Abstract
Ross River virus (RRV) is endemic in Australia and several South Pacific Islands. More than 90,000 cases of RRV disease, which is characterized by debilitating polyarthritis, were reported in Australia in the last 20 years. There is no vaccine available to prevent RRV disease. A phase 3 study was undertaken at 17 sites in Australia to investigate the safety and immunogenicity of an inactivated whole-virus Vero cell culture-derived RRV vaccine in 1,755 healthy younger adults aged 16 to 59 years and 209 healthy older adults aged ≥60 years. Participants received a 2.5-μg dose of Al(OH)3-adjuvanted RRV vaccine, with a second and third dose after 3 weeks and 6 months, respectively. Vaccine-induced RRV-specific neutralizing and total IgG antibody titers were measured after each immunization. Vaccine safety was monitored over the entire study period. The vaccine was safe and well-tolerated after each vaccination. No cases of arthritis resembling RRV disease were reported. The most frequently reported systemic reactions were headache, fatigue, and malaise; the most frequently reported injection site reactions were tenderness and pain. After the third immunization, 91.5% of the younger age group and 76.0% of the older age group achieved neutralizing antibody titers of ≥1:10; 89.1% of the younger age group and 70.9% of the older age group achieved enzyme-linked immunosorbent assay (ELISA) titers of ≥11 PanBio units. A whole-virus Vero cell culture-derived RRV vaccine is well tolerated in an adult population and induces antibody titers associated with protection from RRV disease in the majority of individuals. (This study is registered at www.clinicaltrials.gov under registration no. NCT01242670.)
INTRODUCTION
Ross River virus (RRV) is a mosquito-borne alphavirus, which causes RRV disease and is the most common and widespread arboviral disease in Australia and a number of South Pacific islands (1, 2). RRV disease is characterized by debilitating chronic polyarthritis with severe joint pain, often accompanied by rash, fever, and malaise (2). Almost all RRV disease patients experience painful arthritis, and in 80 to 90% of patients there is also joint stiffness and swelling, typically involving the wrists, knees, ankles, and small joints of the hands and feet. The elbows, shoulders, feet, back, hips, and jaw may also be affected. Inflammation may also cause nerve compression and paresthesia (3). Most patients recover within 4 weeks, but it may take up to 6 months to return to full physical activity. In some patients, joint and muscle pain and fatigue persist for many months or even years (3). A quality-of-life survey conducted in Australia indicated that disability due to RRV disease may be considered comparable to that of patients with chronic rheumatoid arthritis, accompanied by significant depression and anxiety (4).
RRV disease has a substantial financial and social burden on patients and their communities. An epidemiological study conducted in Australia estimated an average wage loss of >4,000 Australian dollars per patient (1). Conservatively estimated, the annual cost of RRV infections in 2001 in Australia alone was estimated to be between 2.8 and 5.7 million Australian dollars (2); however, this estimate does not account for public health surveillance, mosquito control, or all diagnostic and medical costs.
RRV is a nationally notifiable communicable disease in Australia, where between 2,000 and 8,000 cases of RRV disease are reported annually, with an incidence rate of approximately 20 annual cases per 100,000 population (5). RRV epidemics can also occur, as evidenced by large RRV outbreaks in Fiji, Samoa, the Cook Islands, and New Caledonia in 1979-1980 (6), which affected more than 50,000 people (7).
A large number of different mosquito species, some of which are found throughout the Asia Pacific region, are capable of transmitting RRV to humans (3). Because some mosquito species that circulate in the southern states of the United States and New Zealand are also capable of transmitting RRV, these regions could potentially also be affected by RRV disease in the future (1, 8). Prevention of RRV disease is restricted to avoiding mosquito exposure; however, mosquito control programs are costly (annually >20 million Australian dollars in Australia) and have no measurable effect on the incidence of clinical RRV infections. Emerging insecticide resistance is also a concern (9). There is no therapy available to treat RRV disease beyond symptomatic treatment with heat, gentle exercise, and nonsteroidal anti-inflammatory agents (3).
Infection with RRV is considered to afford lifelong immunity against RRV disease because there are no reports of an individual having a second clinical infection with RRV and there is no evidence of a clinical RRV infection in individuals with preexisting RRV-specific IgG antibodies (1). Immunization, therefore, may provide a cost-effective intervention to prevent RRV disease in residents of areas where RRV disease is endemic, in travelers, and in the face of an outbreak such as that in the Pacific in 1978-1980 (1). However, no vaccine is currently available.
We have developed a Vero cell culture-derived whole-virus inactivated RRV vaccine which is highly protective in animal models of viremia and disease (10, 11). In a phase 1/2 dose-finding study, the whole-virus RRV vaccine was safe and well-tolerated in healthy adults, and a 2.5-μg alum-adjuvanted dose was demonstrated to be best tolerated and to induce the highest RRV-specific total IgG and RRV-neutralizing antibody responses. In passive transfer studies (10), administration of human vaccinee sera from the phase 1/2 study protected RRV-challenged mice from viremia and development of arthritic symptoms. Based on the good correlation between neutralizing antibody titers and total IgG antibody titers in human sera and protection of animals, a conservative correlate of protection was defined as a neutralizing antibody titer of ≥1:10. In the present study, we report the data from a pivotal phase 3 study undertaken in Australia to assess the safety and immunogenicity of the inactivated RRV vaccine in adults ≥16 years of age.
MATERIALS AND METHODS
Study design and objectives.
