Abstract
A major impediment to tuberculosis (TB) vaccine development is the lack of reliable correlates of immune protection or biomarkers that would predict vaccine efficacy. Gamma interferon (IFN-γ) produced by CD4+ T cells and, recently, multifunctional CD4+ T cells secreting IFN-γ, tumor necrosis factor (TNF), and interleukin-2 (IL-2) have been used in vaccine studies as a measurable immune parameter, reflecting activity of a vaccine and potentially predicting protection. However, accumulating experimental evidence suggests that host resistance against Mycobacterium tuberculosis infection is independent of IFN-γ and TNF secretion from CD4+ T cells. Furthermore, the booster vaccine MVA85A, despite generating a high level of multifunctional CD4+ T cell response in the host, failed to confer enhanced protection in vaccinated subjects. These findings suggest the need for identifying reliable correlates of protection to determine the efficacy of TB vaccine candidates. This article focuses on alternative pathways that mediate M. tuberculosis control and their potential for serving as markers of protection. The review also discusses the significance of investigating the natural human immune response to M. tuberculosis to identify the correlates of protection in vaccination.
INTRODUCTION
The World Health Organization reported nearly 9 million new cases and about 1.5 million tuberculosis (TB)-related deaths globally in 2013 (1). Additionally, it is estimated that one-third of the world's population is infected with Mycobacterium tuberculosis, and approximately 3 to 10% of these infected individuals are likely to progress to active disease during their lifetime. The risk of reactivation disease and mortality is significantly increased in individuals with HIV coinfection (1). The emergence of multidrug-resistant (MDR) strains of M. tuberculosis further complicates this already grim picture and reinforces the urgent need for an efficacious vaccine against TB.
TB vaccine research is confounded by a conundrum: a candidate biomarker for protective immunity can be validated only in the clinical trial of an effective vaccine. However, clinical trials of an effective vaccine may not be feasible without a validated correlate of protection for the selection of the most promising candidates and for determining dose and schedule of vaccination. Another general issue is that correlates of protective immunity may differ in protection against infection, progression from infection to disease, reactivation, and reinfection. Protection induced by vaccines also may differ from natural infection. Finally, correlates of protection may not be involved in the mechanism of infection—in fact, they may be undiscovered—that is, not previously considered related to protective immunity.
Mycobacterium bovis BCG is effective in preventing disseminated TB only in children, and the protection conferred in adults has been variable, ranging from 0 to 80% in different studies (2). Therefore, widespread vaccination with BCG has not alleviated the public health problem of TB. In the last decade, a great deal of research effort in the TB field has been invested in generating new TB vaccines (3). This concerted effort from several TB investigators and pharmaceutical companies has produced 11 vaccine candidates that currently are in different stages of clinical trials, ranging from phase 1 to phase 2b (4), and are being studied for efficacy in boosting the response to BCG or as a replacement for BCG. The vaccine candidates include live recombinant BCG, viral vector-based vaccines, and subunit vaccines (4). Further, in the pipeline are three live M. tuberculosis vaccines that have been attenuated by deletion of at least two independent genes required for in vivo growth and virulence (4). These modified strains of M. tuberculosis are under preclinical assessments. MVA85A, the first booster vaccine candidate to complete an efficacy trial since BCG, did not provide significantly higher protection (5), despite exhibiting a significantly higher level of antigen-specific T cell responses during preclinical development (6). This setback in TB vaccine development has reinforced the importance of revisiting and revising our understanding of host immune components that can serve as reliable markers of protection in vaccine-mediated immunity. In this article, we first discuss the growing literature which indicates that there is a disconnect between polyfunctional T cells and vaccine efficacy. Next, we deliberate on whether immune cells other than CD4+ T cells potentially correlate with protection and the emerging concept that the innate compartment has memory-like facets. We also discuss the relevance of clinical studies focused on tracking the natural course of human immune response to M. tuberculosis and large-scale data analysis tools to identify correlates of protection. Our aim for this review is to draw attention to mechanisms beyond conventional memory T cells and cytokines. There are exhaustive reviews on host immunity, memory T cells, and cytokines in TB, and therefore, these topics have not been reviewed.
THE PROBLEM: DISCONNECT BETWEEN POLYFUNCTIONAL T CELLS AND VACCINE EFFICACY
Partial or complete gamma interferon (IFN-γ) receptor deficiency in humans leads to disseminated nontuberculous mycobacterial (NTM) infections or BCGosis, and mice deficient in IFN-γ exhibit impaired control of bacterial growth and dissemination (7–10). Furthermore, IFN-γ production is depressed in whole-blood cultures from advanced TB patients (11). Together, these findings led to the assumption that the robust production of IFN-γ is a strong correlate of protection and thus a useful readout for testing immunogenicity of TB vaccine candidates. Subsequently, work from several studies revealed that IFN-γ is not a reliable measure of protection against M. tuberculosis (12, 13). Although systemic production of IFN-γ by M. tuberculosis-stimulated peripheral blood mononuclear cells (PBMC) from patients with moderate and far-advanced pulmonary TB is depressed, the local immune response shows an increased frequency of IFN-γ-producing cells. Indeed, patients with active disease may have higher levels of IFN-γ in plasma and in sputum, suggesting that the levels of this cytokine are a reflection of the intensity of immunopathology and bacterial load in the lungs rather than evidence of a protective response (14–16). It seems likely that IFN-γ production is necessary but insufficient for protection and in the setting of concomitant M. tuberculosis disease may contribute to pathogenesis. Further, the induction of IFN-γ by in vitro stimulation of PBMC may not be relevant to local protective mechanisms following aerosol exposure to viable organisms.
Simultaneous quantification of several immune functions in a single cell can now be achieved due to significant advances in multiparametric flow technology. Polyfunctional T cells show greater association with protective T cell immune responses in infectious diseases than do IFN-γ-secreting monofunctional T cells. For example, HIV nonprogressors expressed a high frequency of polyfunctional CD4+ T cells that simultaneously produced IFN-γ, tumor necrosis factor (TNF), and interleukin-2 (IL-2) (17). Similarly, antigen-specific memory T cells capable of producing these three cytokines were also protective against leishmania infection (18). Consistent with these observations, vaccine-induced protection in mice against M. tuberculosis infection strongly correlated with a high frequency of polyfunctional CD4+ T cells (19, 20). However, in a study that monitored a cohort of BCG-immunized infants for 2 years, the correlation of polyfunctional cytokine profile with protective efficacy of BCG vaccination was not established (21). In this study, flow cytometric analysis of antigen-stimulated whole-blood samples indicated generation of multifunctional T cells in the vaccinated individuals; however, the polyfunctional profiles of these T cells did not correlate with BCG-mediated protection. In another study, T cell cytokine profile in response to the MVA85A (modified vaccinia virus Ankara expressing antigen 85A) was tested in BCG-vaccinated individuals who were given a booster dose of MVA85A. The T cell cytokine profile from these individuals indicated a significantly higher frequency of polyfunctional T cells than that for BCG vaccination alone, leading to the assumption that the booster vaccine would enhance the efficacy of BCG (22). However, despite the expansion of polyfunctional T cells with MVA85A booster immunization, the results obtained from the phase 2b trial in infants given this prime-boost strategy indicated no enhancement in protection (5). These studies suggest that polyfunctional T cells may play a necessary but not sufficient role in protection against TB.
