Abstract
Traditionally, the design of new vaccines directed against Mycobacterium tuberculosis, the most successful bacterial pathogen on the planet, has focused on prophylactic candidates that would be given to individuals while they are still young. It is becoming more apparent, however, that there are several types of vaccine candidates now under development that could be used under various conditions. Thus, in addition to prophylactic vaccines, such as recombinant Mycobacterium bovis BCG or BCG-boosting vaccines, other applications include vaccines that could prevent infection, vaccines that could be given in emergency situations as postexposure vaccines, vaccines that could be used to facilitate chemotherapy, and vaccines that could be used to reduce or prevent relapse and reactivation disease. These approaches are discussed here, including the type of immunity we are trying to specifically target, as well as the limitations of these approaches.
INTRODUCTION
It is currently estimated that >2 billion people have been exposed to Mycobacterium tuberculosis, with >8 million people showing active disease and potentially as many as 2 million people dying each year (1), making tuberculosis (TB) the world's most successful bacterial pathogen. A global emergency by itself, the problem is further compounded by the concomitant HIV epidemic, which renders people highly vulnerable to TB, and the sinister continuing rise in drug resistance among newly emerging isolates (2, 3).
To date, the Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine remains the only licensed vaccine for the prevention of TB. It is traditionally given to newborns, and in that population, it has a protective effect. However, this is not sustained, and the general consensus is now that the vaccine provides little protection in adult individuals (4).
This has prompted a concerted effort over the past 2 decades to develop new candidates, to improve BCG, to boost it, or to replace it altogether (5–8). Although multiple innovative approaches have been proposed and tried, progress has been slow, and only one major candidate has completed a phase IIb efficacy trial, with disappointing results (9). Despite this failure, this experience has been very useful, because it forces the field to reassess exactly how we design vaccines and how we test them (10).
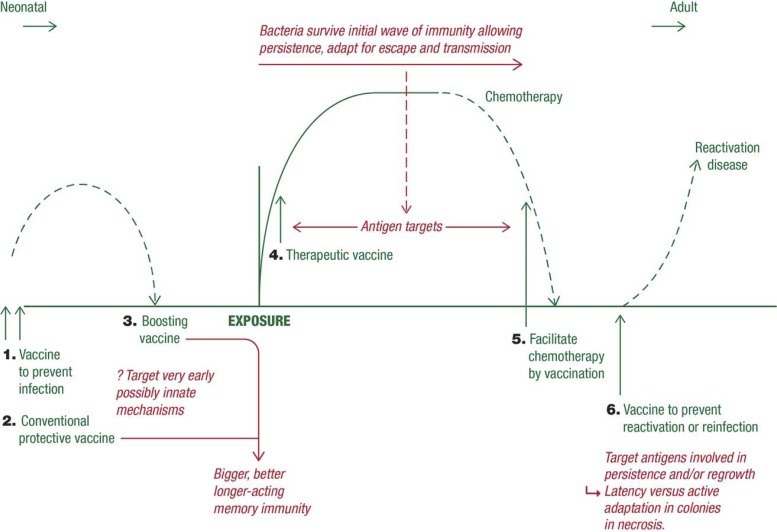
One conventionally regards TB vaccine development in terms of a prophylactic vaccine given to young people that will potentially protect them from infection at a later time (for decades, it is hoped). However, as more and more approaches have been developed, it has emerged that there are multiple potential uses or applications of certain candidates, as illustrated in Fig. 1. Traditionally, prevention or reduction of the severity of the disease process was our primary target, but there is now some speculation that a candidate could be found that could act rapidly to prevent the actual establishment of a site of infection in the first place. In addition, much energy has been expended on finding ways to boost immunity engendered by BCG vaccination, a logical approach given the >80% coverage of this vaccine worldwide.
FIG 1.
Schematic showing multiple potential applications of certain candidate TB vaccines.
Another approach garnering increased attention is the use of therapeutic or postexposure vaccines that could be given to already infected individuals or those potentially exposed deliberately in a bioterror situation. These potentially could also be given as a means to potentiate the administration of chemotherapy and facilitate and improve the rate and degree of bacterial clearance. Finally, given that relapse of disease even after apparently successful chemotherapy is a continuing problem, a sixth type of TB vaccine that could be of substantial usefulness would be one that could be given to prevent reactivation disease and relapse.
Some progress has already been made, at least in some situations. These are reviewed here, including the context of what types of host immunity these new candidates should be inducing and how we can design candidates to achieve this.
VACCINES PREVENTING INFECTION
At this time, a vaccine that could induce a form of immunity that, even years later, would prevent infection rather than disease is purely theoretical, but this idea is now receiving active attention. The problems here are formidable, as recently discussed (11), not only in the identification of such vaccines—which may require substantial lateral thinking—but also in terms of how the action of such candidates could be measured and validated. Above all, such approaches would have to be integrated into the physiological mechanisms present in the lungs, which are themselves complicated (11).
Within the upper respiratory tract, there are antibodies (IgA, IgD) present in the mucus that could potentially be exclusionary (12) or capture bacilli higher up the airway, and several cell populations, including NK cells, γδ T cell receptor cells, and other populations such as mucosa-associated invariant T cells, all of which are potential TH1 cytokine producers. However, even if ways could be found to somehow vaccinate the host to improve the responsiveness of these cells (for example, an earlier attempt to vaccinate via CD1 presentation was not successful [13]), it is hard to envisage how this could influence bacilli passing by in the tidal air. One possibility recently suggested (14) is to enhance the activity of local monocytes, assuming that these could be focused correctly at sites of initial bacterial implantation.
If successful “anti-infection” interventions could be designed, they would most likely have to work at the level of the alveolar macrophage, usually the first cell to encounter the bacterium, and failing that, quickly after the bacillus has reached the pulmonary interstitium (15). One possibility is to design vaccines that could induce opsonizing antibodies that could facilitate bacterial killing by the alveolar macrophage, but an impediment here is that the protein content (including antibodies) of the lung surfactant is very tightly controlled in order to preserve the surface tension properties of this layer.
After the bacteria erode and enter the interstitium and establish a site of infection, there are potentially further options to prevent further progression to a disease state, but these would have to deal with various processes. These include the carriage of bacteria away to the draining lymph nodes, which is needed to trigger the eventual acquired immune response (16), which also causes dissemination of the infection (an early event, particularly in the guinea pig model [17]). The bacilli are carried by dendritic cells, raising the possibility that acceleration of this event could be beneficial if it generated immunity faster (18). In addition, there is a considerable neutrophil response in the interstitium, driven by the local inflammatory conditions, and we have previously suggested (15) that the degranulation of these cells releases hydrolytic enzymes that cause local tissue damage, including that of the capillary blood vessel endothelium, resulting in the formation of initial pockets of necrosis (19, 20). Since we now propose (15, 21) that this necrosis provides a safe harbor for extracellular, persisting bacteria, obviously any infection-preventing vaccination process must be able to completely prevent this.
