on the weekend of the 25th anniversary of the catastrophic Exxon Valdez oil spill in Alaska, the bulk carrier M/V Summer Wind collided with the oil barge Kirby, near Texas City, about 50 km southeast of Houston, Texas. The accident released approximately 168,000 gallons of marine fuel oil (known as RMG-380, a highly viscous, sticky, heavy black oil) into Galveston Bay (GB). After the accident, oil residues began washing up on several beaches along GB. The oil spill also spread into the Gulf of Mexico (GOM) and within a week oil was rapidly transported by shoreline currents to the Matagorda Island Wildlife Management region, located about 200 km south of GB. By the end of March, overflight observers noted beached oil being rapidly buried under clean sand near Matagorda Island [1]. Unfortunately, oil spill incidents like these occur in GB on a regular basis: according to the Texas General Land Office, 3,954 oil spills occurred in GB between 1998 and 2010 [2].
Oil spill residues washing onto shores is a common problem for many northern GOM beach systems. In 2010, the Deepwater Horizon (DWH) accident released about 210 million gallons of Louisiana light sweet crude oil into the GOM impacting over 1,600 miles of shoreline, and depositing oil on Florida, Alabama, Mississippi, Louisiana and Texas beaches. Negative environmental, ecological, social, and economic consequences of this event continue today [3–8]. Impacted beaches and dunes, estuaries, and tidal brackish and freshwater wetlands and the numerous species inhabiting them were, and remain, at risk of long-term detrimental effects as a result the spill [3–6,9,10].
The amount of oil released during the GB accident was relatively small compared to the DWH accident. The physicochemical characteristics of these two oils are also different. Fuel oil released during the GB spill was a heavy, viscous, refined fluid containing low levels of volatile hydrocarbons, while oil released during the DWH accident (MC252 crude oil) was an unrefined, low viscosity, sweet crude enriched in light, volatile hydrocarbons. Another major difference between the two events is that the GB spill was a surface release discharged about a kilometer away from the nearest shoreline; while the DWH event occurred about 75 km from the nearest shoreline, about 1.5 km under water. Uniquely, large volumes of chemical dispersants were also injected subsurface during the DWH spill response. Owing to its proximity to the shoreline, GB oil weathered in marine waters for only a few hours to days before being deposited on nearby beaches. Also, the GB spill occurred close to several sensitive wildlife areas during breeding season of migratory birds and marine species. DWH oil, on the other hand, was weathered by ocean-scale processes such as volatilization, dissolution, emulsification, photo-degradation and/or biodegradation for 3 or more weeks before being deposited on northern GOM shorelines. Table 1 summarizes some of the key features of these two oil spills.
Table 1. Comparisons of Galveston Bay and Deepwater Horizon oil spills.
| Galveston Bay Oil Spill | Deepwater Horizon Oil Spill | |
|---|---|---|
| API at 15°C | ∼ 11 [43] | ∼ 35 [44] |
| Viscosity (cSt) | ∼ 380 at 50°C [43] | ∼5 at 40°C [45] |
| Volume of the spill | ∼168,000 gallons | ∼210 million gallons |
| Type of oil | Refined marine fuel oil | Unrefined Louisiana sweet crude oil |
| Type of accident | Vessels collision | Explosion of oil rig |
| Type of spill | Tanker release | Well head release |
| Point of release | Surface oil spill | Subsurface oil spill, ∼1.5 km below sea |
| Spill location | ∼ 1 km away from beaches | ∼70 km away from beaches |
| Weathering patterns | Fate of remnant oil is yet to be studied | Fate of remnant oil has been studied for ∼4 years [7,8,10,15,16,19,38,46,47] |
The objective of this study is to compare observational and chemical characterization data of first-arrival oil samples collected from GB and DWH spill-impacted beaches. Chemical characterization efforts included the measurement of concentrations of n-alkanes, several biomarkers, five groups of alkylated polycyclic aromatic hydrocarbons (PAHs) and seventeen other PAHs. Biomarker data for the GB oil presented in this study are important since they can be used for identifying and differentiating GB residue from oil residues from other sources, and are also useful for understanding weathering levels. PAH data are useful for comparing and quantifying potential long-term environmental impacts of GB and DWH oil spill residues.
Materials and Methods
Organic solvents used in this study were of analytical or higher grade and were purchased from VWR International (Suwanee, GA). Silica gel (60–200 μm) and anhydrous sodium sulfate (ACS grade) were also purchased from VWR International. Prior to use, silica gel was activated using well-established procedures [11]. C8-C40 alkanes, pristane and phytane mixtures and hexadecane-d34 were purchased from Sigma-Aldrich. Biomarkers, namely C30αβ-hopane (17α(H),21β(H)-hopane), C27ααα(R)-sterane (5α,14α,17α(H) cholestane 20R), and C30ββ-hopane (17β(H),21β(H)-hopane) were purchased form Chiron, Norway. The PAH reference standard consisting of 27 different PAHs (naphthalene, 1-methylnaphthalene, 2-methylnaphthalene, 2,6-dimethylnaphthalene, 2,3,5-trimethylnaphthalene, biphenyl, acenaphthylene, acenaphthene, fluorene, phenanthrene, 1-methylphenanthrene, anthracene, dibenzothiophene, fluoranthene, pyrene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene, benzo[e]pyrene, benzo[a]pyrene, perylene, dibenz[a,c]anthracene, dibenz[a,h]anthracene, indeno[1,2,3,-cd]pyrene and benzo[ghi]perylene) was purchased from Agilent (Wilmington, DE). The reference solution for p-terphenyl-d14 was purchased from AccuStandard (New Haven, CT).