Several investigations have suggested an IFN-γ-independent role of CD4+ T cells in mediating protective immunity to the host. Gallegos et al. showed that the adoptive transfer of antigen-specific CD4+ T effector cells could confer protection on naive hosts, independent of their ability to produce IFN-γ or TNF (23). Interestingly, using a similar approach it was shown that in vitro-differentiated BCG-specific Th17 cells mediated protection in the absence of IFN-γ (24). In another study, BCG-immunized IFN-γ−/− mice exhibited a reduction in bacterial burden on secondary challenge with M. tuberculosis infection (24, 25) but lost this ability when they were depleted of CD4+ T cells at the time of immunization (26); this study further supports the tenet that effector CD4+ T cells can mediate protection against M. tuberculosis in the absence of IFN-γ. Clearly, IFN-γ is indispensable for host protection against M. tuberculosis infection (7–10). Therefore, the requirement for IFN-γ in CD4+ T cell-mediated host resistance may be at the level of skewing CD4+ T cell differentiation toward a Th1 effector phenotype, and perhaps, IFN-γ is expendable after this stage.
Although CD4+ T cells play a predominant role in protective TB immunity, strategies that boost CD8+ function also enhance vaccine efficacy (27). In a nonhuman primate model of tuberculosis disease (28), depletion of CD8+ T cells in immunized monkeys led to reduced protection. Similarly, CD8+ T cell depletion in M. tuberculosis-infected and then antibiotic-treated monkeys led to increased susceptibility to reinfection, indicating their importance in conferring immunity in a vaccination or natural infection setting. Human CD8+ CCR7– CD45RA+ effector memory T cells exhibit significant antimycobacterial activity (29), and their relevance to host protection is somewhat supported by the finding that their numbers are reduced in patients receiving immunotherapy with anti-TNF antibodies (29), a regimen that compromises M. tuberculosis immunity (30). However, whether CD8+ T cell-mediated protection is mediated by multifunctional CD8+ T cells or whether, akin to CD4+ T cells, it is also independent of IFN-γ and TNF needs to be determined.
A CASE FOR Th17 CELLS
Initial studies examining the role of IL-17 in protective immunity against M. tuberculosis found that mice lacking the ability to produce IL-17 (31, 32) and IL-17RA-deficient mice (31) were not compromised in their ability to contain M. tuberculosis growth. In contrast, another study reported that mice lacking IL-17A receptor, despite being able to control acute infection, were unable to stably maintain long-term control of M. tuberculosis infection (33). The increased susceptibility was not related to deficiencies in IFN-γ but correlated with decreased early neutrophil recruitment. However, a major caveat of this study is that the mice were infected either intratracheally or intravenously with a 100-fold-higher inoculum than the normal low-dose aerogenic route. A recent study showed that the requirement for IL-17 in host protection against M. tuberculosis was strain dependent. IL-17 was dispensable for protective immunity against the lab-adapted strain H37Rv while necessary for protection against M. tuberculosis HN878, a hypervirulent M. tuberculosis strain (34). IL-17 exerts a greater influence in vaccine-mediated protection in TB. Cooper and colleagues showed that following BCG (35) and ESAT-6 peptide (36) immunization, antigen-specific Th17 cells localized in the lungs and were critical for the recruitment of Th1 cells to the lung after M. tuberculosis challenge. Another study also found that BCG-induced Th17 cells were protective. In this study, protection was conferred even in the absence of IFN-γ (24). Indeed, other studies have reported an IFN-γ-independent mechanism of protection by Th17 cells. Mucosal vaccination with ESAT-6 peptide with LT-IIB, a mucosal adjuvant, also induced a robust Th17 response that mediated protection against M. tuberculosis infection in an IFN-γ-independent manner (25).
When first identified, Th17 cells were considered to be short-lived cells without the ability to generate long-term memory. Shortly thereafter, several studies showed that Th17 cells were capable of providing protection in immunization settings (37, 38) and transfer (39) models, denoting their capacity for long-term survival. It was then confirmed at the molecular level that indeed Th17 cells are long lived and can form memory cells, despite expressing markers characteristic of terminally differentiated cells (40). In fact, human Th17 cells also exhibited a long-lived effector memory phenotype, possessing a high capacity for self-renewal (41). Th17 cells preserve the molecular signature that is characteristic of T stem cell memory (TSCM) (42–44) and express the Wnt-β-catenin signaling axis (40), a pathway critical for maintaining the self-renewal potential of a cell (45, 46). Interestingly, predating Th17 and TSCM cell discovery, two studies had reported that greater protection against M. tuberculosis challenge infection was seen in mice that were adoptively transferred with T cells bearing a naive phenotype (CD44lo CD62Lhi) than in mice that were transferred with T central memory (TCM) cells (47, 48). Given our current knowledge, it is quite likely that the transferred T cells were Th17 memory cells. Together with these studies, the recent findings that Th17 cells are long lived and can mediate protection in the absence of IFN-γ suggest that the analysis of this subset of T cells is certainly warranted when evaluating new TB vaccine candidates.
OTHER IMMUNE CELLS POTENTIALLY CORRELATING WITH PROTECTION
A number of different innate cell types bearing key resemblance to T cell-like functionalities come into play during TB infection. Traditionally, these innate cells provide immediate protection before the adaptive immune response is generated and thus contribute toward early containment of the pathogen. However, a growing number of studies suggest their involvement in the recall response and protection during secondary challenge.
NK cells.
NK cells lie at the interface of innate and adaptive immune responses and are unique in their ability to recognize antigen and carry out a cytotoxic effector function akin to CD8+ T cells, despite the lack of RAG-mediated diversity (49, 50). NK cells express only a few genes that encode a large number of different antigen-specific receptors. The role of NK cells in protection against TB has not been unequivocally proven. NK cells are present in the lungs of M. tuberculosis-infected mice, but their depletion does not enhance susceptibility to infection (51). However, γc−/− RAG−/− mice that lack both NK and T cells exhibit greater susceptibility to M. tuberculosis infection than do RAG−/− mice, indicating a role of NK cells in host protection against M. tuberculosis infection (52). In vitro studies have shown that human NK cells mediate lysis of M. tuberculosis-infected macrophages that is dependent on expression of the NK cell-activating receptors NKp46 and NKG2D (53, 54). Mechanistically, IL-22 released by the NK cells enhanced phagolysosomal fusion and M. tuberculosis growth inhibition in infected macrophages (55).