A phase 3 study was undertaken between 18 April 2011 and 15 October 2012 at 17 study sites in Australia to investigate the safety, immunogenicity, and lot consistency of three immunizations with a Vero cell culture-derived whole-virus, 2.5-μg dose of alum-adjuvanted RRV vaccine in healthy participants aged 16 years or older. A planned total of 2,010 participants were to be stratified into two groups aged 16 to 59 (n = 1,800) or ≥60 (n = 210) years. Participants in both age groups were randomized 1:1:1 to receive one of three different lots of RRV vaccine, with a second immunization after 3 weeks and a third after 6 months. Subjects were to record daily oral body temperature, solicited injection site reactions, and solicited systemic adverse events (AEs) for 21 days after each vaccination and any other AEs for the entire duration of the study. The study was randomized and blinded with respect to vaccine lot. A subset of subjects in the younger age group and all subjects in the older age group were to be included in the immunogenicity analysis. The vaccine dose, formulation, and vaccination schedule were based on the results of the phase 1/2 dose-finding study (12), which showed that a 2.5-μg dose of alum-adjuvanted vaccine was the best tolerated and provided optimal immune responses. Exclusion criteria included a history of non-trauma-related arthritis, receipt of any vaccination within 30 days prior to study entry, and pregnancy.
The primary immunogenicity endpoints were RRV-specific neutralizing (μNT) antibody titers and the rate of subjects with RRV-specific μNT antibody titers of ≥1:10, 3 weeks after the third vaccination. The primary safety endpoint was the frequency and severity of injection site and systemic reactions within 7 days of each vaccination. Secondary endpoints included RRV-specific μNT titers 3 weeks after each vaccination and 6 months after the first and third vaccinations, the rate of subjects with an RRV-specific IgG enzyme-linked immunosorbent assay (ELISA) titer (defined as ≥11 PanBio units [PBU]) 3 weeks after each vaccination and 6 months after the first and third vaccinations, the frequency and severity of any AE during the entire study period, and the rate of subjects experiencing RRV-like arthritis. This was defined as soft tissue “synovitic” swelling, i.e., joint effusion or synovial tissue thickening, or both, with or without pain localized to the affected joint associated with one or more systemic symptoms consistent with RRV disease (fever, fatigue, malaise, rash, arthralgia, myalgia, lymphadenopathy, splenomegaly, sore throat, diarrhea, paresthesia, headache, neck stiffness, and photophobia) occurring ≥3 days after vaccination and lasting >3 weeks.
The relevant review boards and ethics committees approved the protocol for the study, which was conducted in accordance with good clinical practice guidelines and the Declaration of Helsinki. Subjects were eligible to participate if they were clinically healthy, provided written informed consent, and agreed to keep a daily record of symptoms. The trial is registered at www.clinicaltrials.gov under registration number NCT01242670.
Vaccination and follow-up.
The inactivated Vero cell culture-derived whole-virus RRV vaccine (Baxter) was produced from a viral seed derived from an RRV isolate from a serologically confirmed case of RRV disease in Queensland, Australia (13), as previously described (10–12). After harvest from Vero cells, the virus was inactivated by sequential formalin and UV light treatment and purified by sucrose gradient ultracentrifugation followed by ultrafiltration/diafiltration. The lot consistency was investigated using three vaccine lots in a planned subset of 1,140 participants in the younger age group.
Injection site reactions and fever were analyzed according to the FDA guidelines for toxicity grading for volunteers in preventive vaccine clinical trials (14). Participants who developed symptoms suggestive of RRV infection were asked to contact the study site immediately for clinical evaluation. Blood for serological testing was drawn prior to each immunization, 3 weeks after the second immunization, and 3 weeks and 6 months after the third immunization.
Immunogenicity assessments.
RRV-specific neutralizing antibody responses were assessed using an RRV neutralization assay (μNT), as previously described (12). RRV-specific μNT titers of ≥1:10 were considered positive. RRV-specific total IgG antibody titers were assessed using a commercially available diagnostic RRV IgG ELISA kit (PanBio Diagnostics, Brisbane, Australia), according to the manufacturer's instructions. A positive result is defined as ≥11 PBU.
Statistical analyses.
A sample size of 2,010 subjects was calculated to be sufficient to detect at least one AE with an underlying incidence rate of 1:1,000 with a probability of >86%. The rates of subjects with at least one systemic or injection site reaction occurring within 7 days of each vaccination and their 95% confidence intervals (CIs) were calculated separately for both age groups.
A sample size of 350 subjects receiving a specific lot of the RRV vaccine in the 16- to 59-year age group was calculated to have sufficient power to show equivalence between two study lots. The overall power for the three pairwise comparisons to show immunogenicity equivalence between all three lots within the 16- to 59-year age group was calculated to be approximately 82%. To demonstrate lot consistency, the two-sided 95% CIs of the between-lot ratios of the baseline-adjusted μNT geometric mean titers (GMTs) 21 days after the third vaccination were calculated. Lot consistency was achieved if each of the three 95% CIs of the between-lot ratios was entirely contained in the interval of 0.67 to 1.5. The estimation of the between-lot ratios of GMTs was done using an analysis of covariance (ANCOVA) framework on the log-transformed μNT titers, accounting for the fixed effect of lot and baseline μNT titer as a covariate. For the log-transformed μNT titers, a longitudinal analysis was performed within a repeated mixed-model ANCOVA framework, accounting for the effect of study days, age, gender, and baseline titer as a covariate. Least-square means and least-square mean differences between lots and their 95% CIs were estimated within this ANCOVA framework and back transformed into GMTs and ratios of GMTs and 95% CIs by exponentiation.