The oil spill samples recovered from GB beaches contained sand and other inorganics, and the organic fraction in the sample was estimated to be 65% (w/w) using a standard dichloromethane extraction procedure [12]. The DWH oil spill sample was free of sand and other residues and it fully dissolved in dichloromethane. For biomarker and PAH quantitative assessments, about 20 mg of GB or DWH sample was dissolved in hexane and prepared using a column chromatographic fractionation method [10]. The hexane fraction (F1) was used for n-alkanes and biomarker analysis, and the hexane:dichloromethane (50%, v/v) fraction (F2) was used for PAH analysis. Each sample was prepared in duplicate and analyzed in triplicate.
Both F1 and F2 elutes were analyzed using an Agilent 7890 GC equipped with Agilent 7000B QqQ mass spectrometer detector. Single ion monitoring (SIM) mode was used for F1 analysis with a m/z value of 57 for n-alkanes [13], m/z of 78 for hexadecane-d34, m/z of 191 for hopanes and m/z of 217 for steranes [12]. The five groups of alkylated-PAH homologs and seventeen other PAHs in the F2 fraction were analyzed using SIM and multiple reaction monitoring (MRM) methods, respectively, following previously established analytical approaches [10,14].
Quantification of n-alkanes was achieved by integrating all major chromatographic peaks of n-alkanes observed at the target ion m/z of 57. Hexadecane-d34 was used as the internal standard. The total concentrations of hopanes and steranes were quantified by integrating appropriate peak areas of chromatograms observed at m/z 191 (retention time from 37 to 46 minutes) and m/z 217 (retention time from 32 to 40 minutes), respectively. The reference standards used for quantification were C30αβ-hopane for hopanes, and C27ααα(R)-sterane for steranes. C30ββ-hopane was used as an internal standard to normalize the response factors used for estimating total hopanes and steranes. Based on available alkylated PAH standards, five groups of alkylated PAHs were quantified in this study using previously developed methods [10,11]. The analytical standards used for quantifying various PAHs within these five groups were as follows: in Group-1, naphthalene was used for quantifying C0-naphthalene; 2-methylnaphthalene for C1-naphthalenes; 2,6-dimethylnaphthalene for C2-naphthalenes; and 2,3,5-trimethylnaphthalene for C3- and C4-naphthalenes. In Group-2, phenanthrene was used for C0-phenanthrene, and 1-methylphenanthrene for C1- to C4-phenanthrenes. In Group-3, dibenzothiophene was used for C0- to C3-dibenzothiophenes. In Group-4, fluorene was used for C0- to C3-fluorenes. In Group-5, chrysene was used for C0- to C4-chrysenes. Seventeen other PAHs were also quantified in this study, which included biphenyl, acenaphthylene, acenaphthene, anthracene, fluoranthene, pyrene, benz[a]anthracene, benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene, benzo[e]pyrene, benzo[a]pyrene, perylene, dibenz[a,c]anthracene, dibenz[a,h]anthracene, indeno[1,2,3,-cd]pyrene and benzo[ghi]perylene. These compounds were quantified using the 27-PAH Agilent standard and previously published analytical procedures [10,15]. The internal standard p-terphenyl-d14 was used to normalize all PAH response factors.
Field Observations and Samples Collection
Fig. 1 shows the GB and DWH oil spill sites and our sampling locations. No specific permissions were required for sampling at these locations. Also, the field studies did not involve endangered or protected species. GPS coordinates for our DWH field site in Alabama are: 30°16'42.8"N 87°33'17.1"W; GPS coordinates for our GB field site in Texas are: 29°22'22.6"N 94°49'48.6"W. GB oil began washing on GB beaches within few hours after the spill on March 22, 2014. The GB samples analyzed in this study were collected on March 29, 2014, from an amenity beach located along the Texas City Dike road, about 2 km away from the spill location. The DWH oil first arrived on Alabama’s beaches in early June, 2010, about a month after the accident, and the samples were collected on June 11, 2010 from Orange Beach, Alabama, located about 175 km from oil release location. Further details on the DWH field site, observed contamination patterns, and field sampling methods are discussed in Hayworth et al. [8] and Mulabagal et al. [12]. Fig. 2 shows typical first-arrival oil deposition patterns observed at these two field sites. Although the overall deposition patterns appear similar, the physical characteristics of oil residues were distinctly different. The GB first-arrival oil was black/grayish, highly viscous material, while the DWH first-arrival oil was a brownish, low viscosity emulsion. On the day of sampling (June 11, 2010), DWH oil was actively washing ashore along most of Alabama’s 50 km sandy beach system and public access to these contaminated beaches was unrestricted. In contrast, on the day of GB oil sampling (March 29, 2014) oil was washing ashore only along a limited stretch of GB shoreline, and public access to these active deposition areas was restricted. Our GB oil spill sampling efforts were completed near a sparsely contaminated area, located about 2 km from the spill site, which had previously been cleaned and reopened for public use. Fig. 3 shows the field observations made at this site. Despite active clean-up efforts, the shoreline water along these “cleaned areas” had a strong petroleum odor, and the nearshore water had patches of floating oil sheen (see Fig. 3A). We also observed oil adhering to rocks, beached objects and vegetation (Fig. 3B & C). Furthermore, small blobs of oil (about 2 cm diameter; see Fig. 3D) were randomly scattered in the intertidal zone. During our sampling effort, we collected oil adhered to rocks and beached objects and also collected several beached oil blobs from the intertidal zone. These samples were shipped to our laboratory for chemical analysis.
Fig 1. Locations of the two oil spills and sampling points: a) Galveston Bay spill; and b) Deepwater Horizon spill (maps from OpenStreetMap).