Generation and long-term maintenance of NK cells in response to viral infections such as those with cytomegalovirus (CMV) and hepatitis C virus (HCV) have been reported by many investigators. These memory-like NK cells bear activating C-type lectin-like receptors such as NKG2C and depend on cytokines such as IL-12 and IL-15 for their maintenance (56, 57). Indeed, in a mouse model of CMV infection, NK cells bearing the Ly49H receptor expanded in response to infection and were maintained in the host several months postinfection. These “memory NK cells” exhibited the characteristics of memory lymphocytes by exhibiting a high rate of activation and degranulation and the ability to confer protective status on the recipient host (58). These studies emphasize the importance of investigating memory NK cells in TB vaccine assessments. In support of this, mice vaccinated with BCG demonstrated increased numbers of IFN-γ-expressing NK 1.1 cells, and their depletion led to reduced vaccination efficacy following M. tuberculosis challenge (59).
γδ T cells.
γδ T cells recognize a variety of unrestricted, unprocessed, and small phosphate antigens (60, 61). In M. tuberculosis-infected mice, during the early phase of infection, IFN-γ- and IL-17-secreting γδ T cells with cytotoxic effector functions are recruited to the lungs (62–65). Although antibody-mediated depletion of γδ T cells in mice did not abrogate protection against BCG infection (66), expansion of Vγ2Vδ2 T cells in response to BCG vaccination and their presence in M. tuberculosis-specific recall response were reported in the nonhuman primate macaque model (67). In addition, human Vγ9Vδ2 T cells reduce the viability of intracellular M. tuberculosis via mechanisms dependent on perforin or granulolysin (68–70). These data, together, indicate not only that γδ T cells are present during M. tuberculosis infection and BCG vaccination but that, in humans, they are capable of restricting M. tuberculosis growth. A study carried out in newborn pigs also showed that BCG vaccination leads to an enhanced response from γδ T cells, indicating their probable role in mediating vaccine-induced protection (71). Another study investigating characteristics of cellular response to BCG vaccination in humans found that γδ T cells expanded significantly compared to other cell types after ex vivo PBMC stimulation (72). Together, these studies indicate that it may be worthwhile to include functional studies of γδ T cells in the assessment of TB vaccines.
CD4− CD8− DN T cells.
A rare subset of T cells that are CD3+ TCR-αβ+ but double negative (DN) for CD4 and CD8 expression expand in response to M. tuberculosis infection and restrict bacterial growth in vitro in macrophage cultures (73). Furthermore, other studies demonstrated that both a DNA vaccine cocktail (74) and BCG immunization (75) could induce protective immunity in mice lacking CD4+ T cells but not in mice deficient in CD8+ T cells. In contrast, the live attenuated vaccine strain mc26030 was shown to induce protective immunity, equivalent to that induced by BCG, in CD4−/− mice, but in a CD8+ T cell-independent mechanism (76). Further characterization of the T cell population mediating protection in the CD4−/− vaccinated mice showed that they were CD4− CD8− TCR-αβ+ TCR-γδ– NK1.1– (77). Adoptive transfer of the CD4− CD8− DN T cells from vaccinated CD4−/− mice into naive CD4−/− resulted in significant protection against M. tuberculosis challenge infection (77). These enriched CD4− CD8− TCR-αβ+ T cells had significantly higher mRNA levels for IFN-γ and IL-2, highlighting the need to look for correlates of protection in nontraditional CD4+ and CD8+ T cells. In support of this, IFN-γ-expressing DN T cells were observed in BCG-immunized children (78).
MEMORY IN THE INNATE IMMUNE COMPARTMENT
Due to its inherently nonspecific and short-lived nature, the innate immune response is not associated with the long-term memory immune response in higher vertebrates. However, studies investigating the generation of protective responses to different pathogens in invertebrate species have suggested that these hosts are able to recall prior antigenic experience, despite the lack of a specific and persistent adaptive immune response (79, 80). These protective response mechanisms involve generation of a repertoire of pattern recognition receptor molecules and the Toll pathway (79). For example, DSCAM (Down syndrome cell adhesion molecule) protects Anopheles mosquitoes against the malarial parasite Plasmodium falciparum and molecules of the Toll pathway provide protection against secondary challenge in the fruit fly Drosophila melanogaster (81–83). These evolutionarily conserved mechanisms refute the idea that innate immunity lacks specificity and is short lived (84). In higher vertebrates, pathogen-associated molecular patterns and their recognition via various pathogenic response receptors are well established and are key to providing diversity in pathogen-specific innate responses (85). However, whether this response can have an impact on long-term protection in higher vertebrates, either via generation of unique innate responses on secondary encounter or through modulation of innate responses by memory T cells, has only recently been interrogated. Another issue, of course, is whether the innate mechanisms can be expanded or enhanced through vaccination and, if so, what approaches are likely to do so.
Epigenetic reprogramming in innate cells.
T and B lymphocytes undergo RAG-mediated differentiation, yielding a large repertoire of antigen-specific T and B lymphocytes, via which they then acquire effector and memory phenotypes upon antigen encounter. These memory lymphocytes persist for years and expand rapidly upon antigenic challenge, signifying their role as key targets for studying the correlates of protection in vaccination studies. However, there is emerging evidence that innate cells also undergo epigenetic reprogramming of key inflammatory genes and thereby sustain their innate activation status for long periods following the first insult (86). It is well established that plants and lower invertebrates utilize epigenetic reprogramming to maintain innate resistance in response to pathogenic challenge, but in mammals, this has not been extensively investigated (84). Indeed, BCG infection was shown to enhance the function of monocytes that lasted 3 months postinfection (87). This enhanced functional status was NOD2 dependent, and the increased gene expression of key proinflammatory cytokines was due to increased H3K4 trimethylation. Interestingly, the enhanced proinflammatory response exhibited by BCG-exposed monocytes was not limited to BCG but also included unrelated pathogens such as Candida albicans and Staphylococcus aureus (87). The authors further proposed that this epigenetic reprogramming can account for the nonspecific protection provided by BCG vaccination (84). Additional research comparing two mucosal boosting strategies has demonstrated that innate imprinting can impact the outcome of a vaccine (88). In this study, mice were first immunized parenterally with adenovirus-expressing M. tuberculosis Ag85b and then boosted by the mucosal route with either homologous vaccine or a heterologous vesicular stomatitis virus (VSV) vector expressing M. tuberculosis Ag85. Despite the induction of equivalent antigen-specific T cell responses, only mice boosted with the adenovirus vaccine showed significant protection compared to those boosted with the VSV vaccine. Interestingly, boosting with the VSV vaccine was associated with the production of IFN-β by CD11c+b+/− phagocytes, as well as downregulation of IL-12 and NOS2 (88). These observations further underscore the limitation of considering only the quantity and quality of antigen-specific T cell responses as correlates of immune protection. On the whole, these nascent studies highlight how imprinting of innate phagocytes can be utilized to enhance vaccine efficacy against TB and the need for innovative strategies to test whether vaccine candidates induce such modifications in the innate compartment.
Differential interaction of memory T cells with innate immune cells.
Studies have also begun to address whether innate cells respond differently to effector and memory T cells. In this regard, the study by Strutt et al. (89) demonstrated that adoptive transfer of influenza virus-specific memory T cells to a naive recipient resulted in enhanced innate cytokine and chemokine response during secondary challenge. They observed that this enhanced innate inflammatory response was antigen specific and, importantly, independent of pathogen recognition response (PRR). Interestingly, this modulation of the innate response by memory T cells was specific to the Th1 subset but independent of IFN-γ and TNF. This study suggests that memory T cells probably interact differently with the innate component in comparison to naive T cells in a primary immune response. In a recall response, the differential interaction could lower the activation threshold of the innate cells and allow them to rapidly upregulate their antimicrobial effector response. Consistent with this possibility that effector molecules secreted by primary and secondary effector CD4+ T cells are distinct, comparative microarray analysis of the two subsets revealed approximately 450 differentially expressed genes (90). The signaling pathways between the two CD4+ T cell effector types were also found to be different (90). Another recent study also demonstrated how the innate immune response was specifically modified for enhanced protection by memory T cells in both a systemic and a mucosal model of recall response (91). This study showed that in contrast to unvaccinated hosts, memory T cells in vaccinated hosts rapidly initiated the recruitment of innate cells, including monocytes, dendritic cells, and NK cells. Moreover, only memory T cells induced a differentiation program in the recruited innate cells, which comprised elevated expression of effector cytokines and antimicrobial pathways. However, this study found that memory T cells failed to protect if IFN-γ signaling was disrupted in the innate cells, indicating that in vaccinated hosts T cell-derived IFN-γ was key to the heightened effector response by the innate cells. Interestingly, the study that reported IFN-γ-independent modulation of innate cells investigated the effect of CD4+ T memory cells, whereas the study that found a requirement for IFN-γ to enhance effector functions of the innate cells employed a CD8+ T cell memory model. Whether it is IFN-γ or a yet-to-be-identified effector molecule from T cells that directs innate cells to mediate protection, these studies nonetheless underscore the importance of examining the effects of vaccine-induced memory T cells on innate cells. It is likely that the correlates of protection may be unearthed in the memory T cell-modulated innate cells.
WILL STUDY OF THE NATURAL IMMUNE RESPONSE TO M. TUBERCULOSIS USING “OMICS” TECHNOLOGY PROVIDE THE ANSWER TO THE PROBLEM?
Recent developments in high-throughput “omics” technology provide a global view of the genomic, proteomic, and metabolic status of biological systems under investigation, enabling a comprehensive view of biological processes involved in health or the diseased state (92). Such an approach was successfully employed in predicting the immune response to yellow fever vaccine YF-17D in a cohort of healthy humans (93). In this study, the investigators used blood transcriptome profiling to monitor differences in early innate immune response to vaccination in a group of healthy volunteers and how that correlates with the magnitude of subsequent adaptive T and B cell responses. The analysis established a pattern of innate gene expression profiles in the vaccinated individuals that could predict the T cell and antibody response to 90% and 80% accuracy, respectively. A similar approach was later adopted to test the predictive value of early innate signatures for later-phase adaptive immune response to vaccine candidates against influenza (94). In this study, the investigators compared two vaccine candidates against influenza: the live attenuated influenza vaccine and trivalent inactivated influenza vaccine. In a manner similar to the study with the yellow fever vaccine, early immune response to the two influenza vaccine candidates yielded a gene signature that could be correlated with the ensuing adaptive immune response. A key marker, the calmodulin-dependent protein kinase IV (CAMKIV), identified by this strategy was successfully tested in animal models, and its role in the regulation of adaptive response to vaccination was validated (94). This wider use of “omics” technologies and system biology tools in immunology research has led to the establishment of the Human Immunology Project Consortium, which provides a platform to unite researchers engaged in large-scale data-driven research and could represent a powerful tool to use for the discovery of immune correlates of protection against TB.
Similar approaches could be used in TB to identify the correlates of protection of vaccine-mediated immunity. TB disease itself is associated with an inflammatory transcriptional blood signature absent in healthy or most latently infected individuals (95–101). There are several challenging issues in identifying the true correlates of protection against TB, including the lack of good human challenge models and lack of characterization of immune protective cells for ex vivo measurements. To obviate some of these problems, a reasonable approach would be to study the naturally induced protective immunity using “omics” technologies. The household contact (HHC) platform is a good place to start, as it offers the equivalent of a human challenge model. Household contacts of pulmonary TB cases have an intense and protracted exposure to an infectious case. The >20% that resist infection presumably are a group of heavily exposed individuals, some of whom may be protected by a vigorous innate immune response that can be characterized and potentially mimicked by vaccination. Others “self-cure” the infection, presumably by protective adaptive response. This may be indicated by reversion of tuberculin skin test (TST) and interferon gamma release assay (IGRA) from positive to negative. This is seen in 20% of TST convertors who are treated with isoniazid (INH) (102). Cohorts of household contacts also can define the susceptible phenotype, those who progress from infection to disease. The absence of candidate protective correlates in them and the presence in control populations may be of value in characterizing correlates. In fact, the INH-treated cohort may provide an immunologic profile or biomarker of “cure” of the latent focus that can be applied in a broader study of “self-cure”/protective immunity.
Based on the discussion presented here, an “omics” approach combined with single-cell technology (103) to longitudinally monitor rare cell populations, in addition to T cells, in the household contact (HHC) cohort has the potential to provide a comprehensive view of the natural immune response to M. tuberculosis in HHC. Data obtained from these immune analyses, including gene expression profiling, tetramer technology to identify antigen-specific T cells, multiparametric flow technology, and proteomics of the cohort of HHCs can then be pooled to develop predictive models and identification of biological pathways using systems biology tools (104). Modeling should also include the data related to nonspecific protection induced by BCG vaccination and demographic variation in BCG vaccination (105). The models and pathways identified can then be validated in animal models. Together, this approach has the potential to provide biomarkers predictive of a low risk of progression to disease and cure. These biomarkers would be relevant to vaccine development.