CONVENTIONAL VACCINES ESTABLISHING ACQUIRED SPECIFIC RESISTANCE
(i) Improving BCG.
The gradual realization that the BCG vaccine was far from perfect and exhibited substantial variation in efficacy, coupled with increasing evidence that it had little or no protective effect in adults, drove research into ways it could be improved or replaced altogether.
Much of this research was innovative. The earliest approach was to take the existing BCG vaccine and, given the knowledge that early secreted proteins of the bacillus are immunogenic (22), incorporate them into the bacterium to make a recombinant vaccine. The first attempt to achieve this (23) resulted in a recombinant BCG (rBCG) strain (Tice) overexpressing the 30-kDa antigen (Ag85B) that had protective activity superior to that of the BCG control strain in the relevant guinea pig model. This candidate was not further pursued for regulatory reasons when it was noted that it contained an antibiotic resistance marker, but it nevertheless provided proof of the principle that regular BCG could potentially be improved.
Further development of rBCG candidates then resulted in the new candidate VPM1002 (24), now being produced by a company in Germany, and further new candidates produced by the Aeras Foundation. VPM1002 is an rBCG Prague strain that expresses the lysin molecule from Listeria and in which the urease subunit C gene has been inactivated. The concept here is that this will allow escape of M. tuberculosis antigens from the phagosome into the cytoplasm, giving a broader and more potent T cell response by promoting macrophage apoptosis and cross-presentation of antigens (25). Studies with animals indicate superior protective responses to this candidate, which is now in early clinical trials.
At Aeras, an early prototype was produced that used a different lysin (perfringolysin) and in addition overexpressed the M. tuberculosis antigens Ag85A, Ag85B, and Rv3407. This candidate was very safe and was then tested in a challenge model using the relevant clinical isolate HN878. This isolate is extremely virulent in mice (26, 27), but in the Aeras study, it took >300 days to kill control mice, indicating severe attenuation of the isolate stock. This is in contrast to an earlier study in my laboratory (27) in which HN878 killed mice in 70 to 90 days. These results proved moot, however, when the vaccine was abruptly withdrawn after inducing shingles in volunteers in initial safety studies.
These results apart, there is optimism that an improved BCG candidate can be produced, but whether this can reduce the variability classically seen with the existing vaccine will take time to determine. A further issue, not discussed much yet (21), reflects the finding by Comas and colleagues (28) that shows the hyperconservation of major immunogenic T cell epitopes right across the main families of M. tuberculosis strains. One explanation for this is that it ensures a strong T cell response to the infecting organism, maximizing lung damage and tissue necrosis, thus allowing surviving bacilli to hide in organized biofilm-like communities safe from T cell immunity (and drugs [29, 30]) until they can escape into cavities or airways, allowing their transmission. Whether vaccines that increase T cell recognition of such epitopes (given their presence in several rBCG and fusion protein candidates) will eventually have detrimental outcomes is not known (and, as yet at least, not observed in animal models).
(ii) Fusion proteins.
A second major class of candidate vaccines consists of fusion protein constructs. This approach was initially used to avoid quality control issues in manufacturing, but it is now clear this is an attractive approach that has produced several potentially useful candidates. One of the first candidates in this class was M72, a fusion of Rv1196 and Rv0125 given in AS01 adjuvant, which was found to be protective in mouse and guinea pig challenge models (31, 32), including the capacity to effectively boost BCG (31). Results obtained with nonhuman primates (NHPs) were equally encouraging (33), and this candidate is in clinical trials (34).
Hybrid-4 (H4; Ag85B and TB10.4) and H56 (Ag85B, ESTA-6, and Rv2660), both delivered in IC31 adjuvant, are immunogenic vaccine candidates that have shown good activity in small-animal models (35, 36). The Rv2660 antigen in H56 was included as a “latent-TB” antigen, and while it appears to contribute to efficacy, the actual model in which this was achieved (incomplete chemotherapy of mice after infection) has been criticized (8) as unrealistic. In addition, studies trying to boost BCG with H56 in macaques were less compelling (37), as discussed further below.
ID93 is a fusion polyprotein incorporating Rv2608, Rv3619, Rv3620, and Rv1813. When delivered in a potent glucopyranosyl lipid adjuvant (GLA), this fusion vaccine gives excellent protection in animal models (38, 39).
(iii) Auxotrophs and other mutants.
This area of the field originally sprang from the idea (40) that auxotrophic mutants of M. tuberculosis might be a better option that BCG for new-generation vaccines, as long, of course, as they could be shown to be safe.
An early approach (41) made a mutant of H37Rv lacking both the leuD and panCD genes, producing an auxotroph deficient in leucine and pantothenate biosynthesis. Guinea pigs were then vaccinated with this mutant and challenged by low-dose aerosol with H37Rv. The mutant, whether given once or twice, protected these animals to the same extent as BCG. A single dose of the vaccine gave lung immunopathology similar to that of BCG-vaccinated animals, but animals given the boost had extensive lung pathology (lymphocytic lesions) and lung consolidation (a “too much of a good thing” type of observation others have also made). This was followed (42) by the demonstration that the double mutant was safe and that protection by the mutant was long lived (7-month vaccine-to-challenge interval).
Another mutant that had considerable protective capacity was developed by deletion of the secA2 gene of M. tuberculosis (43). In addition to a strong CD4 response to the mutant, there was also markedly increased priming of antigen-specific CD8 T cells in vivo, and it was then found that vaccination of mice and guinea pigs with this mutant produced significantly greater resistance to an M. tuberculosis challenge than BCG did. Of particular note was the rapid prevention of mediastinal lymph node damage by the ΔsecA2 vaccine, which is an early and prominent event in unprotected animals (17).
A further mutant, designated SO2 (44), has been generated that is based on inactivation of the PhoP response regulator of M. tuberculosis. Good results with this mutant have been reported in in vitro studies, as well as in mice, guinea pigs, and macaques (45–48), and an attraction of this candidate is that, unlike BCG, it appears to be a good inducer of central memory T cell responses.
A completely different approach was the production (49) of a mutant of M. smegmatis in which the esx-3 locus was deleted (IKE) and then replaced with the orthologous esx-3 genes from M. tuberculosis (producing the mutant IKEPLUS). The resulting mutant was readily cleared by mice via an MyD88-dependent mechanism but in the process, an extremely high level of protective immunity generated. This includes a strong central memory response (I. M. Orme and M. Henao-Tamayo, unpublished observation), raising the question of whether a mixture of IKEPLUS and BCG given together could improve the vaccination process simply by providing a broader range of T cell subset responses.
At this time, with the exception of SO2, none of these candidates have been moved toward clinical trials, and there seems to be little enthusiasm among the entities that control this process to do so. This could be a tactical mistake when considered in the context that we may need vaccines with “more muscle” than BCG, given the extreme virulence of at least some of the newly emerging clinical isolates (50–52).
(iv) Immune targets.