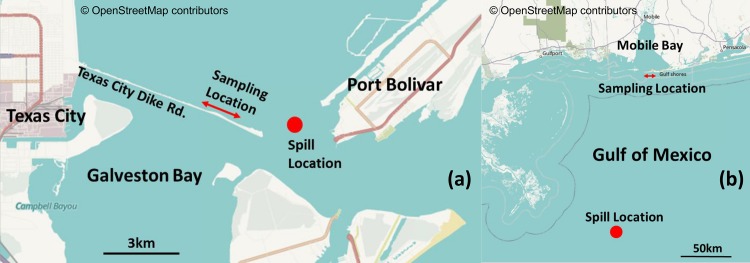
Fig 2. Comparison of Galveston Bay and Deepwater Horizon oil spill deposition patterns: a) blackish oily material deposited on a sandy beach in Galveston Bay, Texas (Photo taken on March 23rd, 2014, by NOAA's Office of Response and Restoration); b) brownish emulsified oil deposited on a sandy beach in Orange Beach, Alabama (Photo taken on June 11th, 2010, by Auburn University team).
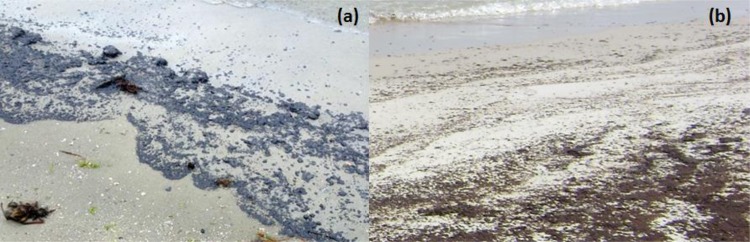
Fig 3. Field observation made at the Texas Dike road (Photographs taken on March 29th 2014, by Auburn University team): a) oil sheen observed in nearshore water; b) oil on a plastic sheet and rocks; c) oil on rocks and on a beached soccer ball and other objects; and d) beached oil blobs observed close to the waterline.
Results and Discussion
Chemical Characterization Data for n-Alkanes
Fig. 4 shows the n-alkane chromatograms (m/z 57) of GB and DWH oil spill residues. The chromatogram for GB residues indicates the presence of n-alkane compounds ranging from C13 to C29. In comparison, the n-alkane profile for DWH oil residue was relatively narrow indicating the presence of compounds ranging from C16 to C30, and the lighter alkanes were absent in this sample. From literature data we know that unweathered DWH crude oil contained a wide range of n-alkanes starting from C9 [16]. Therefore, absence of light n-alkanes (i.e., compounds below C16) in the DWH first-arrival sample is due to ocean-scale weathering effects. The DWH samples were recovered about 50 days after the accident. During this period, the oil traveled over 175 km in marine waters with ocean-scale weathering processes selectively depleting most of the light n-alkanes. In contrast, the GB samples were recovered seven days post-accident; the oil traveled only about 2–3 km and experienced very little natural weathering; hence, the relative distribution of light n-alkanes are expected to be high in these samples. Also, both residues were collected during a similar season (around spring) from beaches with similar water temperatures. Thus, residence time in the marine environment is likely the primary driver for oil evaporation, with temperature playing a minor role [17].
Fig 4. Comparison of extracted ion chromatograms of n-alkanes (m/z of 57) for Galveston Bay and Deepwater Horizon oil spill residues.
We also quantified n-alkane concentrations by integrating all major peaks for m/z 57, and the concentration levels for various n-alkanes ranging from C13 to C30 are presented in Fig. 5. Using the data shown in Fig. 5, the total amount (values reported as mean ± SD) of n-alkanes in GB and DWH samples are estimated to be 9 ± 1 and 37 ± 2 mg/g of oil, respectively. The total concentration of n-alkanes in GB residues is low since it is a highly refined fuel oil. The ratios of pristane/phytane, C17/pristane, and C18/phytane are often used for source identification [14]. Based on peak responses, the ratios of pristane/phytane, C17/pristane and C18/phytane were calculated as: 1.48 ± 0.04, 2.13 ± 0.04, and 3.21 ± 0.08 for GB sample, and 0.91 ± 0.01, 1.73 ± 0.01, and 2.84 ± 0.02 for DWH sample. These ratios are indicative of the differences in chemical characteristics of these two oils.
Fig 5. Concentration levels of various n-alkanes (ranging from C13 to C30) measured in Galveston Bay and Deepwater Horizon oil spill residues.