RESULTS
Study participants.
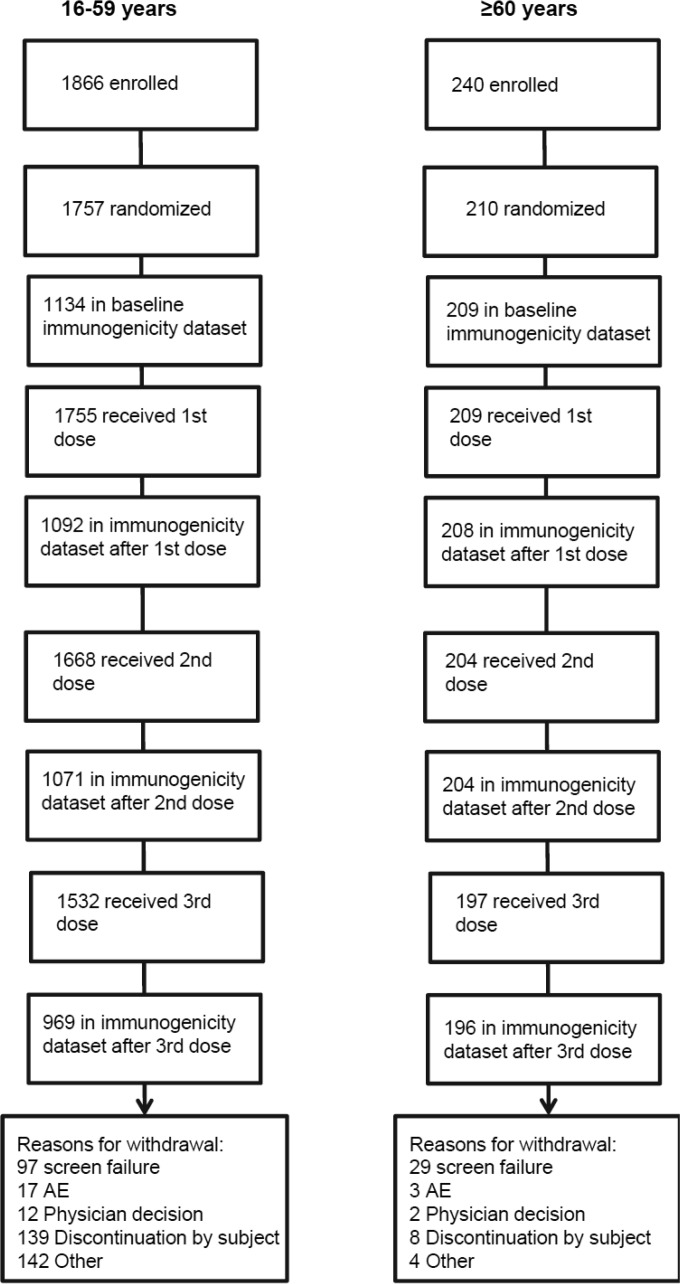
The study profile is shown in Fig. 1. Totals of 1,866 participants aged 16 to 59 years and 240 participants aged ≥60 years were enrolled, of which 1,757 and 210, respectively, were randomized; 1,755 participants aged 16 to 59 years and 209 participants aged ≥60 years received the first immunization. The baseline demographic characteristics of all participants receiving at least one vaccination are shown in Table 1. Both populations were balanced with respect to all demographic parameters, except for gender, with a slightly higher proportion of males in the older age group and a slightly higher proportion of females in the younger age group. The majority (≥90% of vaccinated subjects in both populations) were white. The demographic characteristics of the three groups in the younger population who were randomized to receive three different lots of the vaccine reflected the overall subject population. All vaccinated subjects were included in the safety analysis. A subset of 1,134 subjects in the younger age group and all subjects in the older age group who were immunized at least once and had immunogenicity measurements at baseline and 21 days after the respective vaccination were included in the immunogenicity analysis.
FIG 1.
Study profile.
TABLE 1.
Demographics of the study participants at baseline
| Characteristic | Result for age group: |
|
|---|---|---|
| 16–59 yr (n = 1,755) | ≥60 yr (n = 209) | |
| Age (mean ± SD) (yr) | 33.4 ± 12.9 | 65.6 ± 4.9 |
| Gender (no. [%]) | ||
| Male | 784 (44.7) | 117 (56.0) |
| Female | 971 (55.3) | 92 (44.0) |
| Race (no. [%]) | ||
| White | 1,591 (90.7) | 201 (96.2) |
| Black or African American | 12 (0.7) | 0 (0.0) |
| Asian | 130 (7.4) | 6 (2.9) |
| American Indian or Alaska Native | 0 (0.0) | 0 (0.0) |
| Native Hawaiian or other Pacific Islander | 4 (0.2) | 0 (0.0) |
| Aboriginal or Torres Strait Islander | 5 (0.3) | 0 (0.0) |
| Multiple | 13 (0.7) | 2 (1.0) |
| Wt (mean ± SD) (kg) | 74.1 ± 14.4 | 77.5 ± 14.8 |
| Ht (mean ± SD) (cm) | 171.8 ± 9.5 | 169.9 ± 9.3 |
Safety and tolerability.