CONCLUDING REMARKS
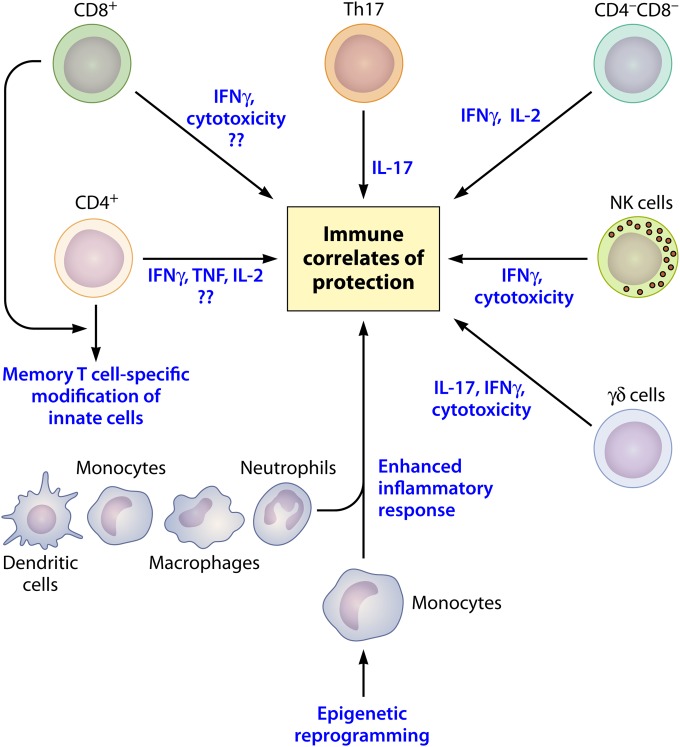
Accumulating evidence is indicative of the deficit that exists in our current understanding of protection in primary TB or vaccination. For logical evolution of newer vaccine candidates and strategies for disease containment, it is of pivotal importance to look beyond assumptions made about the innate and adaptive host immune mechanisms. Although Th1 cells and their cytokine signature form the cornerstone for understanding TB disease manifestation and vaccination outcomes, the discussion presented in this review highlights the importance of analyzing alternate cells and immune mechanisms to better characterize host defense against M. tuberculosis (Fig. 1). These determinants may be T cell effector mechanisms that are IFN-γ independent or may emanate from the interaction of memory T cells with the innate immune system. Study of large cohorts in whom the natural immune response to M. tuberculosis can be followed using “omics” technology is a potentially promising path for uncovering the correlates of protection against TB that will be useful for vaccine studies.
FIG 1.
Finding correlates of vaccine-mediated protection. Protective immunity is dependent on the interactions between innate and adaptive compartments of the immune system. (i) CD4+ and CD8+ T cells exert their effector function via secretion of cytokines and/or cytolysis of infected cells. In addition, a yet-to-be-identified “unknown” mediator may be contributing toward the effector function of these polyfunctional cells. (ii) Th17 cells have a role in host protection via generation of IL-17. (iii) Other immune cells, like NK cells, γδ cells, and CD4− CD8− DN T cells, are also reported to play a role in protective immunity via secretion of inflammatory cytokines and cytotoxic T cell effector-like function. (iv) Additionally, memory T cells generated in response to immunization may modulate the innate immune compartment, resulting in an enhanced proinflammatory cytokine and chemokine response. (v) Epigenetic reprogramming, a key feature of the innate immune system in invertebrates, may also play a role in imprinting the innate cells in vertebrates toward previously encountered antigenic challenge and thereby conferring in them a protective role, independent of the adaptive immune compartment.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI065663 and AI111276 and CRDF Global—USB1-31150-XX-13.
REFERENCES
- 1.World Health Organization. 2014. Global tuberculosis report 2014. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96:29–35. [PubMed] [Google Scholar]
- 3.Ottenhoff TH, Kaufmann SH. 2012. Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog 8:e1002607. doi: 10.1371/journal.ppat.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann SH. 2012. Tuberculosis vaccine development: strength lies in tenacity. Trends Immunol 33:373–379. doi: 10.1016/j.it.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H, Team MATS . 2013. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, Fletcher HA, Hill AVS. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med 10:1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 7.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seneviratne SL, Doffinger R, Macfarlane J, Ceron-Gutierrez L, Amel Kashipaz MR, Robbins A, Patel T, Powell PT, Kumararatne DS, Powell RJ. 2007. Disseminated Mycobacterium tuberculosis infection due to interferon γ deficiency. Response to replacement therapy. Thorax 62:97–99. doi: 10.1136/thx.2005.051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile J-F, Newport M, Levin M, Blanche S, Seboun E, Fischer A, Casanova J-L. 1996. Interferon-γ-receptor deficiency in an infant with fatal Bacille Calmette–Guérin infection. N Engl J Med 335:1956–1962. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, Wallis RS, Edmonds K, Okwera A, Mugerwa R, Peters P, Ellner JJ. 1999. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis 180:2069–2073. doi: 10.1086/315114. [DOI] [PubMed] [Google Scholar]
- 12.Elias D, Akuffo H, Britton S. 2005. PPD induced in vitro interferon gamma production is not a reliable correlate of protection against Mycobacterium tuberculosis. Trans R Soc Trop Med Hyg 99:363–368. doi: 10.1016/j.trstmh.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Mittrücker H-W, Steinhoff U, Köhler A, Krause M, Lazar D, Mex P, Miekley D, Kaufmann SHE. 2007. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc Natl Acad Sci U S A 104:12434–12439. doi: 10.1073/pnas.0703510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, Sanduzzi A, Franken WPJ, Ottenhoff THM, Dieli F. 2010. Multifunctional CD4+ T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol 40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 15.Mattila JT, Diedrich CR, Lin PL, Phuah J, Flynn JL. 2011. Simian immunodeficiency virus-induced changes in T cell cytokine responses in cynomolgus macaques with latent Mycobacterium tuberculosis infection are associated with timing of reactivation. J Immunol 186:3527–3537. doi: 10.4049/jimmunol.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkler S, Necek M, Winkler H, Adegnika AA, Perkmann T, Ramharter M, Kremsner PG. 2005. Increased specific T cell cytokine responses in patients with active pulmonary tuberculosis from Central Africa. Microbes Infect 7:1161–1169. doi: 10.1016/j.micinf.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Duvall MG, Precopio ML, Ambrozak DA, Jaye A, McMichael AJ, Whittle HC, Roederer M, Rowland-Jones SL, Koup RA. 2008. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol 38:350–363. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 19.Lindenstrøm T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, Rosenkrands I, Andersen P. 2009. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol 182:8047–8055. doi: 10.4049/jimmunol.0801592. [DOI] [PubMed] [Google Scholar]