Chemical Characterization Data for Biomarker Compounds
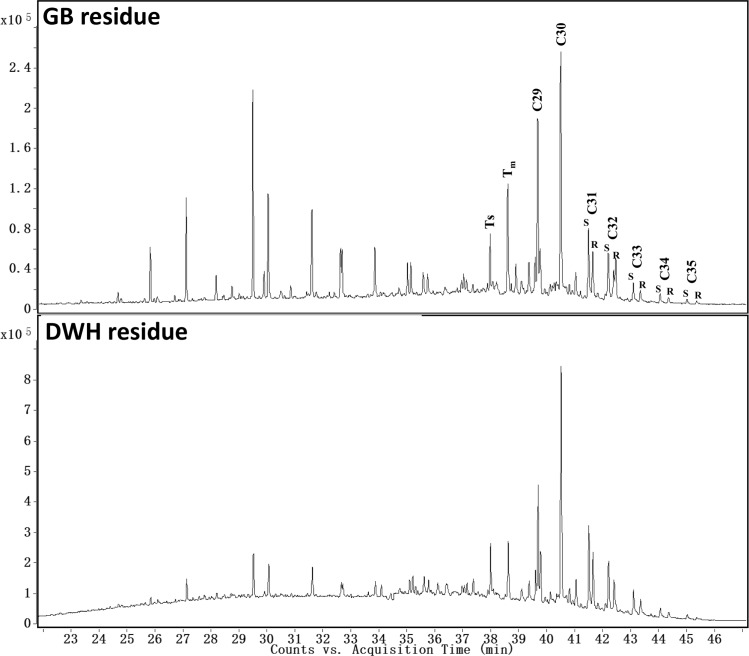
In this study we focused on the biomarker fingerprints of hopanes and steranes, which are the most widely used compounds for fingerprinting oil spill accidents [14,18]. Recently, Aeppli et al. [19] compared the fate of biomarkers in DWH oil spill residues and concluded that hopanes and steranes, quantified at m/z values of 191 and 217, respectively, are the most reliable signatures for fingerprinting DWH oil spill residues. Fig. 6 shows GC/MS chromatograms of hopanes (at m/z 191) present in GB and DWH residues. The total amount of hopanes in GB and DWH samples were estimated to be 380±30 mg/kg oil and 440±20 mg/kg oil, respectively. Analysis of hopane chromatograms show that in the DWH sample, hopane distribution ranged from C27 to C35 with C30αβ-hopane being the most abundant compound. The GB chromatogram, on the other hand, showed higher abundance of C29αβ and C30αβ hopanes; also, the response levels are higher for several other possible tricyclic or tetracyclic terpanes, yielding a wider fingerprint (see Fig. 6). It is well established that C30αβ-hopane is highly resistant to environmental weathering [14,19,20]; thus, the amount of C30αβ-hopane will increase over time, and this effect can be used to estimate the degree of weathering [12]. Furthermore, C30αβ-hopane can also be used as a recalcitrant internal biomarker for quantifying the degradation rates of other chemical compounds [20]. In this study, we estimated the concentrations of C30αβ-hopane in the GB residue as 81±6 mg/kg oil. The concentration of C30αβ-hopane in the DWH residue has already been reported in Mulabagal et al. [12] as 91±6 mg/kg oil. These concentration levels can be used as a starting point for understanding future weathering patterns of these oil residues.
Fig 6. Comparison of extracted ion chromatograms of hopanes (m/z of 191) for Galveston Bay and Deepwater Horizon oil spill residues.
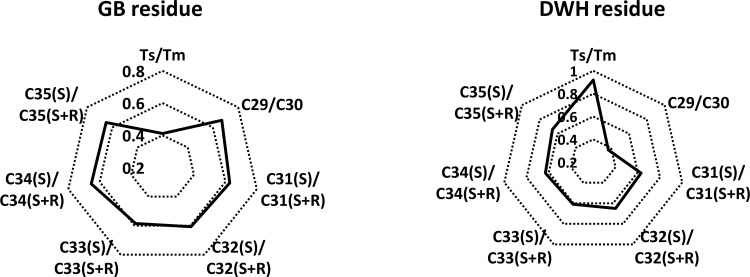
The diagnostic ratios of different types of hopanes can be used to identify and differentiate oil spill sources [12,14,18,21]. Various ratios including those of Ts/Tm, C29/C30, C31(S)/C31(S+R), C32(S)/C32(S+R), C33(S)/C33(S+R), C34(S)/C34(S+R) and C35(S)/C35(S+R) in GB and DWH residues are summarized in Table 2 (a portion of the hopane data for DWH oil are from our previous work [12]). These data are also presented as radar plots in Fig. 7; the plots reveal that unique fingerprint patterns exist for these two oils, and these patterns can be used to differentiate these two spills from other past or future oil spills.
Table 2. Hopane and sterane diagnostic ratios (mean ± SD) estimated for Galveston Bay and Deepwater Horizon oil spill residues.
| Diagnostic ratio | GB residue | DWH residue |
|---|---|---|
| Hopane ratio | ||
| Ts/Tm | 0.41±0.02 | 0.92±0.05 |
| C29/C30 | 0.67±0.03 | 0.37±0.01 |
| C31(S)/C31(S+R) | 0.63±0.01 | 0.63±0.01 |
| C32(S)/C32(S+R) | 0.61±0.02 | 0.65±0.01 |
| C33(S)/C33(S+R) | 0.59±0.02 | 0.61±0.01 |
| C34(S)/C34(S+R) | 0.65±0.02 | 0.63±0.01 |
| C35(S)/C35(S+R) | 0.65±0.04 | 0.65±0.03 |
| Sterane ratio | ||
| DiaC27βα(S)/ DiaC27βα(R) | 1.48±0.06 | 1.47±0.01 |
| C27αββ(R+S)/C29αββ(R+S) | 1.60±0.07 | 3.0±0.3 |
| C27αββ(R+S)/C27(αββ(R+S)+ ααα(S+R)) | 0.50±0.01 | 0.67±0.01 |
| C28αββ(R+S)/C28(αββ(R+S)+ ααα(S+R)) | 0.55±0.03 | 0.62±0.03 |
| C29αββ(R+S)/C29(αββ(R+S)+ ααα(S+R)) | 0.38±0.02 | 0.51±0.01 |
Fig 7. Radar plots of hopane diagnostic ratios of Galveston Bay and Deepwater Horizon oil spill residues.
The total steranes in GB and DWH samples were found to be 221±5 and 720±30 mg/kg oil, respectively. Similar to hopane data, sterane data can also be used for source identification. Fig. 8 shows the chromatograms of steranes (at m/z 217) for both GB and DWH residues. The data show that steranes in GB residue are dominated by several high molecular weight compounds (such as C29-steranes). We have identified several sterane peaks based on published data [22,23] and used them to compute various diagnostic ratios that are suggested in the literature [14,18,19]; these results are summarized in Table 2. The sterane dataset provides an additional line of evidence for identifying and differentiating other residues from these two oil spills.
