Abstract
Vaccination remains the most effective public health tool to prevent infectious diseases. Many vaccines are marginally effective and need enhancement for immunocompromised, elderly, and very young populations. To enhance immunogenicity, we exploited the biphasic property of the (RADA)4 synthetic oligopeptide to create VacSIM (vaccine self-assembling immune matrix), a new delivery method. VacSIM solution can easily be mixed with antigens, organisms, and adjuvants for injection. Postinjection, the peptides self-assemble into hydrated nanofiber gel matrices, forming a depot with antigens and adjuvants in the aqueous phase. We believe the depot provides slow release of immunogens, leading to increased activation of antigen-presenting cells that then drive enhanced immunogenicity. Using recombinant hepatitis B virus surface antigen (rHBsAg) as a model immunogen, we compared VacSIM delivery to delivery in alum or complete Freund's adjuvant (CFA). Delivery of the rHBsAg antigen to mice via VacSIM without adjuvant elicited higher specific IgG responses than when rHBsAg was delivered in alum or CFA. Evaluating IgG subtypes showed a mixed Th1/Th2 type response following immunization with VacSIM, which was driven further toward Th1 with addition of CpG as the adjuvant. Increased specific IgG endpoint titers were observed in both C57BL/6 and BALB/c mice, representative of Th1 and Th2 environments, respectively. Restimulation of splenocytes suggests that VacSIM does not cause an immediate proinflammatory response in the host. Overall, these results suggest that VacSIM, as a new delivery method, has the potential to enhance immunogenicity and efficacy of numerous vaccines.
INTRODUCTION
Vaccines remain the single greatest public health tool to combat infectious diseases. Vaccine formulation and delivery are key to the ability of vaccines to induce the desired immune responses. One goal of vaccine delivery is to present vaccine antigens in a manner that enhances antigen-presenting-cell (APC) activation, leading to antigen/organism uptake and processing of vaccine antigen(s). Delivery methods or adjuvants that safely enhance vaccine immunogenicity/efficacy are desirable for vaccines that are marginally effective and for vaccines administered to low responders or immunocompromised populations. Additional goals are to reduce the number of doses required to induce effective, vaccine responses and to reduce the amount of vaccine/dose, especially when a single dose of vaccine is administered, as with annual influenza vaccines. Lastly, in pandemics, a vaccine that produces high titers after a single administration would be beneficial. Recent advances in the understanding of how innate mechanisms influence adaptive immunity have led to more rational design in the development of new vaccine adjuvants and delivery systems.
Aluminum salts were the first adjuvants licensed for human vaccines in the 1920s. The licensure of non-aluminum salt adjuvants took an additional 80 years (1). One reason for this long gap is that the principles of adjuvant activity were largely unknown; thus, the development of adjuvants was empirical, Moreover, many adjuvants, including Freund's adjuvant, were reactogenic and not acceptable for licensure (2). Recent methods to improve vaccine delivery have taken several approaches, including the use of virosomes (3–5), vector-based methods (6–8), liposome-based methods (9–11), and the use of more traditional formulation with adjuvants (12–17). Each of these methods has some drawbacks, in terms of reactogenicity, regulatory issues, product stability, or time required for formulation; however, each of these methods focuses on presenting the vaccine as a particulate.
To enhance vaccine immunogenicity over that seen when conventional alum-based delivery methods are used, we focused on identifying ways to deliver vaccines such that vaccine antigens are released over time. This led to our development of a new vaccine delivery method we call VacSIM (vaccine self-assembling immune matrix). VacSIM is based on the unique properties of the (RADA)4 synthetic oligopeptide and other biopolymers (18–22). The (RADA)4 synthetic oligopeptide was created by Zhang (22) and commercialized by 3-D Matrix Inc. for cell scaffolding and is currently in clinical trials for wound healing (PuraStat), tissue repair (23), and dental implant scaffolding (PuraMatrix) (24). As such, it has already undergone third-party reactogenicity and toxicity testing (25). VacSIM is composed solely of the (RADA)4 synthetic oligopeptide. Thus, it is biocompatible and biodegradable. Ex vivo, a 1.0% VacSIM solution is in liquid phase, resulting in the flexibility to mix virtually any antigen, organism, and adjuvant. Upon injection into tissue, VacSIM self-assembles into hydrated nanofibers (26), forming a gel matrix depot, which entraps and concentrates vaccine components in the aqueous phase at the injection site (27). We hypothesize that the vaccine depot allows slow egress of antigen out of the gel pores, leading to persistent release of antigen, which is considered to be important in the development of robust adaptive immunity and memory responses (28, 29). We theorize that slow release of antigen and possible cellular infiltration of the VacSIM gel depot increases activation and maturation of antigen-presenting cells, which then drive more robust adaptive responses. Further, it is possible that the gel depot protects vaccine components from degradation.
VacSIM is different from other hydrogels, such as alginates and methacrylates, as well as microneedle and layer-by-layer technology, as all of these require polymerization ex vivo (30–35). Further, it is different from other self-assembling peptide (SAP) technologies, such as the Q11 peptide, where the antigenic motif must be directly conjugated to the SAP (36–38). In contrast, VacSIM is prepared by simply mixing SAP and antigens prior to administration.