When mice and guinea pigs are challenged, usually several weeks after BCG vaccination, the effector immunity engendered by the vaccine controls and contains the infection by way of production of the TH1 cytokine pathway response. This results in the activation of macrophages infected with the bacilli and the formation of a granuloma designed to prevent further dissemination of the infection (53). Because a finite time is needed to fully express this immunity, the initial outcome is a reduction of the bacterial load in the lungs, usually to a 1- to 1.5-log lower level than in controls, rather than any form of sterilization.
Protection is mediated predominantly by CD4 cells, with CD8 cells representing a smaller responding population. The CD4 cells are often multifunctional, in the sense that they secrete gamma interferon (IFN-γ), tumor necrosis factor alpha, and interleukin-2 in various proportions. If the challenge is not given at this point, however, then T cells in the lungs and other organs gradually take on the characteristics of memory T cells, and while these are often “multifunctional” as well, it was recently correctly argued (54) that care is needed in assigning a specific memory function (in studies with children, these cells may actually represent secondary effector cells [55]). In the lungs of mice given BCG 100 to 200 days earlier, most of the secondary effector cells are due to an effector memory T cell response (56), with central memory cells representing only a minor subset—this has been suggested (57) as a cause for the gradual loss of protection in children as they grow older.
Other subsets may also be present. CD4 cells with a naive phenotype are present, mostly in the spleen, and may represent a stem cell-like subset. TH17 cells can also be seen, including in a recall model (58), and may themselves be an important component of the overall memory response (59, 60).
Can these responses be improved? Simply finding ways or candidates to increase IFN-γ responses, once thought logical, may not be the answer since it is now mostly agreed that this does not provide a useful correlate of vaccine protection or efficacy. Another possibility is to try to vaccinate in such a way as to push the memory response more toward the central memory, which is more rapidly reactive than effector immunity (one example is ΔIKEPLUS, which induces good central memory responses, as noted above), and there is now evidence this can also be achieved by antigen delivery in cationic liposomes (61). Another possibility could be to establish a larger reservoir of stem cell-like memory cells, given their very high proliferative capacity (62). At the end of the day, however, our understanding of the memory immune response to TB vaccine candidates, as well as BCG itself, is still very limited, and this is a major impediment to further research.
BCG-BOOSTING VACCINES
A significant proportion of the research in the field has concentrated, quite understandably, on candidates that can be given to individuals already vaccinated with BCG in an effort to improve outcomes. As noted in Fig. 1, the age of the individual when a vaccine is given is a factor, and this applies particularly to BCG-boosting vaccines. BCG is usually given soon after birth, and hence, finding some way to boost immunity engendered by neonatal BCG vaccination is the most practical avenue of approach, unless a highly effective vaccine can be found to replace BCG. The lead candidates in this regard are virus based; MVA85A is based on vaccinia virus, and Aeras-402 uses adenovirus type 35 to deliver the Ag85B and TB10.4 antigens.
In this regard, a very comprehensive review by Brennan and his colleagues (63) lists the growing number of studies that have tried various priming-boosting protocols, rather helpfully divided into those that seemed to work, those that provided no better effect than BCG alone, and (a much smaller number) those where nothing worked at all. Nearly 50 studies were listed in this 2012 review. This is a tribute to the industry of our field, but one must also note that virtually all of those studies used the laboratory-adapted strain H37Rv or Erdman for the challenge infection—it is unclear if similar positive results would have been obtained if virulent clinical strains had been used. This is important, because BCG is less effective against clinical isolates that are capable of inducing regulatory T cells (64), and hence it would be valuable to know if boosting vaccines can overcome these negative effects. Nor is this a new concern, given the results of an important study published over a decade ago (65).
A further weakness, also brought out by this review (63), is the complete lack of standardization of boosting protocols, not just the choice of the challenge strain but the regimens used, vaccine doses, routes used, and so forth, even the conditions of the animals themselves (barrier conditions, etc.).
We know that BCG induces CD4 T cells that behave as memory cells. Their longevity is not known in humans, but “about a decade” is a reasonable assumption given the onset of disease in people already vaccinated. So, when do we apply boosting? The first approach, recently tried (9), is relatively shortly after BCG vaccination, but here we do not know the extent to which effector immunity has transformed into or been replaced by true memory immunity. A second approach is to try to predict when memory immunity has peaked and give a vaccine booster to try to expand this even further. The third approach is to wait until we think memory might be waning, i.e., 8 to 10 years of age, and reinvigorate or reexpand this immunity with a boost.
Because of a combination of funding issues and impatience, these factors have never been seriously addressed in our animal models. One standard protocol is to give BCG to mice and then about 6 weeks later start boosting vaccinations. Here, the boost is being given at the height of effector immunity, not memory, in the animal. In some cases, an improvement can be seen (lower CFU counts in the lungs after a challenge), but often as not, no increase in protection is seen (BCG is protecting well enough and cannot be added to under the constraints of the model itself), or protection can actually decrease because the boost induces effector T cell contraction and exhaustion.
A better option is to let memory immunity to the BCG vaccine develop first, which seems to take a minimum of 10 weeks or so in small-animal models (peak levels are probably reached after about 15 to 20 weeks), before starting boosting. However, to correctly measure the true effect of this in the sense of increased longevity of protection, an extended boost-to-challenge interval is needed to validate this. This substantially increases the expense of the study in terms of per diem animal husbandry costs and is also the reason why attempts have only been occasionally been made to determine the effects of boosting on waning memory immunity (24, 66, 67), which is prohibitively expensive in any model larger than the mouse.
POSTEXPOSURE VACCINES
Postexposure or therapeutic vaccines are those that could be given to individuals after they have been actively infected or exposed. The most advanced candidate is the RUTI vaccine, which contains detoxified bacterial fragments administered in liposomes (68). Positive results have been obtained in animal models, and an early phase I safety trial has been completed (69).
A different approach to vaccine design more recently focused on potential antigen availability occurring in animals after they have been infected. We had previously made the observation that as primary lesions in infected guinea pigs progressed, areas within these structures were stained brightly with Prussian blue, indicating iron accumulation—potentially by the surviving bacilli. Second, it is well established that the bacterium upregulates multiple proteins that are encoded within the important DosR regulon, and we identified four DosR-encoded proteins recognized by lung T cells as the infection was first contained. These two pools, Rv1909, Rv2359, and Rv2711 and Rv1738, Rv2032, Rv3130, and Rv3841, respectively, were given to infected guinea pigs in GLA/CpG-based adjuvant 10 days (or as a boost) 25 days after aerosol exposure to high-virulence clinical Beijing isolates. While these vaccines had no influence on the lung bacterial load, they appeared to significantly prevent infection dissemination and formation of secondary lesions, thus significantly prolonging animal survival (70). In long-term survivors (>200 days), there was evidence of bacterial clearance and lung damage healing (necrosis being replaced with fibrosis).