Fig 8. Comparison of extracted ion chromatograms of steranes (m/z of 217) for Galveston Bay and Deepwater Horizon oil spill residues [Peak 1: DiaC27βα(S); Peak 2: DiaC27βα(R); Peak 3: C27ααα(S); Peak 4: C27αββ(R); Peak 5: C27αββ(S); Peak 6: C27ααα(R); Peak 7: C28ααα(S); Peak 8: C28αββ(R); Peak 9: C28αββ(S); Peak 10: C28ααα(R); Peak 11: C29ααα(S); Peak 12: C29αββ(R); Peak 13: C29αββ(S); Peak 14: C29ααα(R)].
Chemical Characterization Data for PAH Compounds
Fig. 9 presents PAH concentration levels measured in DWH and GB residues. In Table 3 we summarize these concentrations in terms of five groups of alkylated PAHs (namely naphthalenes, phenanthrenes, dibenzothiophenes, fluorenes and chrysenes) with their parents, and seventeen other PAHs. The extracted ion chromatograms used for quantifying the alkylated PAHs in the GB residue are shown in S1 Chromatogram, S2 Chromatogram, S3 Chromatogram, S4 Chromatogram, S5 Chromatogram. For the DWH sample, the total amount of PAHs was estimated to be 1,714 mg/kg oil. The data also show that the five groups of alkylated PAHs were the most dominant compounds and they accounted for about 95% of total PAHs. Among the five groups, phenanthrenes (Group-2) were the most abundant compounds in the DWH sample with a total concentration of 1,183 mg/kg oil (which is about 69% of total PAHs), followed by chrysenes (Group-5) with a total concentration of 178 mg/kg oil (which is 10% of total PAHs), fluorenes (Group-4) with a total concentration of 132 mg/kg oil (which is 8% of total PAHs), dibenzothiophenes (Group-3) with a total concentration of 98 mg/kg oil (which is 6% of total PAHs), and naphthalenes (Group-1) with a total concentration of 46 mg/kg oil (which is 3% of total PAHs). The total concentration of all other 3- to 6-ring parent PAHs was estimated to be 33 mg/kg oil; biphenyl was not detected in the DWH sample.
Fig 9. Concentration levels of various PAHs and alkylated PAH homologs measured in Deepwater Horizon and Galveston Bay oil spill residues.
Table 3. Summary of average PAH concentration levels measured in Deepwater Horizon and Galveston Bay oil spill residues (unit: mg/kg oil).
| Compound | DWH residue [10] | GB residue |
|---|---|---|
| Five groups of alkylated PAHs and their parents | ||
| Group-1: C0- to C4-naphthalenes | 46 | 3,699 |
| Group-2: C0- to C4-phenanthrenes | 1,183 | 5,119 |
| Group-3: C0- to C3-dibenzothiophenes | 98 | 345 |
| Group-4: C0- to C3-fluorenes | 132 | 1,117 |
| Group-5: C0- to C4-chrysenes | 178 | 1,751 |
| Sum of five groups of PAHs | 1,636 | 12,031 |
| Other seventeen PAHs | ||
| Biphenyl (2-ring) | - | 18 |
| Sum of 3 to 6-ring PAHs | 33 | 603 |
| Total amount of PAHs | 1,714 | 12,651 |
The total amount of PAHs measured in the GB sample was 12,651 mg/kg oil, which is about 7 times higher than the levels measured in the DWH sample (see Table 3). Similar to the DWH sample, PAHs in the GB sample were also dominated by the five groups of alkylated PAHs and accounted for about 95% of total PAHs. However, the relative distribution of various types of PAHs in the GB residue was different from the distribution observed for the DWH residue (see Fig. 9). More importantly, individual concentration levels of almost all the PAHs measured in the GB sample were much higher than the levels measured in the DWH sample. Interestingly, phenanthrenes are also the most abundant group of PAHs in GB residue and the total concentration of phenanthrenes (Group-2) estimated was 5,119 mg/kg oil (which is about 40% of total PAHs). This concentration level is about 4 times higher than the level measured in the DWH sample. The second dominant group of compounds are naphthalenes (Group-1) with a total concentration of 3,699 mg/kg oil (which accounted for about 29% of total PAHs); this level is about 80 times higher than the level observed in the DWH sample. The next group is chrysenes (Group-5) with a total concentration of 1,751 mg/kg oil (which is about 14% of total PAHs); followed by fluorenes (Group-4) with a total concentration of 1,117 mg/kg oil (which is about 9% of total PAHs); and dibenzothiophenes (Group-3) with a total concentration of 345 mg/kg oil (which accounted for about 3% of total PAHs). The concentration of biphenyl and the sum of other 3- to 6-ring PAHs were estimated to be 18 mg/kg oil and 603 mg/kg oil, respectively. Since GB residues were collected a short time after the spill, the concentration levels of light molecular weight PAHs, such as naphthalenes and biphenyl (volatile compounds that can easily evaporate during the early stage of a spill) were high, indicating the GB sample experienced very little weathering.
According to the Agency for Toxic Substances and Disease Registry [24], most heavy PAHs are either known or probable human carcinogens. For example, the 5-ring compound benzo[a]pyrene (BaP) has been shown to cause chromosomal replication errors, and can also affect human fertility levels. The concentration levels of BaP in GB and DWH samples were estimated to be 43 and 0.4 mg/kg oil, respectively. These data suggest that, in terms of BaP toxicity effects, GB residues will be substantially more toxic than DWH residues. Furthermore, concentrations of several alkylated PAHs in the GB residue were relatively high. Previous studies have shown that alkylated phenanthrenes, for example, can induce various types of ecological toxic effects in marine organisms [25–27]. Our data show that phenanthrene levels in the GB sample were approximately 4 times higher than the DWH sample, and these values were mostly dominated by alkylated phenanthrenes. These data also indicate that GB residues might be more toxic than DWH residues. Furthermore, recent studies have demonstrated that alkylated chrysenes in oil residues are likely to be recalcitrant for many years [10,28]. Emerging research has shown that although the measured aqueous solubility limits of individual alkylated chrysenes are very low, these chemicals could still induce chronic toxic effects in certain sensitive species, e.g., Japanese medaka embryos (Oryzias latipes) [29]. Additionally, studies have shown that the toxic effects of multiple PAHs present in oil spill residues can be additive [30]. Therefore, based on total PAHs alone, the much higher concentrations present in GB residues are likely to cause far more adverse effects to fishes and other marine species. However, since the toxicity of individual PAHs can vary significantly [31–34], better understanding of overall detrimental ecological effects associated with GB and DWH spills warrants further studies.