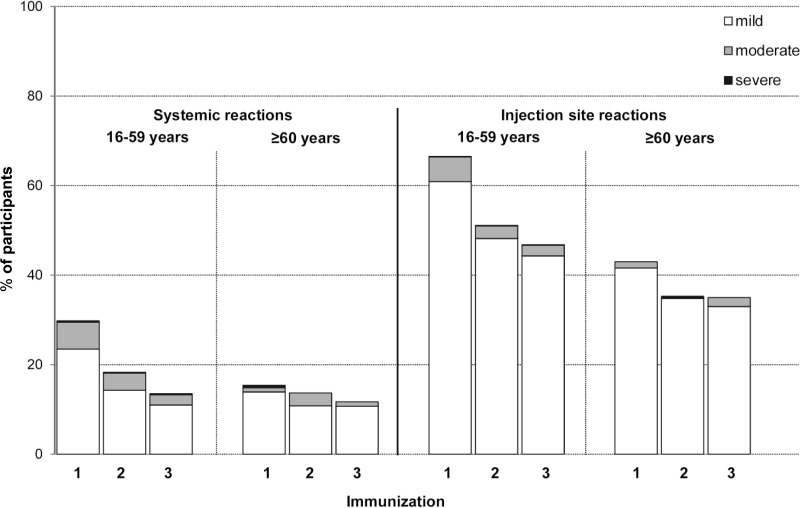
The whole-virus RRV vaccine was safe and well tolerated in both age groups, and adverse reactions were predominantly mild in severity. The proportions of mild, moderate, and severe systemic and injection site reactions within 7 days after each vaccination are shown in Fig. 2. The systemic and injection site reactions decreased with successive vaccination in both age groups. The most frequently reported systemic reactions were headache (≤14.7% in the younger age group and ≤4.9% in the older age group, after any vaccination), fatigue (≤11.6% in the younger age group and ≤5.6% in the older age group), and malaise (≤7.9% in the younger age group and ≤6.2% in the older age group). Fever occurred at a rate of ≤1.4% in the younger age group and ≤0.5% in the older age group. The most frequently reported injection site reactions were tenderness (≤51.7% in the younger age group and ≤36.8% in the older age group) and pain (≤37.8% in the younger age group and ≤17.7% in the older age group). The solicited systemic and injection site reactions reported within 21 days of the first immunization are shown in Table 2. No deaths occurred during the study, and no subjects developed RRV-like arthritis.
FIG 2.
Systemic and injection site reactions occurring within 7 days after each immunization. Data are the percentage of participants with mild, moderate, and severe reactions within 7 days after the first (1), second (2), and third (3) immunizations.
TABLE 2.
Participants with solicited injection site and systemic reactions within 21 days of first immunization
| Solicited reaction | Results (no. [%] [95% CI]) for age group: |
|
|---|---|---|
| 16–59 yr (n = 1,755) | ≥60 yr (n = 209) | |
| Injection site | ||
| Swelling | 29 (1.7) (1.1–2.4) | 3 (1.4) (0.3–4.1) |
| Induration | 32 (1.8) (1.3–2.6) | 6 (2.9) (1.1–6.1) |
| Redness | 25 (1.4) (0.9–2.1) | 3 (1.4) (0.3–4.1) |
| Pain | 664 (37.8) (35.6–40.2) | 37 (17.7) (12.8–23.6) |
| Ecchymosis | 73 (4.2) (3.3–5.2) | 9 (4.3) (2.0–8.0) |
| Tenderness | 907 (51.7) (49.3–54.0) | 77 (36.8) (30.3–43.8) |
| Systemic | ||
| Malaise | 139 (7.9) (6.7–9.3) | 13 (6.2) (3.4–10.4) |
| Fatigue | 204 (11.6) (10.2–13.2) | 10 (4.8) (2.3–8.6) |
| Headache | 258 (14.7) (13.1–16.4) | 10 (4.8) (2.3–8.6) |
| Nausea | 51 (2.9) (2.2–3.8) | 3 (1.4) (0.3–4.1) |
| Vomiting | 8 (0.5) (0.2–0.9) | 0 (0.0) (0.0–1.7) |
| Myalgia | 128 (7.3) (6.1–8.6) | 5 (2.4) (0.8–5.5) |
| Arthralgia | 57 (3.2) (2.5–4.2) | 2 (1.0) (0.1–3.4) |
| Lymph node swelling | 39 (2.2) (1.6–3.0) | 1 (0.5) (0.0–2.6) |
| Fever (≥38.0°C)a | 23 (1.4) (0.9–2.0) | 0 (0.0) (0.0–1.8) |
| RRV-like arthritis | 0 (0.0) (0.0–0.2) | 0 (0.0) (0.0–0.7) |
Within 7 days.
Immunogenicity.