What type of immunity postexposure vaccines must target obviously depends on the length of time between exposure and vaccine administration. In a biodefense scenario (70), the purpose of the vaccine would be to boost or facilitate emerging primary effector immunity, with the main objective being to control the infection while it is being specifically identified, especially in terms of its drug profile. Whether such types of vaccines could be applied to chronic TB is far less clear and has not yet been addressed. Moreover, with these types of approaches, there are potential safety issues (71).
CHEMOTHERAPY-FACILITATING VACCINES
In this day and age, chemotherapy is applied as soon as possible to individuals with diagnosed disease. Here, there is growing evidence that the application of a therapeutic vaccine can facilitate the activity of the drug regimens used. For example, the ID93 candidate described above was shown (72) to increase the survival of a very susceptible inbred mouse strain when given in conjunction with chemotherapy. Less impressive results, however, were seen in an NHP model (72).
“M. indicus pranii” (originally Mw) was initially developed as an immunotherapeutic agent against leprosy, but this candidate has more recently reemerged as a possible facilitator of chemotherapy, although results have mostly been very modest (73).
The application of chemotherapy to animal models usually occurs at a time when effector immunity is predominant and memory immunity has yet to arise. How the animal is actually reacting to the flood of newly available antigens resulting from the destruction of viable bacteria is unclear, but classical studies (74) show no indication that the emergence of memory immunity is compromised. In fact, bacterial clearance by drugs now allows the establishment of both effector memory and central memory (whereas chronic disease only favors the former)—with the caveat that this is unstable if the animal is subsequently reinfected with a virulent isolate (58)—something happening more and more in the field (75). While it is still unclear just how important central memory is in the context of improving TB vaccines, understanding why rapid destruction of the bacterial load seems to push the emergence of these cells could potentially be useful.
REACTIVATION PREVENTION VACCINES
Even today, most reviews of the pathogenesis and immunology of the TB disease process are locked into the concept that the expression of acquired immunity to the bacillus drives it into a dormant or latent state, and this, in turn, centrally influences how we approach vaccine design. Some of us, however, have directly challenged this concept by pointing out multiple holes in this argument (15, 21, 76).
This is directly pertinent to postexposure and “chemotherapy-facilitating” vaccine design. If one considers the concept that latent bacilli survive in macrophages (despite the lack of photographic evidence and the further issue of how macrophages could even survive in lung necrosis) from whence they somehow become resuscitated and cause disease reactivation, this could be targeted. However, no candidate has yet been found that can achieve this, and for that matter, in vitro conditions that simulate latent bacilli under low-oxygen and nutrition-deprived conditions have totally failed to produce the library of new latent-TB drugs we were promised a few years ago. It logically follows, therefore, that the prevailing concept of latent disease could be completely wrong.
As noted above, our own development of therapeutic vaccines was guided by events in the lungs we could directly observe during the containment phase of the infection in the guinea pig model. However, in terms of reactivation prevention vaccines, it is almost certain that the most favorable targets are different. Our working model (15) of reactivation is very different from the prevailing concept that a latent bacillus somehow wakes up, avoids immunity, and creates a new lesion. In our model, reactivation occurs on a periodic basis and, in doing so, rapidly triggers memory immunity to contain it. In our opinion, this explains the basis of the IFN-γ release assay for latent TB; memory cells tend to marginate rather than recirculate, so detecting IFN-γ+ cells in the blood indicates recent reactivation rather than latency. This is all driven, we suggest, by the fact that advanced staining methods indicate the potential existence of large numbers of (possibly biofilm-like) bacterial communities persisting in primary lesion necrosis (15), thus providing the source of periodic reactivation.
If we consider that reactivating bacteria are producing an antigen profile that is similar to or the same as that of a fresh infection, then vaccines targeting early secreted proteins should be the most effective approach. However, this has to be considered also in terms of the existing memory immunity in the host at that time. If immunity triggered by reactivation has similarities to a recent recall model (58), then there is the potential that the introduction by vaccination of a significant quantity of key immunogenic proteins such as Ag85 or ESAT-6 may cause an expansion of both effector and central memory CD4 responses, followed by their contraction and exhaustion (a sort of memory immune Koch reaction). In models of chronic infection at least, memory CD4 T cells do not appear to be terminally differentiated (77) but do appear to have the capacity to become KLRG1+ and PD-1+ when restimulated (78). Whether this applies to T cells remaining after chemotherapy has ceased is not known, but again, this emphasizes our substantial lack of knowledge about these events in this situation.
Conventionally, relapse studies of mice following cessation of a drug regimen are usually conducted for 3 to 6 months, and in addition, if lack of sterilization is suspected, reactivation can be driven by immunosuppression (e.g., with cortisol). If guinea pigs are used, it can take as long as a year before any reactivation disease occurs in drug-treated animals (79). This again illustrates the financial burden of performing such studies, especially under biosafety level 3 conditions.
VACCINE DESIGN AND VALIDATION: PRECLINICAL TESTING IN ANIMAL MODELS
Over the past 10 to 15 years, the number of laboratories capable of performing in-house evaluations of TB vaccine candidates has increased somewhat but is still relatively low. This, of course, reflects both economic and infrastructure limitations, and these rise exponentially with the size of the animal species used. While there is some degree of standardization of the protocols used in different laboratories, there are often still considerable differences in terms of doses and routes of vaccination, in vaccine-to-challenge intervals, and in the route of the challenge itself. In general, there is a concern that vaccine-to-challenge intervals are too short, studies are underpowered, and challenge infections are given long before memory immunity can even develop (80). Finally, since it is now nearly a decade since rival candidates from different laboratories were ever tested in head-to-head comparisons by independent laboratories with no vested interest (81), any prioritization is essentially impossible.
A further concern is that most candidates are tested not against the current clinical isolates sweeping the planet, many of which appear to show substantial virulence in small-animal models, but instead against laboratory-adapted strains H37Rv and Erdman. Recent studies using the clinical strains clearly indicate that many of these isolates induce a much broader T cell response, including TH17 cells and Foxp3+ regulatory T cells. How the balance between these subsets and vaccine-induced memory T cells influences vaccine efficacy is far from clear and, if anything, mostly ignored by the current field (8). TH17 cells themselves can be directly protective under certain conditions (59, 60), and loss of protective immunity induced by BCG vaccination occurs in parallel with regulatory T cell expansion (64). In the latter model, depletion of these cells by antibody or by toxin administration in diphtheria toxin receptor knock-in mice allows the reexpansion of effector CD4 cells (D. J. Ordway, personal communication).
A general consensus in the field is that a progressive pathway of vaccine screening evaluations from small-animal (mouse, guinea pig) models to NHPs is logical, and positive data from each model will increase confidence that the candidate is worthy of clinical evaluation. There is general agreement that the NHP should be the endpoint “gateway,” but despite the close similarity to humans and the opportunity to study “human-like” immune mechanisms, the more this model is thoroughly analyzed, the more serious limitations are starting to be realized. Most of the studies done to date have been performed with macaques, but the two subspecies—rhesus and cynomolgus—can respond differently to vaccination and M. tuberculosis challenge. In addition, there are now three sources of cynomolgus available and again response differences can be observed. Finally, different patterns of disease outcome occur if the challenge is given by bronchoscope—with some animals showing active disease but others a more latent appearance—whereas macaques infected by a more realistic low-dose aerosol exposure uniformly show an active disease pattern (82–84). Importantly, these limitations are further compounded by recent studies that have even had difficulty in demonstrating protection by BCG, a rather serious drawback when the ambition of the study is to demonstrate the ability of the candidate to boost BCG. A recent study (37) testing the H56 candidate ran into this problem, as did a study testing the ability of Aeras-402 to boost BCG (85), in which the vaccinated and/or boosted animals did not even live as long as the unprotected control animals. Accordingly, it would perhaps be wise for the field to continue to utilize smaller-animal models while these serious issues with NHP models are analyzed and resolved prior to eventual dependence on them as the definitive endpoint model.
CONCLUSIONS
Whereas a few decades ago the focus on new, BCG-replacing vaccines was focused strictly on prophylactic vaccines that would be given to neonates, the field has blossomed in the sense that we are realizing that there are multiple situations in which certain candidates could have a practical use. This includes breaking away from the paradigm that a particular type of vaccine must still be administered early in life and instead thinking in terms of whether innovative new candidates could be given to adolescents or even adults. These include fresh approaches, including anti-infection vaccines, vaccines to facilitate chemotherapy, and vaccines specifically designed to prevent the reappearance of persisting/latent bacilli.
In the latter case, we need a new debate on the prevailing concept in the field that bacilli in macrophages in lesions are in a state of latency. This is being increasingly challenged by the alternative concept that these surviving bacteria are, in fact, extracellular and are actively adapting to persistence in necrosis prior to possible escape and transmission. These adaptations include very low energy generation, triglyceride accumulation as an energy source, utilization of cholesterol left behind by dead host cells, and continuing efforts to acquire iron and copper ions (21). Cell wall construction is halted and in fact appears to be actively released within biofilms (86, 87), and thus, the expression of genes encoding proteins involved in cell biosynthesis, including the Ag85 family (88), falls to very low levels since these are not needed at this time. All of these processes could provide valuable new antigen candidate targets.
In a similar vein, a further concern is the “eggs in one basket” continuing emphasis on immunodominant antigens such as the Ag85 family, ESAT-6, etc. The fact is that the bacterium only makes these in large amounts during certain times in its life cycle, not continuously. Moreover, there is growing concern, initiated by Comas et al. (28), that by hyperconserving immunodominant epitopes the bacillus is exploiting an evolutionary advantage, driving its chances of transmission (of course, one can also make the reverse argument—that by generating immunity to these epitopes, the majority of exposed people are protected). Recently, it has been shown instead (89) that subdominant, even weakly immunogenic, proteins of the bacterium can make perfectly good vaccine candidates, giving good protection in the mouse. This is a new approach that deserves further attention.
Finally, as recently discussed by Andersen and Kaufmann (54), it is questionable at this time whether the WHO Stop TB objective of TB elimination by the year 2050 will be achieved. From an optimistic viewpoint, the fact that several candidates are finally moving into clinical trials is promising, as is the development of less conventional candidates such as those that have therapeutic activity, for example. A more pessimistic viewpoint, however, is that there are multiple elements of the host response that are still very poorly understood, such as the nature of memory immunity, or simply ignored, such as the inhibitory role of regulatory T cells induced by newly emerging clinical strains, and efforts are further restricted by pressure to conduct relatively short and often underpowered screening assays with animals (10, 80, 90) because of financial and infrastructure limitations.
ACKNOWLEDGMENT
I have no conflicts of interest to declare.
REFERENCES
- 1.World Health Organization. 2013. Global tuberculosis report 2013. World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. [Google Scholar]
- 2.Cohen T, Dye C, Colijn C, Williams B, Murray M. 2009. Mathematical models of the epidemiology and control of drug-resistant TB. Expert Rev Respir Med 3:67–79. doi: 10.1586/17476348.3.1.67. [DOI] [PubMed] [Google Scholar]
- 3.Dye C. 2009. Doomsday postponed? Preventing and reversing epidemics of drug-resistant tuberculosis. Nat Rev Microbiol 7:81–87. doi: 10.1038/nrmicro2048. [DOI] [PubMed] [Google Scholar]
- 4.Smith KC, Orme IM, Starke J. 2012. The BCG vaccine, p 789–811. In Plotkin S, Orenstein W, Offit P (ed), Vaccines, 6th ed. W. B. Saunders, Philadelphia, PA. [Google Scholar]
- 5.Kaufmann SH, Lange C, Rao M, Balaji KN, Lotze M, Schito M, Zumla AI, Maeurer M. 2014. Progress in tuberculosis vaccine development and host-directed therapies-a state of the art review. Lancet Respir Med 2:301–320. doi: 10.1016/S2213-2600(14)70033-5. [DOI] [PubMed] [Google Scholar]
- 6.Orme IM. 1999. Vaccination against tuberculosis: recent progress. Adv Vet Med 41:135–143. [DOI] [PubMed] [Google Scholar]
- 7.Orme IM. 2001. The search for new vaccines against tuberculosis. J Leukoc Biol 70:1–10. [PubMed] [Google Scholar]
- 8.Orme IM. 2013. Vaccine development for tuberculosis: current progress. Drugs 73:1015–1024. doi: 10.1007/s40265-013-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H, Team MATS . 2013. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McShane H, Williams A. 2014. A review of preclinical animal models utilised for TB vaccine evaluation in the context of recent human efficacy data. Tuberculosis (Edinb) 94:105–110. doi: 10.1016/j.tube.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orme IM. 27 November 2014. Vaccines to prevent tuberculosis infection rather than disease: physiological and immunological aspects. Tuberculosis (Edinb) doi: 10.1016/j.tube.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Williams A, Reljic R, Naylor I, Clark SO, Falero-Diaz G, Singh M, Challacombe S, Marsh PD, Ivanyi J. 2004. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology 111:328–333. doi: 10.1111/j.1365-2567.2004.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dascher CC, Hiromatsu K, Xiong X, Morehouse C, Watts G, Liu G, McMurray DN, LeClair KP, Porcelli SA, Brenner MB. 2003. Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. Int Immunol 15:915–925. doi: 10.1093/intimm/dxg091. [DOI] [PubMed] [Google Scholar]
- 14.Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, Saeed S, Jacobs C, van Loenhout J, de Jong D, Stunnenberg HG, Xavier RJ, van der Meer JW, van Crevel R, Netea MG. 2012. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orme IM. 2014. A new unifying theory of the pathogenesis of tuberculosis. Tuberculosis (Edinb) 94:8–14. doi: 10.1016/j.tube.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ernst JD. 2012. The immunological life cycle of tuberculosis. Nat Rev Immunol 12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 17.Basaraba RJ, Dailey DD, McFarland CT, Shanley CA, Smith EE, McMurray DN, Orme IM. 2006. Lymphadenitis as a major element of disease in the guinea pig model of tuberculosis. Tuberculosis (Edinb) 86:386–394. doi: 10.1016/j.tube.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Festjens N, Bogaert P, Batni A, Houthuys E, Plets E, Vanderschaeghe D, Laukens B, Asselbergh B, Parthoens E, De Rycke R, Willart MA, Jacques P, Elewaut D, Brouckaert P, Lambrecht BN, Huygen K, Callewaert N. 2011. Disruption of the SapM locus in Mycobacterium bovis BCG improves its protective efficacy as a vaccine against M. tuberculosis. EMBO Mol Med 3:222–234. doi: 10.1002/emmm.201000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner OC, Basaraba RJ, Frank AA, Orme IM. 2003. Granuloma formation in mouse and guinea pig models of experimental tuberculosis, p 65–84. In Boros DL. (ed), Granulomatous infections and inflammation: cellular and molecular mechanisms. ASM Press, Washington, DC. [Google Scholar]
- 20.Turner OC, Basaraba RJ, Orme IM. 2003. Immunopathogenesis of pulmonary granulomas in the guinea pig after infection with Mycobacterium tuberculosis. Infect Immun 71:864–871. doi: 10.1128/IAI.71.2.864-871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orme IM. 2011. Development of new vaccines and drugs for TB: limitations and potential strategic errors. Future Microbiol 6:161–177. doi: 10.2217/fmb.10.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orme IM, Andersen P, Boom WH. 1993. T cell response to Mycobacterium tuberculosis. J Infect Dis 167:1481–1497. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz MA, Harth G, Dillon BJ, Maslesa-Galic S. 2000. Recombinant bacillus Calmette-Guérin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci U S A 97:13853–13858. doi: 10.1073/pnas.250480397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, Mann P, Goosmann C, Bandermann S, Smith D, Bancroft GJ, Reyrat JM, van Soolingen D, Raupach B, Kaufmann SH. 2005. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J Clin Invest 115:2472–2479. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K, Modlin RL, Brinkmann V, Kaufmann SH. 2003. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med 9:1039–1046. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- 26.Manca C, Tsenova L, Freeman S, Barczak AK, Tovey M, Murray PJ, Barry C, Kaplan G. 2005. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res 25:694–701. doi: 10.1089/jir.2005.25.694. [DOI] [PubMed] [Google Scholar]
- 27.Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, Basaraba RJ, Orme IM. 2007. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J Immunol 179:522–531. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- 28.Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, Ernst JD, Gagneux S. 2010. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet 42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ackart DF, Hascall-Dove L, Caceres SM, Kirk NM, Podell BK, Melander C, Orme IM, Leid JG, Nick JA, Basaraba RJ. 2014. Expression of antimicrobial drug tolerance by attached communities of Mycobacterium tuberculosis. Pathog Dis 70:359–369. doi: 10.1111/2049-632X.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackart DF, Lindsey EA, Podell BK, Melander RJ, Basaraba RJ, Melander C. 2014. Reversal of Mycobacterium tuberculosis phenotypic drug resistance by 2-aminoimidazole-based small molecules. Pathog Dis 70:370–378. doi: 10.1111/2049-632X.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brandt L, Skeiky YA, Alderson MR, Lobet Y, Dalemans W, Turner OC, Basaraba RJ, Izzo AA, Lasco TM, Chapman PL, Reed SG, Orme IM. 2004. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect Immun 72:6622–6632. doi: 10.1128/IAI.72.11.6622-6632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skeiky YA, Alderson MR, Ovendale PJ, Guderian JA, Brandt L, Dillon DC, Campos-Neto A, Lobet Y, Dalemans W, Orme IM, Reed SG. 2004. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol 172:7618–7628. doi: 10.4049/jimmunol.172.12.7618. [DOI] [PubMed] [Google Scholar]
- 33.Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ, Orme IM, Skeiky YA, Alderson MR, Cowgill KD, Prieels JP, Abalos RM, Dubois MC, Cohen J, Mettens P, Lobet Y. 2009. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci U S A 106:2301–2306. doi: 10.1073/pnas.0712077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day CL, Tameris M, Mansoor N, van Rooyen M, de Kock M, Geldenhuys H, Erasmus M, Makhethe L, Hughes EJ, Gelderbloem S, Bollaerts A, Bourguignon P, Cohen J, Demoitie MA, Mettens P, Moris P, Sadoff JC, Hawkridge A, Hussey GD, Mahomed H, Ofori-Anyinam O, Hanekom WA. 2013. Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults. Am J Respir Crit Care Med 188:492–502. doi: 10.1164/rccm.201208-1385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P. 2011. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med 17:189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- 36.Billeskov R, Elvang TT, Andersen PL, Dietrich J. 2012. The HyVac4 subunit vaccine efficiently boosts BCG-primed anti-mycobacterial protective immunity. PLoS One 7:e39909. doi: 10.1371/journal.pone.0039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, Bigbee M, Milk L, Gideon HP, Rodgers M, Cochran C, Guinn KM, Sherman DR, Klein E, Janssen C, Flynn JL, Andersen P. 2012. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J Clin Invest 122:303–314. doi: 10.1172/JCI46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duthie MS, Coler RN, Laurance JD, Sampaio LH, Oliveira RM, Sousa AL, Stefani MM, Maeda Y, Matsuoka M, Makino M, Reed SG. 2014. Protection against Mycobacterium leprae infection by the ID83/GLA-SE and ID93/GLA-SE vaccines developed for tuberculosis. Infect Immun 82:3979–3985. doi: 10.1128/IAI.02145-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orr MT, Beebe EA, Hudson TE, Moon JJ, Fox CB, Reed SG, Coler RN. 2014. A dual TLR agonist adjuvant enhances the immunogenicity and protective efficacy of the tuberculosis vaccine antigen ID93. PLoS One 9:e83884. doi: 10.1371/journal.pone.0083884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hondalus MK, Bardarov S, Russell R, Chan J, Jacobs WR Jr, Bloom BR. 2000. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect Immun 68:2888–2898. doi: 10.1128/IAI.68.5.2888-2898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sampson SL, Dascher CC, Sambandamurthy VK, Russell RG, Jacobs WR Jr, Bloom BR, Hondalus MK. 2004. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infect Immun 72:3031–3037. doi: 10.1128/IAI.72.5.3031-3037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambandamurthy VK, Derrick SC, Jalapathy KV, Chen B, Russell RG, Morris SL, Jacobs WR Jr. 2005. Long-term protection against tuberculosis following vaccination with a severely attenuated double lysine and pantothenate auxotroph of Mycobacterium tuberculosis. Infect Immun 73:1196–1203. doi: 10.1128/IAI.73.2.1196-1203.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, Chan J, Braunstein M, Orme IM, Derrick SC, Morris SL, Jacobs WR Jr, Porcelli SA. 2007. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest 117:2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nambiar JK, Pinto R, Aguilo JI, Takatsu K, Martin C, Britton WJ, Triccas JA. 2012. Protective immunity afforded by attenuated, PhoP-deficient Mycobacterium tuberculosis is associated with sustained generation of CD4+ T-cell memory. Eur J Immunol 42:385–392. doi: 10.1002/eji.201141903. [DOI] [PubMed] [Google Scholar]
- 45.Verreck FA, Vervenne RA, Kondova I, van Kralingen KW, Remarque EJ, Braskamp G, van der Werff NM, Kersbergen A, Ottenhoff TH, Heidt PJ, Gilbert SC, Gicquel B, Hill AV, Martin C, McShane H, Thomas AW. 2009. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS One 4:e5264. doi: 10.1371/journal.pone.0005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aporta A, Arbues A, Aguilo JI, Monzon M, Badiola JJ, de Martino A, Ferrer N, Marinova D, Anel A, Martin C, Pardo J. 2012. Attenuated Mycobacterium tuberculosis SO2 vaccine candidate is unable to induce cell death. PLoS One 7:e45213. doi: 10.1371/journal.pone.0045213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cardona PJ, Asensio JG, Arbues A, Otal I, Lafoz C, Gil O, Caceres N, Ausina V, Gicquel B, Martin C. 2009. Extended safety studies of the attenuated live tuberculosis vaccine SO2 based on phoP mutant. Vaccine 27:2499–2505. doi: 10.1016/j.vaccine.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 48.Etna MP, Giacomini E, Severa M, Pardini M, Aguilo N, Martin C, Coccia EM. 2014. A human dendritic cell-based in vitro model to assess Mycobacterium tuberculosis SO2 vaccine immunogenicity. ALTEX 31:397–406. doi: 10.14573/altex.1311041. [DOI] [PubMed] [Google Scholar]
- 49.Sweeney KA, Dao DN, Goldberg MF, Hsu T, Venkataswamy MM, Henao-Tamayo M, Ordway D, Sellers RS, Jain P, Chen B, Chen M, Kim J, Lukose R, Chan J, Orme IM, Porcelli SA, Jacobs WR Jr. 2011. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat Med 17:1261–1268. doi: 10.1038/nm.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palanisamy GS, DuTeau N, Eisenach KD, Cave DM, Theus SA, Kreiswirth BN, Basaraba RJ, Orme IM. 2009. Clinical strains of Mycobacterium tuberculosis display a wide range of virulence in guinea pigs. Tuberculosis (Edinb) 89:203–209. doi: 10.1016/j.tube.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Palanisamy GS, Smith EE, Shanley CA, Ordway DJ, Orme IM, Basaraba RJ. 2008. Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis (Edinb) 88:295–306. doi: 10.1016/j.tube.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shang S, Harton M, Tamayo MH, Shanley C, Palanisamy GS, Caraway M, Chan ED, Basaraba RJ, Orme IM, Ordway DJ. 2011. Increased Foxp3 expression in guinea pigs infected with W-Beijing strains of M. tuberculosis. Tuberculosis (Edinb) 91:378–385. doi: 10.1016/j.tube.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orme IM, Basaraba RJ. 2014. The formation of the granuloma in tuberculosis infection. Semin Immunol 26:601–609. doi: 10.1016/j.smim.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 54.Andersen P, Kaufmann SH. 2 June 2014. Novel vaccination strategies against tuberculosis. Cold Spring Harb Perspect Med doi: 10.1101/cshperspect.a018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soares AP, Kwong Chung CK, Choice T, Hughes EJ, Jacobs G, van Rensburg EJ, Khomba G, de Kock M, Lerumo L, Makhethe L, Maneli MH, Pienaar B, Smit E, Tena-Coki NG, van Wyk L, Boom WH, Kaplan G, Scriba TJ, Hanekom WA. 2013. Longitudinal changes in CD4(+) T-cell memory responses induced by BCG vaccination of newborns. J Infect Dis 207:1084–1094. doi: 10.1093/infdis/jis941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henao-Tamayo MI, Ordway DJ, Irwin SM, Shang S, Shanley C, Orme IM. 2010. Phenotypic definition of effector and memory T-lymphocyte subsets in mice chronically infected with Mycobacterium tuberculosis. Clin Vaccine Immunol 17:618–625. doi: 10.1128/CVI.00368-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orme IM. 2010. The Achilles heel of BCG. Tuberculosis (Edinb) 90:329–332. doi: 10.1016/j.tube.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Henao-Tamayo M, Obregon-Henao A, Ordway DJ, Shang S, Duncan CG, Orme IM. 2012. A mouse model of tuberculosis reinfection. Tuberculosis (Edinb) 92:211–217. doi: 10.1016/j.tube.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wozniak TM, Saunders BM, Ryan AA, Britton WJ. 2010. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect Immun 78:4187–4194. doi: 10.1128/IAI.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lindenstrøm T, Woodworth J, Dietrich J, Aagaard C, Andersen P, Agger EM. 2012. Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect Immun 80:3533–3544. doi: 10.1128/IAI.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aagaard C, Hoang TT, Izzo A, Billeskov R, Troudt J, Arnett K, Keyser A, Elvang T, Andersen P, Dietrich J. 2009. Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS One 4:e5930. doi: 10.1371/journal.pone.0005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henao-Tamayo M, Ordway DJ, Orme IM. 2014. Memory T cell subsets in tuberculosis: what should we be targeting? Tuberculosis (Edinb) 94:455–461. doi: 10.1016/j.tube.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Brennan MJ, Clagett B, Fitzgerald H, Chen V, Williams A, Izzo AA, Barker LF. 2012. Preclinical evidence for implementing a prime-boost vaccine strategy for tuberculosis. Vaccine 30:2811–2823. doi: 10.1016/j.vaccine.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ordway DJ, Shang S, Henao-Tamayo M, Obregon-Henao A, Nold L, Caraway M, Shanley CA, Basaraba RJ, Duncan CG, Orme IM. 2011. Mycobacterium bovis BCG-mediated protection against W-Beijing strains of Mycobacterium tuberculosis is diminished concomitant with the emergence of regulatory T cells. Clin Vaccine Immunol 18:1527–1535. doi: 10.1128/CVI.05127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.López B, Aguilar D, Orozco H, Burger M, Espitia C, Ritacco V, Barrera L, Kremer K, Hernandez-Pando R, Huygen K, van Soolingen D. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol 133:30–37. doi: 10.1046/j.1365-2249.2003.02171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brooks JV, Frank AA, Keen MA, Bellisle JT, Orme IM. 2001. Boosting vaccine for tuberculosis. Infect Immun 69:2714–2717. doi: 10.1128/IAI.69.4.2714-2717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romano M, D'Souza S, Adnet PY, Laali R, Jurion F, Palfliet K, Huygen K. 2006. Priming but not boosting with plasmid DNA encoding mycolyl-transferase Ag85A from Mycobacterium tuberculosis increases the survival time of Mycobacterium bovis BCG vaccinated mice against low dose intravenous challenge with M. tuberculosis H37Rv. Vaccine 24:3353–3364. doi: 10.1016/j.vaccine.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 68.Vilaplana C, Gil O, Caceres N, Pinto S, Diaz J, Cardona PJ. 2011. Prophylactic effect of a therapeutic vaccine against TB based on fragments of Mycobacterium tuberculosis. PLoS One 6:e20404. doi: 10.1371/journal.pone.0020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nell AS, D'Lom E, Bouic P, Sabate M, Bosser R, Picas J, Amat M, Churchyard G, Cardona PJ. 2014. Safety, tolerability, and immunogenicity of the novel antituberculous vaccine RUTI: randomized, placebo-controlled phase II clinical trial in patients with latent tuberculosis infection. PLoS One 9:e89612. doi: 10.1371/journal.pone.0089612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shanley CA, Ireton GC, Baldwin SL, Coler RN, Reed SG, Basaraba RJ, Orme IM. 2014. Therapeutic vaccination against relevant high virulence clinical isolates of Mycobacterium tuberculosis. Tuberculosis (Edinb) 94:140–147. doi: 10.1016/j.tube.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orme IM. 2006. Safety issues regarding new vaccines for tuberculosis, with an emphasis on post-exposure vaccination. Tuberculosis (Edinb) 86:68–73. doi: 10.1016/j.tube.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 72.Coler RN, Bertholet S, Pine SO, Orr MT, Reese V, Windish HP, Davis C, Kahn M, Baldwin SL, Reed SG. 2013. Therapeutic immunization against Mycobacterium tuberculosis is an effective adjunct to antibiotic treatment. J Infect Dis 207:1242–1252. doi: 10.1093/infdis/jis425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faujdar J, Gupta P, Natrajan M, Das R, Chauhan DS, Katoch VM, Gupta UD. 2011. Mycobacterium indicus pranii as stand-alone or adjunct immunotherapeutic in treatment of experimental animal tuberculosis. Indian J Med Res 134:696–703. doi: 10.4103/0971-5916.90999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orme IM. 1988. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol 140:3589–3593. [PubMed] [Google Scholar]
- 75.Chiang CY, Riley LW. 2005. Exogenous reinfection in tuberculosis. Lancet Infect Dis 5:629–636. doi: 10.1016/S1473-3099(05)70240-1. [DOI] [PubMed] [Google Scholar]
- 76.Cardona PJ. 2009. A dynamic reinfection hypothesis of latent tuberculosis infection. Infection 37:80–86. doi: 10.1007/s15010-008-8087-y. [DOI] [PubMed] [Google Scholar]
- 77.Lindenstrøm T, Knudsen NP, Agger EM, Andersen P. 2013. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1-IL-2-secreting central memory cells. J Immunol 190:6311–6319. doi: 10.4049/jimmunol.1300248. [DOI] [PubMed] [Google Scholar]
- 78.Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM, Woodland DL. 2010. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A 107:19408–19413. doi: 10.1073/pnas.1006298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shang S, Shanley CA, Caraway ML, Orme EA, Henao-Tamayo M, Hascall-Dove L, Ackart D, Lenaerts AJ, Basaraba RJ, Orme IM, Ordway DJ. 2011. Activities of TMC207, rifampin, and pyrazinamide against Mycobacterium tuberculosis infection in guinea pigs. Antimicrob Agents Chemother 55:124–131. doi: 10.1128/AAC.00978-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams A, Hall Y, Orme IM. 2009. Evaluation of new vaccines for tuberculosis in the guinea pig model. Tuberculosis (Edinb) 89:389–397. doi: 10.1016/j.tube.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Williams A, Hatch GJ, Clark SO, Gooch KE, Hatch KA, Hall GA, Huygen K, Ottenhoff TH, Franken KL, Andersen P, Doherty TM, Kaufmann SH, Grode L, Seiler P, Martin C, Gicquel B, Cole ST, Brodin P, Pym AS, Dalemans W, Cohen J, Lobet Y, Goonetilleke N, McShane H, Hill A, Parish T, Smith D, Stoker NG, Lowrie DB, Kallenius G, Svenson S, Pawlowski A, Blake K, Marsh PD. 2005. Evaluation of vaccines in the EU TB vaccine cluster using a guinea pig aerosol infection model of tuberculosis. Tuberculosis (Edinb) 85:29–38. doi: 10.1016/j.tube.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 82.Lin PL, Flynn JL. 2010. Understanding latent tuberculosis: a moving target. J Immunol 185:15–22. doi: 10.4049/jimmunol.0903856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharpe SA, Eschelbach E, Basaraba RJ, Gleeson F, Hall GA, McIntyre A, Williams A, Kraft SL, Clark S, Gooch K, Hatch G, Orme IM, Marsh PD, Dennis MJ. 2009. Determination of lesion volume by MRI and stereology in a macaque model of tuberculosis. Tuberculosis (Edinb) 89:405–416. doi: 10.1016/j.tube.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Sharpe SA, McShane H, Dennis MJ, Basaraba RJ, Gleeson F, Hall G, McIntyre A, Gooch K, Clark S, Beveridge NE, Nuth E, White A, Marriott A, Dowall S, Hill AV, Williams A, Marsh PD. 2010. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin Vaccine Immunol 17:1170–1182. doi: 10.1128/CVI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Darrah PA, Bolton DL, Lackner AA, Kaushal D, Aye PP, Mehra S, Blanchard JL, Didier PJ, Roy CJ, Rao SS, Hokey DA, Scanga CA, Sizemore DR, Sadoff JC, Roederer M, Seder RA. 2014. Aerosol vaccination with AERAS-402 elicits robust cellular immune responses in the lungs of rhesus macaques but fails to protect against high-dose Mycobacterium tuberculosis challenge. J Immunol 193:1799–1811. doi: 10.4049/jimmunol.1400676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kulka K, Hatfull G, Ojha AK. 2012. Growth of Mycobacterium tuberculosis biofilms. J Vis Exp pii:3820. doi: 10.3791/3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ojha AK, Baughn AD, Sambandan D, Hsu T, Trivelli X, Guerardel Y, Alahari A, Kremer L, Jacobs WR Jr, Hatfull GF. 2008. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol Microbiol 69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi L, North R, Gennaro ML. 2004. Effect of growth state on transcription levels of genes encoding major secreted antigens of Mycobacterium tuberculosis in the mouse lung. Infect Immun 72:2420–2424. doi: 10.1128/IAI.72.4.2420-2424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orr MT, Ireton GC, Beebe EA, Huang PW, Reese VA, Argilla D, Coler RN, Reed SG. 2014. Immune subdominant antigens as vaccine candidates against Mycobacterium tuberculosis. J Immunol 193:2911–2918. doi: 10.4049/jimmunol.1401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McShane H, Jacobs WR, Fine PE, Reed SG, McMurray DN, Behr M, Williams A, Orme IM. 2012. BCG: myths, realities, and the need for alternative vaccine strategies. Tuberculosis (Edinb) 92:283–288. doi: 10.1016/j.tube.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]