Conclusions
This is the first study that reports field observations and chemical characterization data for the 2014 GB oil spill and compares the results against observations made during another major spill (the DWH oil spill). Our data document the differences in weathering patterns of GB and DWH oil spill residues. When compared to DWH first-arrival oil residue, the GB first-arrival sample experienced much less weathering due to the proximity of the accident site to the shoreline. Furthermore, the environmental weathering characteristics of the heavy, highly refined GB fuel oil are vastly different from the characteristics of raw light, sweet crude oil released during the DWH spill. For example, heavy fuel oil (like the GB oil) is expected to have a very low evaporation rate [35]. In comparison, as documented in previous studies, the evaporation rate of DWH raw crude oil is very high and evaporation process alone likely removed over 40% of the DWH oil mass within a week of surface weathering [10,36].
The hopane fingerprinting data show that GB residue has a wider m/z 191 chromatogram and displays a distinctly different fingerprint compared to the DWH fingerprint. Interestingly, both GB and DWH residues had similar amounts of total hopanes; however, the relative ratios of various types of hopane were different, yielding distinctly different fingerprints. GB residue also showed distinctly different sterane fingerprints from the DWH residue; also, the total amount of sterane measured in the GB residue was about three times lower than the DWH residue.
DWH oil spill residues arriving along GOM beaches interacted with suspended sand in nearshore waters and formed submerged oil mats (SOMs; often called tar mats). SOMs are continuously worked by waves and other nearshore processes yielding surface residual oil balls (SRBs; often called tar balls). Field studies have shown that the presence of SRBs continue to be a problem along Florida and Alabama beaches more than four years after the DWH spill, especially after major storm events [8,12,37]. Also, PAHs and their post-spill derivatives (such as oxygenated species whose effects are yet to be determined), trapped in buried DWH oil spill residues, continue to pose serious environmental concerns [16,38–42]. For the GB spill, early predictions indicated that the oil would be carried by northeasterly winds out into the GOM, and onshore winds would deposit oil onto various beaches [35]. Later, overflight observations documented beached oil being rapidly buried under clean sand on Matagorda Island, located about 200 km away from the spill location [1]. Based on both predicted and observed oil spill trajectories, and also based on the prior knowledge gained from studying the DWH accident, GB oil should have formed SRBs containing heavy fuel oil that are potentially distributed along various beaches located to the southwest of the GB. Since GB residues contain much higher levels of PAHs, these SRBs could pose long-term environmental risks. However, the total amount of oil released from the GB spill is substantially low when compared to the DWH spill and hence the spatial extent of these impacts will likely be small. Managing oil spill impacts to beach systems is a significant environmental challenge, and it becomes more complex in systems that experience multiple spill events (such as the GB system). The biomarker and PAH datasets provided in this study are important baseline information for monitoring the long-term fate and the potential environmental impacts of the GB oil spill event.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported, in part, by funding received from the Alabama Emergency Management Agency (AEMA). The GC/MS/MS facility and the characterization methods were developed using grant funds received from the National Science Foundation (NSF-MRI grant #G00006697). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.NOAA (2014) Progress at the Texas City oil spill in Galveston Bay. http://usresponserestoration.wordpress.com/2014/03/28/progress-at-the-texas-city-y-oil-spill-in-galveston-bay/.
- 2.TGLO (2010) Galveston Bay status and trends. http://www.galvbaydata.org/WaterandSediment/OilSpills/tabid/218/Default.aspx.