- 20.Derrick SC, Yabe IM, Yang A, Morris SL. 2011. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine 29:2902–2909. doi: 10.1016/j.vaccine.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Kagina BMN, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, Gamieldien H, Sidibana M, Hatherill M, Gelderbloem S, Mahomed H, Hawkridge A, Hussey G, Kaplan G, Hanekom WA. 2010. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guérin vaccination of newborns. Am J Respir Crit Care Med 182:1073–1079. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkridge T, Scriba TJ, Gelderbloem S, Smit E, Tameris M, Moyo S, Lang T, Veldsman A, Hatherill M, Merwe L, Fletcher HA, Mahomed H, Hill AV, Hanekom WA, Hussey GD, McShane H. 2008. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis 198:544–552. doi: 10.1086/590185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallegos AM, van Heijst JW, Samstein M, Su X, Pamer EG, Glickman MS. 2011. A gamma interferon independent mechanism of CD4 T cell mediated control of M. tuberculosis infection in vivo. PLoS Pathog 7:e1002052. doi: 10.1371/journal.ppat.1002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wozniak TM, Saunders BM, Ryan AA, Britton WJ. 2010. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect Immun 78:4187–4194. doi: 10.1128/IAI.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, Reinhart TA, Kolls J, Randall TD, Connell TD, Khader SA. 2013. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol 6:972–984. doi: 10.1038/mi.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowley SC, Elkins KL. 2003. CD4+ T cells mediate IFN-gamma-independent control of Mycobacterium tuberculosis infection both in vitro and in vivo. J Immunol 171:4689–4699. doi: 10.4049/jimmunol.171.9.4689. [DOI] [PubMed] [Google Scholar]
- 27.Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, Chan J, Braunstein M, Orme IM, Derrick SC, Morris SL, Jacobs WR Jr, Porcelli SA. 2007. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest 117:2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, Shen Y, Halliday L, Fortman J, McAllister M, Estep J, Hunt R, Vasconcelos D, Du G, Porcelli SA, Larsen MH, Jacobs WR Jr, Haynes BF, Letvin NL, Chen ZW. 2009. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog 5:e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, Modlin RL, Antoni C, Stenger S. 2009. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest 119:1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehlers S. 2005. Why does tumor necrosis factor targeted therapy reactivate tuberculosis? J Rheumatol Suppl 74:35–39. [PubMed] [Google Scholar]
- 31.Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Junecko BA, Fountain JJ, Martino C, Pearl JE, Tighe M, Lin YY, Slight S, Kolls JK, Reinhart TA, Randall TD, Cooper AM. 2011. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol 187:5402–5407. doi: 10.4049/jimmunol.1101377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. 2005. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-{gamma} responses if IL-12p70 is available. J Immunol 175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- 33.Freches D, Korf H, Denis O, Havaux X, Huygen K, Romano M. 2013. Mice genetically inactivated in interleukin-17A receptor are defective in long-term control of Mycobacterium tuberculosis infection. Immunology 140:220–231. doi: 10.1111/imm.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gopal R, Monin L, Slight S, Uche U, Blanchard E, Fallert Junecko BA, Ramos-Payan R, Stallings CL, Reinhart TA, Kolls JK, Kaushal D, Nagarajan U, Rangel-Moreno J, Khader SA. 2014. Unexpected role for IL-17 in protective immunity against hypervirulent Mycobacterium tuberculosis HN878 infection. PLoS Pathog 10:e1004099. doi: 10.1371/journal.ppat.1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, Ahmed M, Kalinski P, Khader SA. 2012. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol 42:364–373. doi: 10.1002/eji.201141569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 37.Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, Alcorn JF, Weaver CT, Kolls JK. 2011. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity 35:997–1009. doi: 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole G, Klein B. 2011. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest 121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. 2009. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood 113:1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP. 2011. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J, Klimczak A, Lange A, Zou W. 2011. Human TH17 cells are long-lived effector memory cells. Sci Transl Med 3:104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. 2005. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med 11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 43.Zhao DM, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, Harty JT, Xue HH. 2010. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol 184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP. 2009. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med 15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Staal FJ, Luis TC, Tiemessen MM. 2008. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol 8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 46.Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. 2008. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell 2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andersen P, Smedegaard B. 2000. CD4(+) T-cell subsets that mediate immunological memory to Mycobacterium tuberculosis infection in mice. Infect Immun 68:621–629. doi: 10.1128/IAI.68.2.621-629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kipnis A, Irwin S, Izzo AA, Basaraba RJ, Orme IM. 2005. Memory T lymphocytes generated by Mycobacterium bovis BCG vaccination reside within a CD4 CD44lo CD62 ligand(hi) population. Infect Immun 73:7759–7764. doi: 10.1128/IAI.73.11.7759-7764.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paust S, von Andrian UH. 2011. Natural killer cell memory. Nat Immunol 12:500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 50.Colucci F, Caligiuri MA, Di Santo JP. 2003. What does it take to make a natural killer? Nat Rev Immunol 3:413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 51.Junqueira-Kipnis AP, Kipnis A, Jamieson A, Juarrero MG, Diefenbach A, Raulet DH, Turner J, Orme IM. 2003. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol 171:6039–6045. doi: 10.4049/jimmunol.171.11.6039. [DOI] [PubMed] [Google Scholar]
- 52.Feng CG, Kaviratne M, Rothfuchs AG, Cheever A, Hieny S, Young HA, Wynn TA, Sher A. 2006. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J Immunol 177:7086–7093. doi: 10.4049/jimmunol.177.10.7086. [DOI] [PubMed] [Google Scholar]
- 53.Vankayalapati R, Wizel B, Weis SE, Safi H, Lakey DL, Mandelboim O, Samten B, Porgador A, Barnes PF. 2002. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol 168:3451–3457. doi: 10.4049/jimmunol.168.7.3451. [DOI] [PubMed] [Google Scholar]
- 54.Vankayalapati R, Garg A, Porgador A, Griffith DE, Klucar P, Safi H, Girard WM, Cosman D, Spies T, Barnes PF. 2005. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol 175:4611–4617. doi: 10.4049/jimmunol.175.7.4611. [DOI] [PubMed] [Google Scholar]
- 55.Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV, Vankayalapati R. 2009. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol 183:6639–6645. doi: 10.4049/jimmunol.0902587. [DOI] [PubMed] [Google Scholar]
- 56.Gasteiger G, Hemmers S, Bos PD, Sun JC, Rudensky AY. 2013. IL-2-dependent adaptive control of NK cell homeostasis. J Exp Med 210:1179–1187. doi: 10.1084/jem.20122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Firth MA, Madera S, Beaulieu AM, Gasteiger G, Castillo EF, Schluns KS, Kubo M, Rothman PB, Vivier E, Sun JC. 2013. Nfil3-independent lineage maintenance and antiviral response of natural killer cells. J Exp Med 210:2981–2990. doi: 10.1084/jem.20130417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun JC, Beilke JN, Lanier LL. 2009. Adaptive immune features of natural killer cells. Nature 457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dhiman R, Periasamy S, Barnes PF, Jaiswal AG, Paidipally P, Barnes AB, Tvinnereim A, Vankayalapati R. 2012. NK1.1+ cells and IL-22 regulate vaccine-induced protective immunity against challenge with Mycobacterium tuberculosis. J Immunol 189:897–905. doi: 10.4049/jimmunol.1102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boom WH. 1999. Gammadelta T cells and Mycobacterium tuberculosis. Microbes Infect 1:187–195. doi: 10.1016/S1286-4579(99)80033-1. [DOI] [PubMed] [Google Scholar]
- 61.Poccia F, Malkovsky M, Pollak A, Colizzi V, Sireci G, Salerno A, Dieli F. 1999. In vivo gammadelta T cell priming to mycobacterial antigens by primary Mycobacterium tuberculosis infection and exposure to nonpeptidic ligands. Mol Med 5:471–476. [PMC free article] [PubMed] [Google Scholar]
- 62.Lockhart E, Green AM, Flynn JL. 2006. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 63.Dieli F, Ivanyi J, Marsh P, Williams A, Naylor I, Sireci G, Caccamo N, Di Sano C, Salerno A. 2003. Characterization of lung gamma delta T cells following intranasal infection with Mycobacterium bovis bacillus Calmette-Guerin. J Immunol 170:463–469. doi: 10.4049/jimmunol.170.1.463. [DOI] [PubMed] [Google Scholar]
- 64.Janis EM, Kaufmann SH, Schwartz RH, Pardoll DM. 1989. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science 244:713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- 65.Okamoto Yoshida Y, Umemura M, Yahagi A, O'Brien RL, Ikuta K, Kishihara K, Hara H, Nakae S, Iwakura Y, Matsuzaki G. 2010. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol 184:4414–4422. doi: 10.4049/jimmunol.0903332. [DOI] [PubMed] [Google Scholar]
- 66.Nabeshima S, Hiromatsu K, Matsuzaki G, Mukasa A, Takada H, Yoshida S, Nomoto K. 1995. Infection of Mycobacterium bovis bacillus Calmette-Guerin in antibody-mediated gamma delta T-cell-depleted mice. Immunology 84:317–321. [PMC free article] [PubMed] [Google Scholar]
- 67.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW. 2002. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science 295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dieli F, Troye-Blomberg M, Ivanyi J, Fournie JJ, Bonneville M, Peyrat MA, Sireci G, Salerno A. 2000. Vgamma9/Vdelta2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur J Immunol 30:1512–1519. doi:. [DOI] [PubMed] [Google Scholar]
- 69.Spencer CT, Abate G, Sakala IG, Xia M, Truscott SM, Eickhoff CS, Linn R, Blazevic A, Metkar SS, Peng G, Froelich CJ, Hoft DF. 2013. Granzyme A produced by gamma(9)delta(2) T cells induces human macrophages to inhibit growth of an intracellular pathogen. PLoS Pathog 9:e1003119. doi: 10.1371/journal.ppat.1003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dieli F, Troye-Blomberg M, Ivanyi J, Fournie JJ, Krensky AM, Bonneville M, Peyrat MA, Caccamo N, Sireci G, Salerno A. 2001. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis 184:1082–1085. doi: 10.1086/323600. [DOI] [PubMed] [Google Scholar]
- 71.Lee J, Choi K, Olin MR, Cho SN, Molitor TW. 2004. Gammadelta T cells in immunity induced by Mycobacterium bovis bacillus Calmette-Guerin vaccination. Infect Immun 72:1504–1511. doi: 10.1128/IAI.72.3.1504-1511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoft DF, Brown RM, Roodman ST. 1998. Bacille Calmette-Guerin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol 161:1045–1054. [PubMed] [Google Scholar]
- 73.Cowley SC, Hamilton E, Frelinger JA, Su J, Forman J, Elkins KL. 2005. CD4-CD8- T cells control intracellular bacterial infections both in vitro and in vivo. J Exp Med 202:309–319. doi: 10.1084/jem.20050569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Derrick SC, Repique C, Snoy P, Yang AL, Morris S. 2004. Immunization with a DNA vaccine cocktail protects mice lacking CD4 cells against an aerogenic infection with Mycobacterium tuberculosis. Infect Immun 72:1685–1692. doi: 10.1128/IAI.72.3.1685-1692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J, Santosuosso M, Ngai P, Zganiacz A, Xing Z. 2004. Activation of CD8 T cells by mycobacterial vaccination protects against pulmonary tuberculosis in the absence of CD4 T cells. J Immunol 173:4590–4597. doi: 10.4049/jimmunol.173.7.4590. [DOI] [PubMed] [Google Scholar]
- 76.Sambandamurthy VK, Derrick SC, Hsu T, Chen B, Larsen MH, Jalapathy KV, Chen M, Kim J, Porcelli SA, Chan J, Morris SL, Jacobs WR Jr. 2006. Mycobacterium tuberculosis DeltaRD1 DeltapanCD: a safe and limited replicating mutant strain that protects immunocompetent and immunocompromised mice against experimental tuberculosis. Vaccine 24:6309–6320. doi: 10.1016/j.vaccine.2006.05.097. [DOI] [PubMed] [Google Scholar]
- 77.Derrick SC, Evering TH, Sambandamurthy VK, Jalapathy KV, Hsu T, Chen B, Chen M, Russell RG, Junqueira-Kipnis AP, Orme IM, Porcelli SA, Jacobs WR Jr, Morris SL. 2007. Characterization of the protective T-cell response generated in CD4-deficient mice by a live attenuated Mycobacterium tuberculosis vaccine. Immunology 120:192–206. doi: 10.1111/j.1365-2567.2006.02491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zufferey C, Germano S, Dutta B, Ritz N, Curtis N. 2013. The contribution of non-conventional T cells and NK cells in the mycobacterial-specific IFNgamma response in Bacille Calmette-Guerin (BCG)-immunized infants. PLoS One 8:e77334. doi: 10.1371/journal.pone.0077334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun JC, Ugolini S, Vivier E. 2014. Immunological memory within the innate immune system. EMBO J 33:1295–1303. doi: 10.1002/embj.201387651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kurtz J, Franz K. 2003. Innate defence: evidence for memory in invertebrate immunity. Nature 425:37–38. doi: 10.1038/425037a. [DOI] [PubMed] [Google Scholar]