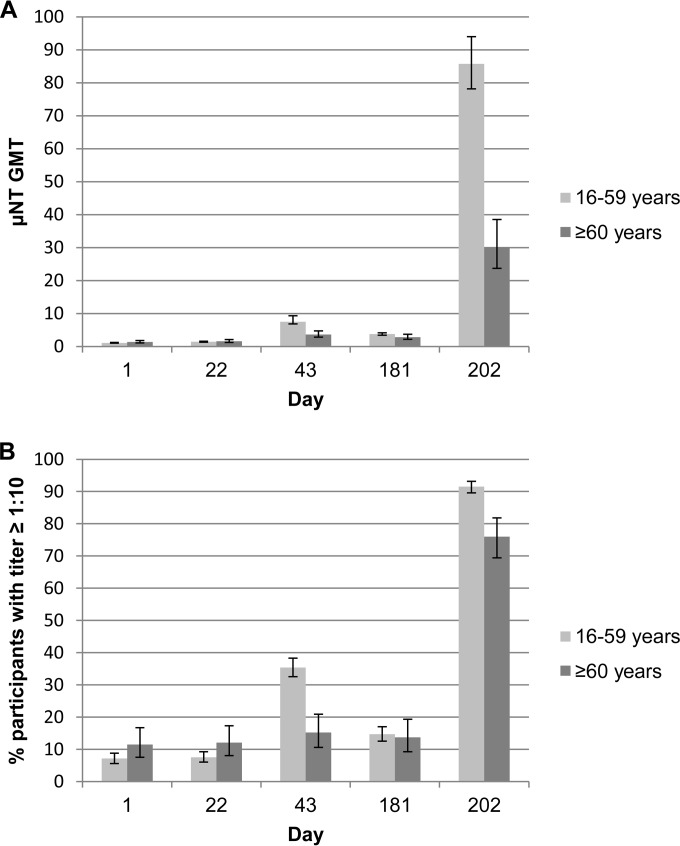
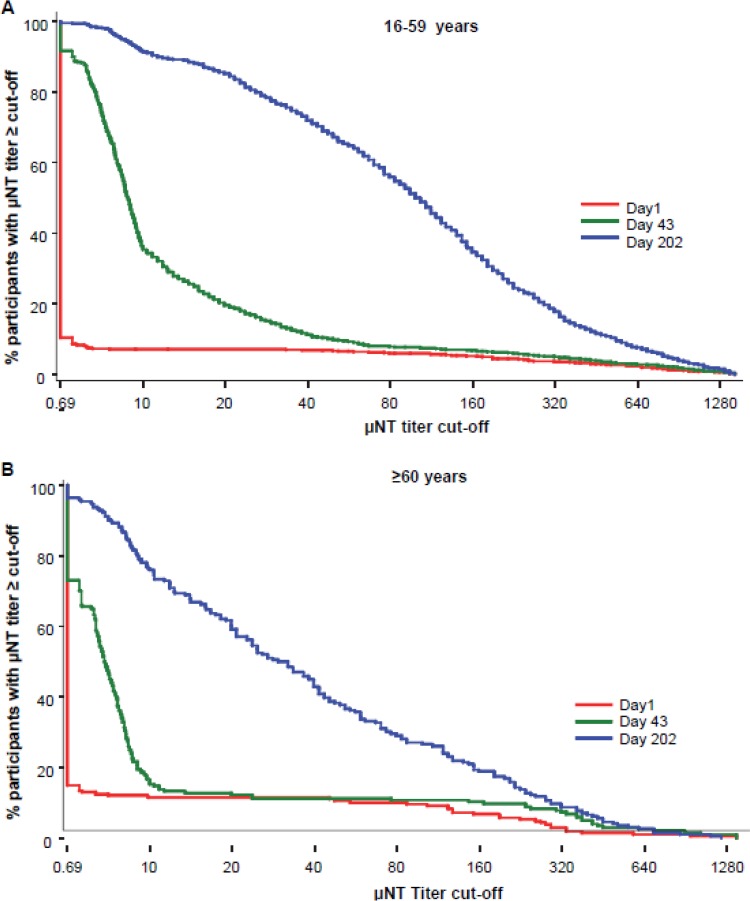
Substantial neutralizing and total IgG ELISA titers were induced in both age groups after three doses of the whole-virus RRV vaccine. The neutralizing antibody GMTs and the proportion of participants who achieved μNT titers of ≥1:10 are shown in Fig. 3. At 21 days after the third immunization, μNT GMTs in the 16- to 59- and ≥60-year age groups were 85.7 (95% CI, 78.18 to 93.95) and 30.2 (95% CI, 23.69 to 38.50), respectively, with 91.5% (95% CI, 89.6% to 93.2%) of the younger age group and 76.0% (95% CI, 69.4% to 81.8%) of the older age group achieving μNT titers of ≥1:10. Reverse cumulative distributions of neutralizing antibody titers in both populations are shown in Fig. 4.
FIG 3.
Neutralizing antibody responses. Geometric mean titer (GMT) of the μNT responses (A) and percentage of participants with μNT titers of ≥1:10 (B) at baseline (day 1), 3 weeks after the first immunization (day 22), 3 weeks after the second immunization (day 43), 6 months after the first immunization (day 181), and 3 weeks after the third immunization (day 202).
FIG 4.
Reverse cumulative distributions of neutralizing antibody responses. Data are the percentage of participants with μNT titers above each titer cutoff at baseline (day 1), 3 weeks after the second immunization (day 43), and 3 weeks after the third immunization (day 202) in participants aged 16 to 59 years (A) and participants aged ≥60 years (B).
The RRV-specific neutralizing titers were significantly affected by time (P < 0.001), the logarithmic RRV-specific neutralizing titer at baseline (P < 0.001), age (P < 0.001), and gender (P < 0.001). The comparisons of GMTs of RRV-specific neutralizing titers at 21 days after the third vaccination demonstrated lot consistency for the three different vaccine lots tested, based on the demonstration that the 95% CIs for the ratios of GMTs are entirely contained in the interval of 0.67 to 1.5 for any pairwise lot comparison.
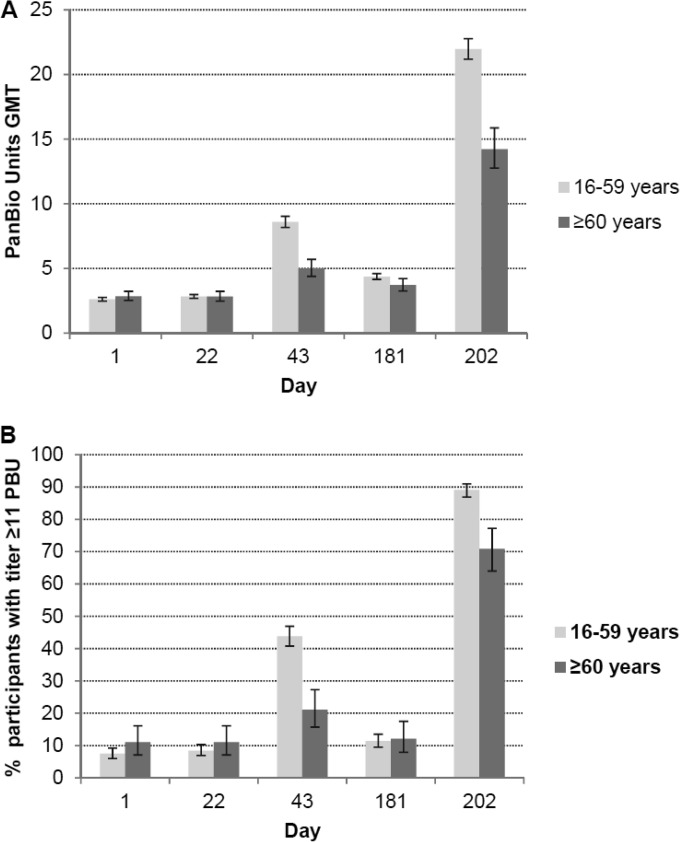
The total IgG ELISA antibody GMTs and the proportion of participants who achieved ELISA titers of ≥11 PBU are shown in Fig. 5. After the third immunization, ELISA GMTs in the 16- to 59- and ≥60-year age groups were 22.0 PBU and 14.2 PBU, respectively, with 89.1% of the younger age group and 70.9% of the older age group achieving ELISA titers of ≥11 PBU.
FIG 5.
Total IgG ELISA antibody responses. Geometric mean titer (GMT) of total IgG ELISA (PBU) responses (A) and percentage of participants with ELISA titers of ≥11 PBU (B) at baseline (day 1), 3 weeks after the first immunization (day 22), 3 weeks after the second immunization (day 43), 6 months after the first immunization (day 181), and 3 weeks after the third immunization (day 202).
DISCUSSION
A Vero cell culture-derived whole-virus RRV vaccine is well tolerated and immunogenic in a healthy adult population. No vaccine-related serious AEs, no cases of arthritis associated with RRV disease, and low rates of fever were reported. Lot consistency was demonstrated for 3 different manufacturing lots.
Following natural infection with RRV, which is thought to result in lifelong immunity, seropositivity is defined, using the standard diagnostic RRV-specific PanBio IgG ELISA, by the presence of RRV-specific serum IgG antibodies corresponding to a value of ≥11 PBU. A previous study using human vaccinee sera from a phase 1/2 study of the whole-virus RRV vaccine demonstrated that a highly significant correlation existed between data generated using the PanBio IgG ELISA and the μNT assay (r2 = 0.91) and that the ≥11 PBU cutoff is equivalent to a neutralizing antibody titer of 1:5.7 (10). In mouse passive transfer studies using the human vaccinee sera from the phase 1/2 study, an RRV-specific neutralizing antibody titer of ≥1:3 was sufficient to provide complete protection against an RRV challenge (10). Based on these data, a neutralizing antibody titer of ≥1:10 is considered to be a conservative titer cutoff for an indicator of protection against RRV disease in humans. In this phase 3 study, after the third immunization, 91.5% and 76.0% of the 16- to 59- and ≥60-year age groups achieved μNT titers of ≥1:10, and 89.1% of participants in the younger age group and 70.9% in the older age group, respectively, achieved titers of ≥11 PBU. Thus, the majority of participants in both populations had seroprotective μNT titers after three immunizations with the whole-virus RRV vaccine, and titers of serum IgG antibodies after three immunizations were higher than the serological IgG ELISA titer threshold (≥11 PBU) associated with protection after natural infection with RRV.
The immunogenicity profile of the 2.5-μg adjuvanted RRV vaccine in the younger age group is highly consistent with that previously demonstrated for this dose and formulation in a phase 1/2 study in adults aged 18 to 49 years (12). There are no other reported clinical studies of RRV vaccines. Several other inactivated whole-virus candidate vaccines against other alphaviruses such as Venezuelan equine encephalomyelitis virus, Eastern equine encephalomyelitis virus, and Western equine encephalomyelitis virus have been developed (15–20), but none have been licensed for human use and clinical data are limited. More extensive clinical data are available for inactivated, whole-virus vaccines to prevent diseases caused by flaviviruses, which structurally resemble alphaviruses, such as tick-borne encephalitis virus (TBEV), Japanese encephalitis virus, and yellow fever virus (21–23). The most extensively studied flavivirus vaccine is an alum-adjuvanted inactivated whole-virus TBEV vaccine, which has been demonstrated to be safe and immunogenic in a multitude of clinical studies (24–30) and which has been used in Europe for several decades. In field studies, it has been demonstrated that three immunizations with a 2.4-μg dose of the inactivated whole-virus TBEV vaccine provide approximately 99% effectiveness in preventing tick-borne encephalitis (22).
A limitation of our study is that we could not demonstrate vaccine efficacy in this phase 3 trial. Because of the relatively low incidence of RRV disease in Australia, it would have been necessary to enroll between 40,000 and 60,000 participants in order to evaluate vaccine efficacy or effectiveness in a phase 3 study. Substantial data from animal models which demonstrate the protective efficacy of the vaccine are available. However, postlicensure studies such as a field effectiveness study need to be conducted to confirm the validity of the immunological correlate, as well as safety studies to further evaluate the safety of the vaccine, particularly with regard to the occurrence of unexpected rare AEs. Further studies will also need to be undertaken to investigate the long-term seropersistence of the antibody response to provide long-term protection and to determine the requirement for any booster immunizations.
ACKNOWLEDGMENTS
We thank the members of the Baxter research and development team, Birgit Schaefer, Jens Modroff, Christine Hohenadl, Kathleen O'Hara, Randee Kalkstein, Veronika Schuetz-Dirnboeck, Debra McCarthy, Roopashree Dwarakanath, and Laura Simons, for their role in this study; the members of the independent data-monitoring committee, David Harley, Egon Marth, Herwig Kollaritsch, Klaus Machold, and Peter Nasveld; and Tanya Stoney, Fiona McDonald, and Jennifer Kent for their contribution to study conduction in Perth.
The study was designed and funded by Baxter. Data were held, analyzed, and interpreted by Baxter. Baxter employees were involved in drafting the manuscript and in the decision to submit the paper for publication.
N.W., M.V.W.V.D.V., D.P., W.D., P.N.B., and G.A. are employees of Baxter. N.W., M.V.W.V.D.V., W.D., P.N.B., and G.A. report having stocks or stock options in Baxter. All authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest.
REFERENCES
- 1.Aaskov J. 2009. Ross River virus: epidemic polyarthritis, p 631–644. In Barrett ADT, Stanberry LR (ed), Vaccines for biodefense and emerging and neglected diseases. Elsevier Academic Press, Amsterdam, The Netherlands. [Google Scholar]
- 2.Harley D, Sleigh A, Ritchie S. 2001. Ross River virus transmission, infection, and disease: a cross-disciplinary review. Clin Microbiol Rev 14:909–932. doi: 10.1128/CMR.14.4.909-932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith DW, Speers DJ, Mackenzie JS. 2011. The viruses of Australia and the risk to tourists. Travel Med Infect Dis 9:113–125. doi: 10.1016/j.tmaid.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Suhrbier A, La Linn M. 2004. Clinical and pathologic aspects of arthritis due to Ross River virus and other alphaviruses. Curr Opin Rheumatol 16:374–379. doi: 10.1097/01.bor.0000130537.76808.26. [DOI] [PubMed] [Google Scholar]
- 5.Australian Government National Notifiable Diseases Surveillance System. 1991. National Notifiable Diseases Surveillance System: number of notifications of Ross River virus infection, received from State and Territory health authorities in the period of to 2013 and year-to-date notifications for 2014. Australian Government, Department of Health, Canberra, Australia. [Google Scholar]
- 6.Jacups SP, Whelan PI, Currie BJ. 2008. Ross River virus and Barmah Forest virus infections: a review of history, ecology, and predictive models, with implications for tropical northern Australia. Vector Borne Zoonotic Dis 8:283–297. doi: 10.1089/vbz.2007.0152. [DOI] [PubMed] [Google Scholar]
- 7.Tong S. 2004. Ross River virus disease in Australia: epidemiology, socioecology and public health response. Intern Med J 34:58–60. doi: 10.1111/j.1444-0903.2004.00520.x. [DOI] [PubMed] [Google Scholar]
- 8.Kramer LD, Chin P, Cane RP, Kauffman EB, Mackereth G. 2011. Vector competence of New Zealand mosquitoes for selected arboviruses. Am J Trop Med Hyg 85:182–189. doi: 10.4269/ajtmh.2011.11-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aaskov J, Williams L, Yu S. 1997. A candidate Ross River virus vaccine: preclinical evaluation. Vaccine 15:1396–1404. doi: 10.1016/S0264-410X(97)00051-0. [DOI] [PubMed] [Google Scholar]
- 10.Holzer GW, Coulibaly S, Aichinger G, Savidis-Dacho H, Mayrhofer J, Brunner S, Schmid K, Kistner O, Aaskov JG, Falkner FG, Ehrlich H, Barrett PN, Kreil TR. 2011. Evaluation of an inactivated Ross River virus vaccine in active and passive mouse immunization models and establishment of a correlate of protection. Vaccine 29:4132–4141. doi: 10.1016/j.vaccine.2011.03.089. [DOI] [PubMed] [Google Scholar]
- 11.Kistner O, Barrett N, Brühmann A, Reiter M, Mundt W, Savidis-Dacho H, Schober-Bendixen S, Dorner F, Aaskov J. 2007. The preclinical testing of a formaldehyde inactivated Ross River virus vaccine designed for use in humans. Vaccine 25:4845–4852. doi: 10.1016/j.vaccine.2007.01.103. [DOI] [PubMed] [Google Scholar]
- 12.Aichinger G, Ehrlich HJ, Aaskov JG, Fritsch S, Thomasser C, Draxler W, Wolzt M, Muller M, Pinl F, Van Damme P, Hens A, Levy J, Portsmouth D, Holzer G, Kistner O, Kreil TR, Barrett PN. 2011. Safety and immunogenicity of an inactivated whole virus Vero cell-derived Ross River virus vaccine: a randomized trial. Vaccine 29:9376–9384. doi: 10.1016/j.vaccine.2011.09.125. [DOI] [PubMed] [Google Scholar]
- 13.Yu S, Aaskov JG. 1994. Development of a candidate vaccine against Ross River virus infection. Vaccine 12:1118–1124. doi: 10.1016/0264-410X(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research 2007. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, Washington, DC. [Google Scholar]
- 15.DeMeio JL, DeSanctis A, Thomas WJ. 1979. Persistence in humans of antibody after immunization with four alphavirus vaccines. Asian J Infect Dis 3:119–124. [PubMed] [Google Scholar]
- 16.Edelman R, Ascher MS, Oster CN, Ramsburg HH, Cole FE, Eddy GA. 1979. Evaluation in humans of a new, inactivated vaccine for Venezuelan equine encephalitis virus (C-84). J Infect Dis 140:708–715. doi: 10.1093/infdis/140.5.708. [DOI] [PubMed] [Google Scholar]
- 17.Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. 2000. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg 62:681–685. [DOI] [PubMed] [Google Scholar]
- 18.Engler RJ, Mangiafico JA, Jahrling P, Ksiazek TG, Pedrotti-Krueger M, Peters CJ. 1992. Venezuelan equine encephalitis-specific immunoglobulin responses: live attenuated TC-83 versus inactivated C-84 vaccine. J Med Virol 38:305–310. doi: 10.1002/jmv.1890380414. [DOI] [PubMed] [Google Scholar]
- 19.Pittman PR, Makuch RS, Mangiafico JA, Cannon TL, Gibbs PH, Peters CJ. 1996. Long-term duration of detectable neutralizing antibodies after administration of live-attenuated VEE vaccine and following booster vaccination with inactivated VEE vaccine. Vaccine 14:337–343. doi: 10.1016/0264-410X(95)00168-Z. [DOI] [PubMed] [Google Scholar]
- 20.Steele KE, Reed DS, Glass PJ. 2007. Alphavirus Encephalitides, p 241–270. In Dembek ZF. (ed), Medical aspects of biological warfare. Office of the Surgeon General, U.S. Army Medical Department Center and School, Borden Institute, Washington DC. [Google Scholar]
- 21.Halstead SB, Thomas SJ. 2010. Japanese encephalitis: new options for active immunization. Clin Infect Dis 50:1155–1164. doi: 10.1086/651271. [DOI] [PubMed] [Google Scholar]
- 22.Heinz FX, Holzmann H, Essl A, Kundi M. 2007. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine 25:7559–7567. doi: 10.1016/j.vaccine.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Monath TP, Fowler E, Johnson CT, Balser J, Morin MJ, Sisti M, Trent DW. 2011. An inactivated cell-culture vaccine against yellow fever. N Engl J Med 364:1326–1333. doi: 10.1056/NEJMoa1009303. [DOI] [PubMed] [Google Scholar]
- 24.Barrett PN, Portsmouth D, Ehrlich HJ. 2013. Tick-borne encephalitis virus vaccine, p 773–788. In Plotkin SA, Orenstein WA, Offit PA (ed), Vaccines, 6th ed. Saunders Elsevier, Philadelphia, PA. [Google Scholar]
- 25.Ehrlich HJ, Pavlova BG, Fritsch S, Pöllabauer EM, Löw-Baselli A, Obermann-Slupetzky O, Maritsch F, Cil I, Dorner F, Barrett PN. 2003. Randomized, phase II dose-finding studies of a modified tick-borne encephalitis vaccine: evaluation of safety and immunogenicity. Vaccine 22:217–223. doi: 10.1016/S0264-410X(03)00563-2. [DOI] [PubMed] [Google Scholar]
- 26.Loew-Baselli A, Poellabauer EM, Pavlova BG, Fritsch S, Koska M, Bobrovsky R, Konior R, Ehrlich HJ. 2009. Seropersistence of tick-borne encephalitis antibodies, safety and booster response to FSME-IMMUN 0.5 ml in adults aged 18-67 years. Hum Vaccin 5:551–556. doi: 10.4161/hv.5.8.8571. [DOI] [PubMed] [Google Scholar]
- 27.Loew-Baselli A, Poellabauer EM, Pavlova BG, Fritsch S, Firth C, Petermann R, Barrett NP, Ehrlich HJ. 2011. Prevention of tick-borne encephalitis by FSME-IMMUN vaccines: review of a clinical development programme. Vaccine 29:7307–7319. doi: 10.1016/j.vaccine.2011.07.089. [DOI] [PubMed] [Google Scholar]
- 28.Loew-Baselli A, Konior R, Pavlova BG, Fritsch S, Pöllabauer E, Maritsch F, Harmacek P, Krammer M, Barrett PN, Ehrlich HJ, FSME-IMMUN Study Group . 2006. Safety and immunogenicity of the modified adult tick-borne encephalitis vaccine FSME-IMMUN: results of two large phase 3 clinical studies. Vaccine 24:5256–5263. doi: 10.1016/j.vaccine.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 29.Pöllabauer EM, Fritsch S, Pavlova BG, Löw-Baselli A, Firth C, Koska M, Maritsch F, Barrett PN, Ehrlich HJ. 2010. Clinical evaluation to determine the appropriate paediatric formulation of a tick-borne encephalitis vaccine. Vaccine 28:4558–4568. doi: 10.1016/j.vaccine.2010.04.075. [DOI] [PubMed] [Google Scholar]
- 30.Pöllabauer EM, Pavlova BG, Löw-Baselli A, Fritsch S, Draxler W, Firth C, Bosman J, Valenta B, Harmacek P, Maritsch F, Barrett PN, Ehrlich HJ. 2010. Comparison of immunogenicity and safety between two paediatric TBE vaccines. Vaccine 28:4680–4685. doi: 10.1016/j.vaccine.2010.04.047. [DOI] [PubMed] [Google Scholar]