- 3. White HK, Hsing PY, Cho W, Shank TM, Cordes EE, et al. (2012) Impact of the Deepwater Horizon oil spill on a deep-water coral community in the Gulf of Mexico. Proceedings of the National Academy of Sciences of the United States of America 109: 20303–20308. 10.1073/pnas.1118029109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCrea-Strub A, Kleisner K, Sumaila UR, Swartz W, Watson R, et al. (2011) Potential Impact of the Deepwater Horizon Oil Spill on Commercial Fisheries in the Gulf of Mexico. Fisheries 36: 332–336. [Google Scholar]
- 5. Fisher CR, Hsing PY, Kaiser CL, Yoerger DR, Roberts HH, et al. (2014) Footprint of Deepwater Horizon blowout impact to deep-water coral communities. Proceedings of the National Academy of Sciences of the United States of America 111: 11744–11749. 10.1073/pnas.1403492111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mason OU, Scott NM, Gonzalez A, Robbins-Pianka A, Baelum J, et al. (2014) Metagenomics reveals sediment microbial community response to Deepwater Horizon oil spill. Isme Journal 8: 1464–1475. 10.1038/ismej.2013.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hayworth JS, Clement TP, Valentine JF (2011) Deepwater Horizon oil spill impacts on Alabama beaches. Hydrology and Earth System Sciences 15: 3639–3649. [Google Scholar]
- 8. Hayworth JS, Clement TP, John GF, Yin F (2015) Fate of Deepwater Horizon Oil in Alabama's Beach System: Conceptual Framework for Physical Evolution Based on Observational Data. Marine Pollution Bulletin 90: 95–105. 10.1016/j.marpolbul.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 9. Martinez ML, Feagin RA, Yeager KM, Day J, Costanza R, et al. (2012) Artificial modifications of the coast in response to the Deepwater Horizon oil spill: quick solutions or long-term liabilities? Frontiers in Ecology and the Environment 10: 44–49. [Google Scholar]
- 10. Yin F, John GF, Hayworth JS, Clement TP (2015) Long-term monitoring data to describe the fate of polycyclic aromatic hydrocarbons in Deepwater Horizon oil submerged off Alabama's beaches. Science of The Total Environment 508: 46–56. 10.1016/j.scitotenv.2014.10.105 [DOI] [PubMed] [Google Scholar]
- 11. Wang ZD, Fingas M, Li K (1994) Fractionation of a Light Crude-Oil and Identification and Quantitation of Aliphatic, Aromatic, and Biomarker Compounds by GC-FID and GC-MS. 1. Journal of Chromatographic Science 32: 361–366. [Google Scholar]
- 12. Mulabagal V, Yin F, John GF, Hayworth JS, Clement TP (2013) Chemical fingerprinting of petroleum biomarkers in Deepwater Horizon oil spill samples collected from Alabama shoreline. Marine Pollution Bulletin 70: 147–154. 10.1016/j.marpolbul.2013.02.026 [DOI] [PubMed] [Google Scholar]
- 13. Smith LL, Strickland JR (2007) Improved GC/MS method for quantitation of n-alkanes in plant and fecal material. Journal of Agricultural and Food Chemistry 55: 7301–7307. [DOI] [PubMed] [Google Scholar]
- 14. Wang ZD, Fingas M, Page DS (1999) Oil spill identification. Journal of Chromatography A 843: 369–411. [Google Scholar]
- 15. Xia K, Hagood G, Childers C, Atkins J, Rogers B, et al. (2012) Polycyclic Aromatic Hydrocarbons (PAHs) in Mississippi Seafood from Areas Affected by the Deepwater Horizon Oil Spill. Environmental Science & Technology 46: 5310–5318. 10.1016/j.scitotenv.2015.01.041 [DOI] [PubMed] [Google Scholar]
- 16. Liu ZF, Liu JQ, Zhu QZ, Wu W (2012) The weathering of oil after the Deepwater Horizon oil spill: insights from the chemical composition of the oil from the sea surface, salt marshes and sediments. Environmental Research Letters 7 23284587 [Google Scholar]
- 17. Fingas MF (1997) Studies on the evaporation of crude oil and petroleum products: I. the relationship between evaporation rate and time. Journal of Hazardous Materials 56: 227–236. [Google Scholar]
- 18. Wang ZD, Fingas MF (2003) Development of oil hydrocarbon fingerprinting and identification techniques. Marine Pollution Bulletin 47: 423–452. [DOI] [PubMed] [Google Scholar]
- 19. Aeppli C, Nelson RK, Radović JR, Carmichael CA, Valentine DL, et al. (2014) Recalcitrance and Degradation of Petroleum Biomarkers upon Abiotic and Biotic Natural Weathering of Deepwater Horizon Oil. Environmental Science & Technology 48: 6726–6734. 10.1021/es500825q [DOI] [PubMed] [Google Scholar]
- 20. Douglas GS, Bence AE, Prince RC, McMillen SJ, Butler EL (1996) Environmental stability of selected petroleum hydrocarbon source and weathering ratios. Environmental Science & Technology 30: 2332–2339. [Google Scholar]
- 21. Yim UH, Ha SY, An JG, Won JH, Han GM, et al. (2011) Fingerprint and weathering characteristics of stranded oils after the Hebei Spirit oil spill. Journal of Hazardous Materials 197: 60–69. 10.1016/j.jhazmat.2011.09.055 [DOI] [PubMed] [Google Scholar]
- 22. Peters K, Walters C, Moldowan J (2004) The biomarker guide: Volume 1, Biomarkers and isotopes in the environment and human history: Cambridge university press; [Google Scholar]
- 23. Rosenbauer RJ, Campbell PL, Lam A, Lorenson TD, Hostettler FD, et al. (2010) Reconnaissance of Macondo-1 well oil in sediment and tarballs from the Northern Gulf of Mexico shoreline, Texas to Florida: U. S. Geological Survey; [Google Scholar]
- 24.ATSDR (1995) Chemical and physical information, in: Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs). Agency for Toxic Substances and Disease Registry. [PubMed]
- 25. Fallahtafti S, Rantanen T, Brown RS, Snieckus V, Hodson PV (2012) Toxicity of hydroxylated alkyl-phenanthrenes to the early life stages of Japanese medaka (Oryzias latipes). Aquatic Toxicology 106: 56–64. 10.1016/j.aquatox.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 26. Turcotte D, Akhtar P, Bowerman M, Kiparissis Y, Brown RS, et al. (2011) Measuring the Toxicity of Alkyl-Phenanthrenes to Early Life Stages of Medaka (Oryzias Latipes) Using Partition-Controlled Delivery. Environmental Toxicology and Chemistry 30: 487–495. 10.1002/etc.404 [DOI] [PubMed] [Google Scholar]