- 81.Dong Y, Cirimotich CM, Pike A, Chandra R, Dimopoulos G. 2012. Anopheles NF-kappaB-regulated splicing factors direct pathogen-specific repertoires of the hypervariable pattern recognition receptor AgDscam. Cell Host Microbe 12:521–530. doi: 10.1016/j.chom.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. 2007. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathog 3:e26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leclerc V, Reichhart JM. 2004. The immune response of Drosophila melanogaster. Immunol Rev 198:59–71. doi: 10.1111/j.0105-2896.2004.0130.x. [DOI] [PubMed] [Google Scholar]
- 84.Netea MG. 2013. Training innate immunity: the changing concept of immunological memory in innate host defence. Eur J Clin Invest 43:881–884. doi: 10.1111/eci.12132. [DOI] [PubMed] [Google Scholar]
- 85.Akira S. 2006. TLR signaling. Curr Top Microbiol Immunol 311:1–16. [DOI] [PubMed] [Google Scholar]
- 86.Foster SL, Hargreaves DC, Medzhitov R. 2007. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 87.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JW, van Crevel R, Netea MG. 2012. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jeyanathan M, Damjanovic D, Shaler CR, Lai R, Wortzman M, Yin C, Zganiacz A, Lichty BD, Xing Z. 2013. Differentially imprinted innate immunity by mucosal boost vaccination determines antituberculosis immune protective outcomes, independent of T-cell immunity. Mucosal Immunol 6:612–625. doi: 10.1038/mi.2012.103. [DOI] [PubMed] [Google Scholar]
- 89.Strutt TM, McKinstry KK, Dibble JP, Winchell C, Kuang Y, Curtis JD, Huston G, Dutton RW, Swain SL. 2010. Memory CD4+ T cells induce innate responses independently of pathogen. Nat Med 16:558–564. doi: 10.1038/nm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Strutt TM, McKinstry KK, Kuang Y, Bradley LM, Swain SL. 2012. Memory CD4+ T-cell-mediated protection depends on secondary effectors that are distinct from and superior to primary effectors. Proc Natl Acad Sci U S A 109:E2551–E2560. doi: 10.1073/pnas.1205894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soudja SM, Chandrabos C, Yakob E, Veenstra M, Palliser D, Lauvau G. 2014. Memory-T-cell-derived interferon-gamma instructs potent innate cell activation for protective immunity. Immunity 40:974–988. doi: 10.1016/j.immuni.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tanca A, Deligios M, Addis MF, Uzzau S. 2013. High throughput genomic and proteomic technologies in the fight against infectious diseases. J Infect Dev Ctries 7:182–190. doi: 10.3855/jidc.3027. [DOI] [PubMed] [Google Scholar]
- 93.Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, Kennedy K, Wu H, Bennouna S, Oluoch H, Miller J, Vencio RZ, Mulligan M, Aderem A, Ahmed R, Pulendran B. 2009. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol 10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li G-M, McCausland M, Kanchan V, Kokko KE, Li S, Elbein R, Mehta AK, Aderem A, Subbarao K, Ahmed R, Pulendran B. 2011. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol 12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O'Garra A. 2010. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mistry R, Cliff JM, Clayton CL, Beyers N, Mohamed YS, Wilson PA, Dockrell HM, Wallace DM, van Helden PD, Duncan K, Lukey PT. 2007. Gene-expression patterns in whole blood identify subjects at risk for recurrent tuberculosis. J Infect Dis 195:357–365. doi: 10.1086/510397. [DOI] [PubMed] [Google Scholar]
- 97.Maertzdorf J, Ota M, Repsilber D, Mollenkopf HJ, Weiner J, Hill PC, Kaufmann SHE. 2011. Functional correlations of pathogenesis-driven gene expression signatures in tuberculosis. PLoS One 6:e26938. doi: 10.1371/journal.pone.0026938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ottenhoff THM, Dass RH, Yang N, Zhang MM, Wong HEE, Sahiratmadja E, Khor CC, Alisjahbana B, van Crevel R, Marzuki S, Seielstad M, van de Vosse E, Hibberd ML. 2012. Genome-wide expression profiling identifies type 1 interferon response pathways in active tuberculosis. PLoS One 7:e45839. doi: 10.1371/journal.pone.0045839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lesho E, Forestiero FJ, Hirata MH, Hirata RD, Cecon L, Melo FF, Paik SH, Murata Y, Ferguson EW, Wang Z, Ooi GT. 2011. Transcriptional responses of host peripheral blood cells to tuberculosis infection. Tuberculosis 91:390–399. doi: 10.1016/j.tube.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 100.Bloom CI, Graham CM, Berry MPR, Rozakeas F, Redford PS, Wang Y, Xu Z, Wilkinson KA, Wilkinson RJ, Kendrick Y, Devouassoux G, Ferry T, Miyara M, Bouvry D, Dominique V, Gorochov G, Blankenship D, Saadatian M, Vanhems P, Beynon H, Vancheeswaran R, Wickremasinghe M, Chaussabel D, Banchereau J, Pascual V, Ho L-P, Lipman M, O'Garra A. 2013. Transcriptional blood signatures distinguish pulmonary tuberculosis, pulmonary sarcoidosis, pneumonias and lung cancers. PLoS One 8:e70630. doi: 10.1371/journal.pone.0070630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jacobsen M, Repsilber D, Gutschmidt A, Neher A, Feldmann K, Mollenkopf H, Ziegler A, Kaufmann SE. 2007. Candidate biomarkers for discrimination between infection and disease caused by Mycobacterium tuberculosis. J Mol Med 85:613–621. doi: 10.1007/s00109-007-0157-6. [DOI] [PubMed] [Google Scholar]
- 102.Johnson DF, Malone LL, Zalwango S, Mukisa Oketcho J, Chervenak KA, Thiel B, Mayanja-Kizza H, Stein CM, Boom WH, Lancioni CL, Tuberculosis Research Unit . 2014. Tuberculin skin test reversion following isoniazid preventive therapy reflects diversity of immune response to primary Mycobacterium tuberculosis infection. PLoS One 9:e96613. doi: 10.1371/journal.pone.0096613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chattopadhyay PK, Gierahn TM, Roederer M, Love JC. 2014. Single-cell technologies for monitoring immune systems. Nat Immunol 15:128–135. doi: 10.1038/ni.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aderem A, Adkins JN, Ansong C, Galagan J, Kaiser S, Korth MJ, Law GL, McDermott JG, Proll SC, Rosenberger C, Schoolnik G, Katze MG. 2011. A systems biology approach to infectious disease research: innovating the pathogen-host research paradigm. mBio 2(1):e00325-10. doi: 10.1128/mBio.00325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fine PEM. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339–1345. doi: 10.1016/S0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]