The biphasic property of VacSIM, coupled with the inert nature of the resultant vaccine gel depot, provides novel technology that can be translated for use in a multitude of vaccines. In this study, we tested the ability of VacSIM to enhance specific immune responses to the recombinant hepatitis B virus surface antigen.
MATERIALS AND METHODS
Immunizations.
Five- to seven-week-old female BALB/c or C57BL/6 mice were purchased from Harlan Laboratories, housed under specific-pathogen-free conditions, and allowed to acclimate for 1 week prior to manipulation. All animal work was performed in accordance with all applicable policies and approved by the University of Georgia institutional animal care and use committee. Mice were immunized subcutaneously with recombinant hepatitis B virus surface antigen (rHBsAg), subtype adw (5 μg; Fitzgerald Industries, Inc., North Acton, MA, USA), with or without CpG (50 μg; ODN 1826; InvivoGen, Inc., San Diego, CA, USA), via alhydrogel (250 μg; Inject Alum; Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA) with or without CpG, by VacSIM with or without CpG, or in Freund's (13 μl; Sigma-Aldrich Co., St. Louis, MO, USA) or sham immunized with VacSIM or phosphate-buffered saline (PBS), at a maximum volume of 200 μl per injection site. Three or four weeks later, as indicated in the figures, mice were administered a second, identical immunization.
Antibody responses.
For the kinetic experiments, blood samples were collected from all immunized and control mice weekly, beginning at week −1 prior to primary immunization. rHBsAg-specific antibodies in sera were analyzed by enzyme-linked immunosorbent assay (ELISA). Briefly, plates were coated with 4 μg/ml rHBsAg before singly diluted serum (1:800 for IgA and IgG2a and 1:1,200 for IgM, IgG, and IgG1) was added. Antibodies specific for rHBsAg were detected by horseradish peroxidase (HRP)-conjugated secondary antibodies (1:2,000 anti-IgA, 1:2,500 anti-IgM, and 1:2,500 anti-IgG from Santa Cruz; 1:1,000 anti-IgG1 and 1:1,000 IgG2a from Invitrogen). Plates were developed with SureBlue TMB (3,3′,5,5′-tetramethylbenzidine) 1-component substrate (KPL), and the reaction was stopped by the addition of 2 N sulfuric acid. To evaluate the IgG1/IgG2a ratio, we multiplied the absorbance values by the dilution factors of the sera to normalize prior to dividing. For nonkinetic studies, all methods remained the same except that mice were bled prior to immunization and after each subsequent immunization. Each sample of serum was diluted serially for endpoint titer antibody analysis, and samples with undetectable antibody were assigned a value below the detection limit.
ELISpots.
Splenocytes were obtained 3 weeks postprime or 2 weeks postboost and then stimulated for 20 h to evaluate HBsAg-specific cell-mediated responses using gamma interferon (IFN-γ) enzyme-linked immunospot assay (ELISpot), according to the manufacturer's instructions (BD Biosciences, San Francisco, CA, USA). Briefly, single-cell suspensions (3 × 105 and 1.5 × 105 cells per well) were cultured at 37°C with 5% CO2 for 20 h in complete medium (RPMI 1640 [HyClone, Thermo Scientific, Logan, UT, USA] supplemented with 10% fetal bovine serum [FBS], 100 U/ml penicillin, 100 μg/ml streptomycin, antimycotic, nonessential amino acids, and β-mercaptoethanol [Sigma-Aldrich, St. Louis, MO, USA]). Cells were stimulated with 1 μg/ml concanavalin A (ConA), 20 μM HBs-specific peptide [S28-39; IPQSLDSWWTSL (synthesized at more than 95% purity [Biosynthesis Inc., Lewisville, TX, USA] and dissolved in dimethyl sulfoxide [DMSO] prior to dilution in culture medium) or left unstimulated. ELISpot plates were developed with 3-amino-9-ethylcarbazole (AEC) substrate, and spot-forming units (SFU) were counted using an immunospot analyzer (Cellular Technology Limited). The SFU value was expressed as the mean for triplicate cultures per mouse.
Flow cytometry.
Splenocytes collected at 3 weeks postprime and 2 weeks postboost were stimulated for 3 days with 5 μM HBs S28-39 peptide and 40 U/ml recombinant interleukin 2 (rIL-2) (Peprotech) for assessment of molecular specificity. Briefly, HBs peptide was bound with H2-Ld DimerX reagent (BD) at 37°C overnight and incubated with secondary antibody (A85-1-PE; BD) and isotype control (mouse IgG1λ [mIgG1λ]; BD). Two million restimulated splenocytes were stained with DimerX-HB reagent, anti-CD8 Pacific Blue antibody (BD), and viability dye (live/dead near-infrared fixable dye; Invitrogen). Live cells were acquired using a BD LSRII flow cytometer and analyzed with FACSDiva software (BD).
Cytokine responses.
Splenocytes were isolated 2 weeks postboost and tested for rHBsAg-specific cytokine production. Single-cell suspensions (1.5 × 106/ml) were cultured at 37°C with 5% CO2 in complete medium and stimulated with 1 μg/ml ConA or 5 μg/ml rHBsAg or left unstimulated. Levels of tumor necrosis factor alpha (TNF-α) and IFN-γ were quantified after 24 and 48 h culture, IL-5 after 48 h, IL-4 at 48 and 72 h, and IL-10 after 72 h, each in triplicate, by ELISA, according to the manufacturer's instructions (BD).
Statistical analyses.
Except for the kinetic studies whose results are shown in Fig. 1A, all data are represented by box-and-whisker plots, ranging from the minimum value to the maximum, with the mean displayed as a plus sign. Statistical analyses (one- or two-way analyses of variance [ANOVAs] with Bonferroni posttests) were performed using Prism 5 (GraphPad, La Jolla, CA, USA). Differences were considered statistically significant when P values were ≤0.05, as indicated by asterisks in the figures.
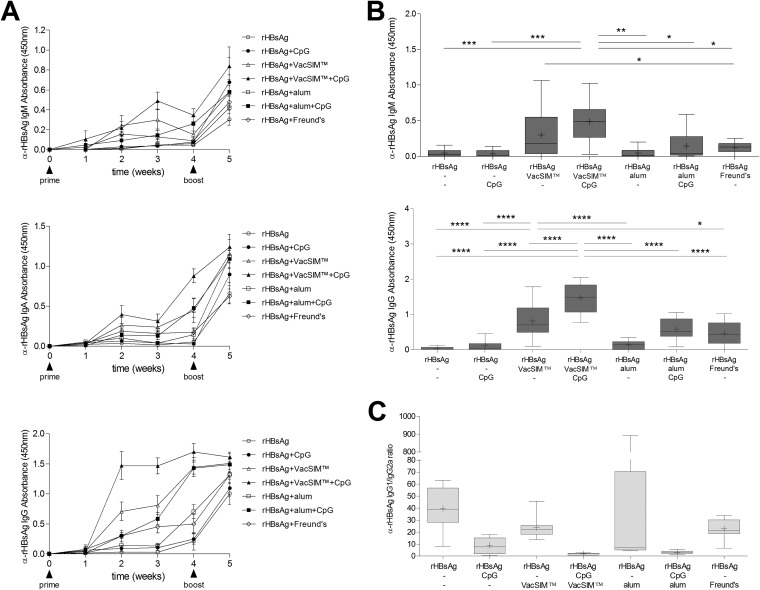
FIG 1.
rHBsAg-specific humoral responses are enhanced and sustained by VacSIM delivery. (A) Kinetic evaluation of rHBsAg-specific IgA, IgM, and IgG antibodies in sera of immunized BALB/c mice (n = 10, pooled from 2 independent experiments) were determined by ELISA. Immunization times are indicated by arrowheads. (B) rHBsAg-specific IgM (top) and IgG (bottom) levels from sera collected 21 days postimmunization. (C) The IgG1:IgG2a ratio was determined at 35 days postimmunization. Statistical differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001) were determined by two-way ANOVA with Bonferroni posttest.
RESULTS
Using recombinant hepatitis B surface antigen (rHBsAg) as a model immunogen, we compared the VacSIM delivery to that of rHBsAg in alum or Freund's, with or without CpG as a model adjuvant for use with VacSIM and alum.
VacSIM enhances and sustains specific humoral immunity.
To assess humoral immunity, total rHBsAg-specific IgA, IgM, and IgG titers were quantified at various times postimmunization and are presented in Fig. 1A. Mice immunized with VacSIM-delivered rHBsAg had higher specific IgM levels than CFA-delivered antigen (Fig. 1B, top) (P < 0.05) 21 days after a single injection. When mice were given rHBsAg administered with CpG adjuvant and delivered by VacSIM, IgM levels were higher than those in all other groups except those receiving antigen by VacSIM without CpG adjuvant. Mice immunized with rHBsAg in VacSIM developed anti-rHBsAg IgG antibody within 2 weeks postprime. At 21 days postprime (Fig. 1B, bottom), mice receiving rHBsAg in VacSIM had significantly higher levels of rHBsAg-specific IgG antibodies than mice immunized with rHBsAg alone (P < 0.0001) or rHBsAg with the standard adjuvant/delivery methods, CpG (P < 0.0001), alum (P < 0.0001), or Freund's (P < 0.05). When rHBsAg was delivered by CpG-adjuvanted VacSIM, the IgG levels postprime were higher than those obtained with CpG-adjuvanted alum delivery of rHBsAg (P < 0.0001) and all other groups (P < 0.0001). One week postboost (Fig. 1A), levels of antibodies were similar between groups, with the exception that the group immunized with rHBsAg in VacSIM plus CpG had higher specific IgA levels than mice immunized with rHBsAg in Freund's. Immunization via VacSIM, compared to immunization with CpG, alum, or Freund's adjuvant, generated higher levels of specific antibody responses after only a single injection, which remained elevated postboost.
To determine if immunization using VacSIM would alter rHBsAg-specific IgG isotype responses, we measured rHBsAg-specific IgG1 and IgG2a levels in mice after the prime and boost. As shown in Fig. 1C, the IgG1/IgG2a ratio elicited in vivo shows that VacSIM delivery drove a mixed Th1/Th2 response, which could be skewed further toward the Th1 type by the inclusion of CpG. As expected, mice immunized with Freund's trended toward a Th1 response, and those immunized with alum trended toward a Th2 response, which could be driven toward Th1 by addition of CpG. Overall, these data show that single immunization with VacSIM enhances specific antibody responses over that seen with single immunization in the absence of adjuvants or with conventional adjuvants such as alum or Freund's.
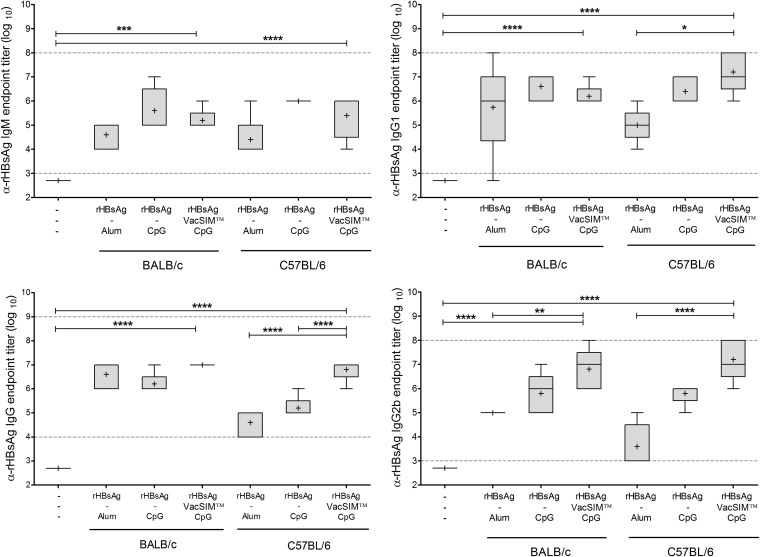
To determine if host Th1/Th2 bias would alter the effect of VacSIM, both C57BL/6 and BALB/c mice (Th1 and Th2 biased, respectively) were immunized in a prime-boost manner, 3 weeks apart, and serum was collected 3 weeks postboost. Tenfold serial dilutions of the serum were analyzed by ELISA, as described in Materials and Methods. The reciprocal value of the last dilution showing a positive value after baseline correction was recorded as the endpoint titer. As shown in Fig. 2, rHBsAg delivered by VacSIM and CpG had high titers of all antibody types tested. Evaluating specific antibodies in serum showed high IgM, IgG, IgG1, and IgG2b endpoint titers in both C57BL/6 and BALB/c mice.
FIG 2.
Immunization with VacSIM increases rHBsAg-specific antibody titers in both Th1- and Th2-biased mouse models. Comparison of rHBsAg-specific antibodies induced in mice immunized 3 weeks apart. The immunized and naïve groups had 5 and 4 mice per group, respectively. There are only 3 IgM values for rHBsAg + CpG-immunized C57BL/6 mice, as sera were limited. Serum was collected 3 weeks postboost, and rHBsAg-specific IgM, IgG, IgG1 and IgG2b endpoint titers were determined by indirect ELISA. Dashed lines indicate detection limits of the assay. Statistical differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001) were determined by one-way ANOVA with the Bonferroni posttest.
Together, these results indicate that immunization of mice with rHBsAg using VacSIM substantially improves and sustains humoral immunity while requiring fewer doses and that it can be used in a variety of host environments.
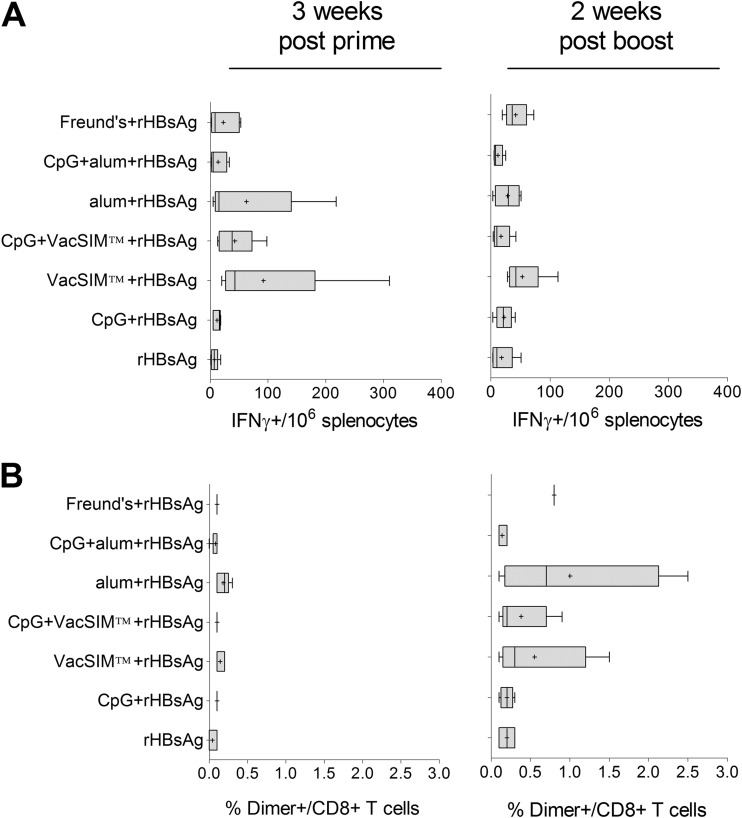
VacSIM induces rHBsAg-specific cellular responses.
rHBsAg-specific cytotoxic-T-lymphocyte (CTL) responses were evaluated after the prime and boost by IFN-γ ELISpot and flow cytometry upon restimulation of splenocytes with the dominant CD8-restricted peptide epitope (Fig. 3). All delivery methods tested resulted in specific IFN-γ ELISpot responses. Responses in groups administered rHBsAg via VacSIM with and without CpG were not significantly higher than those in other groups. When molecular specificity of the CTL was examined by flow cytometry, all groups showed an increase in percentage of CD8+ T cells with T cell receptors specific for the immunodominant epitope. Responses in the group immunized with rHBsAg adjuvanted with alum were significantly higher than the others, but only after the boost.
FIG 3.
Cell-mediated immunity is induced following immunization via VacSIM. Mice were immunized 4 weeks apart and rHBsAg-specific CD8+ T cells were enumerated by IFN-γ ELISpot (A) and flow cytometry (B) of splenocytes harvested from mice (n = 5, except n = 1 for rHBsAg + Freund's, due to lost samples) at 3 weeks postprime (left) and 2 weeks postboost (right).
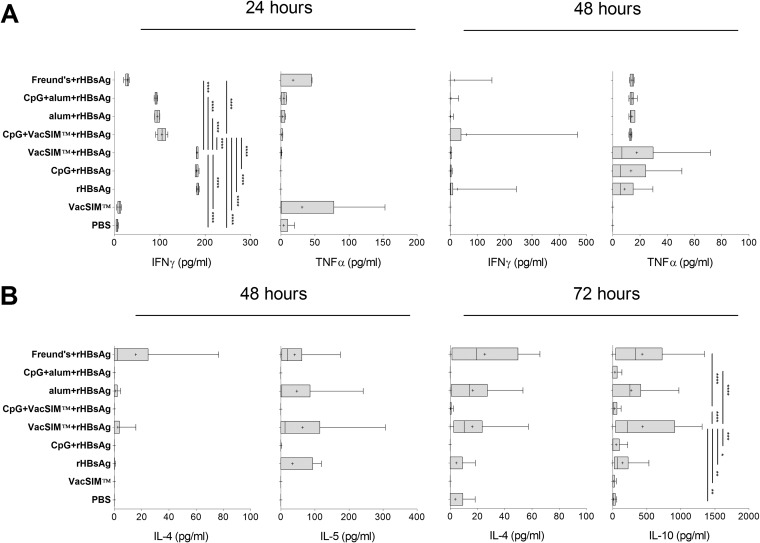
We analyzed cytokine responses to rHBsAg at 2 weeks postboost. Splenocytes were stimulated with rHBsAg ex vivo for 24 to 72 h, and levels of Th1 and Th2 cytokines were determined as an estimate of the Th1/Th2 cytokine balance of the host. Figure 4A shows the levels of the proinflammatory cytokines IFN-γ and TNF-α, collected at 24 and 48 h after ex vivo rHBsAg restimulation of splenocytes. As expected, splenocytes from mice administered phosphate-buffered saline (PBS) or VacSIM, without rHBsAg coadministration, had minimal IFN-γ secretion, whereas immunization with rHBsAg alone resulted in a dramatic increase in IFN-γ at 24 h. Similarly, splenocytes from mice immunized via VacSIM without CpG made amounts of IFN-γ and TNF-α similar to those in splenocytes from mice immunized with only rHBsAg. Interestingly, splenocytes from mice immunized using Freund's adjuvant delivery of rHBsAg had the lowest levels of IFN-γ expression at 24 h, close to the amounts produced from splenocytes from PBS (sham)- or VacSIM only-immunized mice., Surprisingly, splenocytes from VacSIM with CpG-immunized mice had reduced IFN-γ expression at 24 h, compared to splenocytes from VacSIM-rHBsAg, CpG-rHBsAg, and rHBsAg-alone groups. This result suggests that VacSIM does not cause an immediate proinflammatory response in the host. All remaining comparisons were not significantly different from that obtained with antigen alone.
FIG 4.
VacSIM delivery produces a mixed Th1/Th2 type cellular response. Mice were immunized 4 weeks apart, and splenocytes were prepared from mice 2 weeks postboost. For rHBsAg-immunized mice, there are 10 mice per group and data were pooled from 2 independent experiments, whereas control groups (PBS- and VacSIM-immunized) had only 5 mice per group. Splenocytes were restimulated with 5 μg/ml rHBsAg, and levels of proinflammatory (IFN-γ and TNF-α) (A) and anti-inflammatory (IL-4, IL-5, and IL-10) (B) cytokines were measured at 24, 48 or 72 h by ELISA. Statistical differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001) were determined by two-way ANOVA with Bonferroni posttest.
We assayed for IL-4, IL-5, and IL-10 at 48 and 72 h after rHBsAg restimulation by ELISA (Fig. 4B). Cells from mice immunized with rHBsAg in VacSIM or Freund's adjuvant showed increased levels of anti-inflammatory cytokines at both time points, with significantly higher levels of IL-10 at 72 h than cells from rHBsAg-only mice. Immunization via alum also induced a not statistically significant increase in production of anti-inflammatory cytokines. Addition of CpG to the immunogens inhibited production of Th2 and anti-inflammatory cytokines.
Overall, these results suggest that VacSIM by itself is not proinflammatory and that it has significant potential to enhance immunogenicity and efficacy of various immunogens.
DISCUSSION
Design and development of novel, nonreactogenic adjuvants, including synthetic LPS derivatives such as glucopyranosyl lipid A (GLA), are aimed at improving vaccine immunogenicity and efficacy (14). Candidate vaccines have been defined for a number of diseases that are currently unable to drive reasonable levels of efficacy. For example, vaccines that need to drive robust CTL responses to kill intracellular pathogens induce weak Th1-biased responses (39–41). Here, we compared a new delivery method (VacSIM) to alhydrogel and CFA delivery of the rHBsAg immunogen, to determine if VacSIM delivery would drive enhanced hepatitis B-specific humoral and cellular responses. For VacSIM, alum and rHBsAg alone, we also compared immunization plus or minus CpG as an adjuvant. The results demonstrate that delivery of rHBsAg with VacSIM with or without CpGs functioned to increase specific humoral (Fig. 1 and 2) and cellular (Fig. 3 and 4) responses. Alum has been considered a reasonable, nonreactogenic adjuvant for years, promoting antigen presentation in a particulate form and enhancing internalization by APCs (42). The results presented here show that for the rHBsAg, VacSIM is superior to alum, significantly enhancing the humoral response postprime, by increasing antibody titers at earlier time points and maintaining them in a sustained manner (Fig. 1). The ability of VacSIM to increase early rHBsAg-specific adaptive immune responses may be due to the slow release of antigen from the gel depot, possibly enhancing antigen uptake by APCs compared to that seen when aluminum hydroxide particles or oil-in-water emulsions are used.
A major focus for enhancing immunogenicity is activation of the innate immune system by incorporating agents that ligate one or more innate immune pattern recognition receptors on antigen-presenting cells. Maturation of APCs is essential for priming antigen-specific naive T cells, influencing both the magnitude and the type of the T and B cell responses as well as the induction of memory cells (2). Furthermore, the interaction of T cells and APCs in the presence of immunomodulatory molecules (IL-4, IL-2, IFN-γ, IL-12, TGF-β, and/or other cytokines) defines the lineage commitment of CD4+ T cells to Th1 and Th2 subtypes.
We examined the T helper biasing of the humoral response by evaluating IgG subtypes produced by the various immunization schemes. Immunization using VacSIM resulted in a mixed Th1/Th2 type antibody response, with more IgG1 than IgG2a. Adding CpG to VacSIM skewed the response toward Th1. We also tested biasing of cellular responses by analyzing antigen-specific restimulation of splenocytes after the boost. In that context, VacSIM induced a mixed Th1/Th2 type response, with high levels of all cytokines. Interestingly, when CpG was added to rHBsAg and VacSIM, the levels of all cytokines were reduced.
Conclusions.
The VacSIM delivery system tested in this study was superior to conventional delivery/adjuvants in driving early immune responses. Given the flexibility afforded by VacSIM, differential administration of adjuvants and delivery methods can be employed to produce a Th2 or mixed-type response, depending on the outcome desired for each vaccine target. In addition to our studies on VacSIM delivery of recombinant subunit vaccines, we are also evaluating VacSIM as a vaccine delivery method for parasitic and viral vaccines. In this regard, we are evaluating VacSIM for delivery of influenza vaccines, including both whole inactivated virus and subunit vaccines. A major goal of ongoing studies is to determine the mechanism(s) whereby VacSIM delivery of vaccines results in enhanced vaccine immunogenicity and efficacy. Lastly, we are working to define optimal parameters for VacSIM delivery, particularly the concentration of the (RADA)4 peptide, as gel pore size will get smaller/larger with higher/lower (RADA)4 concentration, respectively, altering the rate of antigen release from the gel depot. Because VacSIM delivery leads to release of antigen from the gel depot over time, we also need to optimize the interval between prime and boost and determine whether VacSIM will function to deliver mucosal vaccines. In summary, VacSIM delivery is a flexible “plug-and-play” platform technology, representing a new approach for delivery of vaccines.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI071883 and AI036657 awarded to D.A.H. and Coordination for the Improvement of Higher Level Education Personnel (CAPES) and The Council of the International Educational Exchange of Scholars (Fulbright, U.S. Department of State), both awarded to R.F.Q.G.
We thank Cac Bui and Lindsay Nyhoff for technical assistance and the University of Georgia College of Veterinary Medicine Cytometry Core Facility.
Patent applications have been filed on behalf of the authors for the use of the polypeptide matrix as a vaccine delivery system.
REFERENCES
- 1.De Gregorio E, Tritto E, Rappuoli R. 2008. Alum adjuvanticity: unraveling a century old mystery. Eur J Immunol 38:2068–2071. doi: 10.1002/eji.200838648. [DOI] [PubMed] [Google Scholar]
- 2.Miyaji EN, Carvalho E, Oliveira ML, Raw I, Ho PL. 2011. Trends in adjuvant development for vaccines: DAMPs and PAMPs as potential new adjuvants. Braz J Med Biol Res 44:500–513. doi: 10.1590/S0100-879X2011007500064. [DOI] [PubMed] [Google Scholar]
- 3.Easterbrook JD, Schwartzman LM, Gao J, Kash JC, Morens DM, Couzens L, Wan H, Eichelberger MC, Taubenberger JK. 2012. Protection against a lethal H5N1 influenza challenge by intranasal immunization with virus-like particles containing 2009 pandemic H1N1 neuraminidase in mice. Virology 432:39–44. doi: 10.1016/j.virol.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng MP, Hu ZH, Wang HL, Deng F. 2012. Developments of subunit and VLP vaccines against influenza A virus. Virol Sin 27:145–153. doi: 10.1007/s12250-012-3241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hossain MJ, Bourgeois M, Quan FS, Lipatov AS, Song JM, Chen LM, Compans RW, York I, Kang SM, Donis RO. 2011. Virus-like particle vaccine containing hemagglutinin confers protection against 2009 H1N1 pandemic influenza. Clin Vaccine Immunol 18:2010–2017. doi: 10.1128/CVI.05206-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mooney AJ, Li Z, Gabbard JD, He B, Tompkins SM. 2013. Recombinant parainfluenza virus 5 vaccine encoding the influenza virus hemagglutinin protects against H5N1 highly pathogenic avian influenza virus infection following intranasal or intramuscular vaccination of BALB/c mice. J Virol 87:363–371. doi: 10.1128/JVI.02330-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornelissen LA, de Leeuw OS, Tacken MG, Klos HC, de Vries RP, de Boer-Luijtze EA, van Zoelen-Bos DJ, Rigter A, Rottier PJ, Moormann RJ, de Haan CA. 2012. Protective efficacy of Newcastle disease virus expressing soluble trimeric hemagglutinin against highly pathogenic H5N1 influenza in chickens and mice. PLoS One 7:e44447. doi: 10.1371/journal.pone.0044447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashem A, Jaentschke B, Gravel C, Tocchi M, Doyle T, Rosu-Myles M, He R, Li X. 2012. Subcutaneous immunization with recombinant adenovirus expressing influenza A nucleoprotein protects mice against lethal viral challenge. Hum Vaccin Immunother 8:425–430. doi: 10.4161/hv.19109. [DOI] [PubMed] [Google Scholar]
- 9.Rosenkrands I, Vingsbo-Lundberg C, Bundgaard TJ, Lindenstrom T, Enouf V, van der Werf S, Andersen P, Agger EM. 2011. Enhanced humoral and cell-mediated immune responses after immunization with trivalent influenza vaccine adjuvanted with cationic liposomes. Vaccine 29:6283–6291. doi: 10.1016/j.vaccine.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 10.Even-Or O, Joseph A, Itskovitz-Cooper N, Samira S, Rochlin E, Eliyahu H, Goldwaser I, Balasingam S, Mann AJ, Lambkin-Williams R, Kedar E, Barenholz Y. 2011. A new intranasal influenza vaccine based on a novel polycationic lipid-ceramide carbamoyl-spermine (CCS). II. Studies in mice and ferrets and mechanism of adjuvanticity. Vaccine 29:2474–2486. doi: 10.1016/j.vaccine.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Dong L, Liu F, Fairman J, Hong DK, Lewis DB, Monath T, Warner JF, Belser JA, Patel J, Hancock K, Katz JM, Lu X. 2012. Cationic liposome-DNA complexes (CLDC) adjuvant enhances the immunogenicity and cross-protective efficacy of a pre-pandemic influenza A H5N1 vaccine in mice. Vaccine 30:254–264. doi: 10.1016/j.vaccine.2011.10.103. [DOI] [PubMed] [Google Scholar]
- 12.Principi N, Esposito S. 2012. Adjuvanted influenza vaccines. Hum Vaccin Immunother 8:59–66. doi: 10.4161/hv.8.1.18011. [DOI] [PubMed] [Google Scholar]
- 13.Zuccotti GV, Pariani E, Scaramuzza A, Santoro L, Giani E, Macedoni M, Gazzarri A, Anselmi G, Amendola A, Zanetti A. 2011. Long-lasting immunogenicity and safety of a 2009 pandemic influenza A(H1N1) MF59-adjuvanted vaccine when co-administered with a 2009-2010 seasonal influenza vaccine in young patients with type 1 diabetes mellitus. Diabet Med 28:1530–1536. doi: 10.1111/j.1464-5491.2011.03449.x. [DOI] [PubMed] [Google Scholar]
- 14.Behzad H, Huckriede AL, Haynes L, Gentleman B, Coyle K, Wilschut JC, Kollmann TR, Reed SG, McElhaney JE. 2012. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. J Infect Dis 205:466–473. doi: 10.1093/infdis/jir769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clegg CH, Roque R, Van Hoeven N, Perrone L, Baldwin SL, Rininger JA, Bowen RA, Reed SG. 2012. Adjuvant solution for pandemic influenza vaccine production. Proc Natl Acad Sci U S A 109:17585–17590. doi: 10.1073/pnas.1207308109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, Lu X, DeVos J, Hancock K, Katz JM, Vedvick TS, Duthie MS, Clegg CH, Van Hoeven N, Reed SG. 2010. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One 5:e13677. doi: 10.1371/journal.pone.0013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manon MJ, Cox PSC. 9 August 2012. Safety and immunogenicity of PanBlok influenza vaccine in healthy adults. ClinicalTrials.gov http://clinicaltrials.gov/ct2/show/NCT01147068 Accessed 29 October 2012.
- 18.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. 2002. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci U S A 99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwai Y, Matsuda Y, Nakatsuka M, Mikami Y, Kumabe S. 2012. A preliminary study of the dental implant therapy–initial osteogenesis of human mesenchymal stem (HMS0014) cells on titanium discs with different surface modifications. Okajimas Folia Anat Jpn 88:133–140. doi: 10.2535/ofaj.88.133. [DOI] [PubMed] [Google Scholar]
- 20.Horii A, Wang X, Gelain F, Zhang S. 2007. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS One 2:e190. doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henriksson H, Hagman M, Horn M, Lindahl A, Brisby H. 2011. Investigation of different cell types and gel carriers for cell-based intervertebral disc therapy, in vitro and in vivo studies. J Tissue Eng Regen Med 6:738–747. doi: 10.1002/term.480. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S. 2003. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol 21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 23.Kondo Y, Nagasaka T, Kobayashi S, Kobayashi N, Fujiwara T. 2014. Management of peritoneal effusion by sealing with a self-assembling nanofiber polypeptide following pelvic surgery. Hepatogastroenterology 61:349–353. [PubMed] [Google Scholar]
- 24.Rosa V, Zhang Z, Grande RH, Nor JE. 2013. Dental pulp tissue engineering in full-length human root canals. J Dent Res 92:970–975. doi: 10.1177/0022034513505772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Ellis-Behnke R, Zhao X, Spirio L. 2010. PuraMatrix: self-assembling peptide nanofiber scaffolds, p 217–240. In Ma PX, Elisseeff J (ed), Scaffolding in tissue engineering. CRC Press, Boca Raton, FL. [Google Scholar]
- 26.Zhang S, Holmes TC, DiPersio CM, Hynes RO, Su X, Rich A. 1995. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials 16:1385–1393. doi: 10.1016/0142-9612(95)96874-Y. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura A, Hayakawa T, Yamamoto Y, Hamori M, Tabata K, Seto K, Shibata N. 2012. Controlled release of insulin from self-assembling nanofiber hydrogel, PuraMatrix: application for the subcutaneous injection in rats. Eur J Pharm Sci 45:1–7. doi: 10.1016/j.ejps.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Sacks DL. 2014. Vaccines against tropical parasitic diseases: a persisting answer to a persisting problem. Nat Immunol 15:403–405. doi: 10.1038/ni.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray D, Skarvall H. 1988. B-cell memory is short-lived in the absence of antigen. Nature 336:70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- 30.Ingavle GC, Gehrke SH, Detamore MS. 2014. The bioactivity of agarose-PEGDA interpenetrating network hydrogels with covalently immobilized RGD peptides and physically entrapped aggrecan. Biomaterials 35:3558–3570. doi: 10.1016/j.biomaterials.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Browning MB, Cereceres SN, Luong PT, Cosgriff-Hernandez EM. 2014. Determination of the in vivo degradation mechanism of PEGDA hydrogels. J BiomedMater Res A 102:4244–4251. doi: 10.1002/jbm.a.35096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jose S, Hughbanks ML, Binder BY, Ingavle GC, Leach JK. 2014. Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels. Acta Biomater 10:1955–1964. doi: 10.1016/j.actbio.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong Y, Yan K, Bentley WE, Deng H, Du Y, Payne GF, Shi XW. 2014. Compartmentalized multilayer hydrogel formation using a stimulus-responsive self-assembling polysaccharide. ACS Appl Mater Interfaces 6:2948–2957. doi: 10.1021/am405544r. [DOI] [PubMed] [Google Scholar]
- 34.Jorquera PA, Choi Y, Oakley KE, Powell TJ, Boyd JG, Palath N, Haynes LM, Anderson LJ, Tripp RA. 2013. Nanoparticle vaccines encompassing the respiratory syncytial virus (RSV) G protein CX3C chemokine motif induce robust immunity protecting from challenge and disease. PLoS One 8:e74905. doi: 10.1371/journal.pone.0074905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JW, Choi SO, Felner EI, Prausnitz MR. 2011. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small 7:531–539. doi: 10.1002/smll.201001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudra JS, Tian YF, Jung JP, Collier JH. 2010. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci U S A 107:622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chesson CB, Huelsmann EJ, Lacek AT, Kohlhapp FJ, Webb MF, Nabatiyan A, Zloza A, Rudra JS. 2014. Antigenic peptide nanofibers elicit adjuvant-free CD8(+) T cell responses. Vaccine 32:1174–1180. doi: 10.1016/j.vaccine.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 38.Rudra JS, Mishra S, Chong AS, Mitchell RA, Nardin EH, Nussenzweig V, Collier JH. 2012. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials 33:6476–6484. doi: 10.1016/j.biomaterials.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandtzaeg P. 2007. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 25:5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Roy MJ, Wu MS, Barr LJ, Fuller JT, Tussey LG, Speller S, Culp J, Burkholder JK, Swain WF, Dixon RM, Widera G, Vessey R, King A, Ogg G, Gallimore A, Haynes JR, Heydenburg Fuller D. 2000. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 19:764–778. doi: 10.1016/S0264-410X(00)00302-9. [DOI] [PubMed] [Google Scholar]
- 41.Wang R, Doolan DL, Le TP, Hedstrom RC, Coonan KM, Charoenvit Y, Jones TR, Hobart P, Margalith M, Ng J, Weiss WR, Sedegah M, de Taisne C, Norman JA, Hoffman SL. 1998. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 42.Morefield GL, Sokolovska A, Jiang D, HogenEsch H, Robinson JP, Hem SL. 2005. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine 23:1588–1595. doi: 10.1016/j.vaccine.2004.07.050. [DOI] [PubMed] [Google Scholar]