- 27. Meador JP, Buzitis J, Bravo CF (2008) Using fluorescent aromatic compounds in bile from juvenile salmonids to predict exposure to polycyclic aromatic hydrocarbons. Environmental Toxicology and Chemistry 27: 845–853. 10.1897/07-434.1 [DOI] [PubMed] [Google Scholar]
- 28. Wang ZD, Fingas M, Sergy G (1994) Study of 22-Year-Old Arrow Oil Samples Using Biomarker Compounds by GC/MS. Environmental Science & Technology 28: 1733–1746. 10.1016/j.scitotenv.2015.01.041 [DOI] [PubMed] [Google Scholar]
- 29.Lin H, Morandi GD, Brown RS, Snieckus V, Rantanen T, et al. (2015) Quantitative structure-activity relationships for chronic toxicity of alkyl-chrysenes and alkyl-benz[a]anthracenes to Japanese medaka embryos (Oryzias latipes). Aquatic Toxicology. In press. [DOI] [PubMed]
- 30. Barata C, Calbet A, Saiz E, Ortiz L, Bayona JM (2005) Predicting single and mixture toxicity of petrogenic polycyclic aromatic hydrocarbons to the copepod Oithona davisae. Environmental Toxicology and Chemistry 24: 2992–2999. [DOI] [PubMed] [Google Scholar]
- 31. Bellas J, Saco-Alvarez L, Nieto O, Beiras R (2008) Ecotoxicological evaluation of polycyclic aromatic hydrocarbons using marine invertebrate embryo-larval bioassays. Marine Pollution Bulletin 57: 493–502. 10.1016/j.marpolbul.2008.02.039 [DOI] [PubMed] [Google Scholar]
- 32. Di Toro DM, McGrath JA, Stubblefield WA (2007) Predicting the toxicity of neat and weathered crude oil: Toxic potential and the toxicity of saturated mixtures. Environmental Toxicology and Chemistry 26: 24–36. [DOI] [PubMed] [Google Scholar]
- 33. Incardona JP, Gardner LD, Linbo TL, Brown TL, Esbaugh AJ, et al. (2014) Deepwater Horizon crude oil impacts the developing hearts of large predatory pelagic fish. Proc Natl Acad Sci U S A 111: E1510–1518. 10.1073/pnas.1320950111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Incardona JP, Vines CA, Linbo TL, Myers MS, Sloan CA, et al. (2012) Potent Phototoxicity of Marine Bunker Oil to Translucent Herring Embryos after Prolonged Weathering. Plos One 7 10.1371/journal.pone.0051204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.NOAA (2014) Kirby Barge Oil Spill, Houston/Texas City Ship Channel, Port Bolivar, Texas. http://response.restoration.noaa.gov/oil-and-chemical-spills/oil-spills/kirby-barge-oil-spill-houstontexas-city-ship-channel-port-bolivar.
- 36. John GF, Yin F, Mulabagal V, Hayworth JS, Clement TP (2014) Development and application of an analytical method using gas chromatography/triple quadrupole mass spectrometry for characterizing alkylated chrysenes in crude oil samples. Rapid Commun Mass Spectrom 28: 948–956. 10.1002/rcm.6868 [DOI] [PubMed] [Google Scholar]
- 37.Plant NG, Long JW, Dalyander PS, Thompson DM, Raabe EA (2013) Application of a hydrodynamic and sediment transport model for guidance of response efforts related to the Deepwater Horizon oil spill in the Northern Gulf of Mexico along the coast of Alabama and Florida: U.S. Geological Survey Open-File Report 2012–1234, 46 p.
- 38. Aeppli C, Carmichael CA, Nelson RK, Lemkau KL, Graham WM, et al. (2012) Oil Weathering after the Deepwater Horizon Disaster Led to the Formation of Oxygenated Residues. Environmental Science & Technology 46: 8799–8807. 10.1016/j.scitotenv.2015.01.041 [DOI] [PubMed] [Google Scholar]
- 39. Dubansky B, Whitehead A, Miller JT, Rice CD, Galvez F (2013) Multitissue molecular, genomic, and developmental effects of the Deepwater Horizon oil spill on resident Gulf killifish (Fundulus grandis). Environmental Science & Technology 47: 5074–5082. 10.1016/j.scitotenv.2015.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sammarco PW, Kolian SR, Warby RAF, Bouldin JL, Subra WA, et al. (2013) Distribution and concentrations of petroleum hydrocarbons associated with the BP/Deepwater Horizon Oil Spill, Gulf of Mexico. Marine Pollution Bulletin 73: 129–143. 10.1016/j.marpolbul.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 41. Ruddy BM, Huettel M, Kostka JE, Lobodin VV, Bythell BJ, et al. (2014) Targeted Petroleomics: Analytical Investigation of Macondo Well Oil Oxidation Products from Pensacola Beach. Energy & Fuels 28: 4043–4050. 10.3928/02793695-20141218-04 [DOI] [PubMed] [Google Scholar]
- 42. Zuijdgeest A, Huettel M (2012) Dispersants as Used in Response to the MC252-Spill Lead to Higher Mobility of Polycyclic Aromatic Hydrocarbons in Oil-Contaminated Gulf of Mexico Sand. Plos One 7 10.1371/journal.pone.0051204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vermeire MB (2012) Everything You Need to Know About Marine Fuels. Published by Chevron Global Marine Products.
- 44. Atlas RM, Hazen TC (2011) Oil Biodegradation and Bioremediation: A Tale of the Two Worst Spills in US History. Environmental Science & Technology 45: 6709–6715. 10.1016/j.scitotenv.2015.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Somasundaran P, Patra P, Farinato RS, Papadopoulos K (2014) Oil Spill Remediation: Colloid Chemistry-based Principles and Solutions. pp.302.
- 46.OSAT-3 (2014) Investigation of Recurring Residual Oil in Discrete Shoreline Areas in the Eastern Area of Responsibility Operational Science Advisory Team, Unified Area Command.
- 47. Radovic JR, Aeppli C, Nelson RK, Jimenez N, Reddy CM, et al. (2014) Assessment of photochemical processes in marine oil spill fingerprinting. Marine Pollution Bulletin 79: 268–277. 10.1016/j.marpolbul.2013.11.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper.