Abstract
Human parainfluenza virus type 1 (hPIV-1) is the most common cause of laryngotracheobronchitis (croup), resulting in tens of thousands of hospitalizations each year in the United States alone. No licensed vaccine is yet available. We have developed murine PIV-1 (Sendai virus [SeV]) as a live Jennerian vaccine for hPIV-1. Here, we describe vaccine testing in healthy 3- to 6-year-old hPIV-1-seropositive children in a dose escalation study. One dose of the vaccine (5 × 105, 5 × 106, or 5 × 107 50% egg infectious doses) was delivered by the intranasal route to each study participant. The vaccine was well tolerated by all the study participants. There was no sign of vaccine virus replication in the airway in any participant. Most children exhibited an increase in antibody binding and neutralizing responses toward hPIV-1 within 4 weeks from the time of vaccination. In several children, antibody responses remained above incoming levels for at least 6 months after vaccination. Data suggest that SeV may provide a benefit to 3- to 6-year-old children, even when vaccine recipients have preexisting cross-reactive antibodies due to previous exposures to hPIV-1. Results encourage the testing of SeV administration in young seronegative children to protect against the serious respiratory tract diseases caused by hPIV-1 infections.
INTRODUCTION
Human parainfluenza virus type 1 (hPIV-1) is a member of the Paramyxoviridae family. It is the major cause of laryngotracheobronchitis (croup) and can also mediate bronchiolitis and pneumonia, most commonly in children (1, 2). There have been previous attempts to develop a vaccine against hPIV-1, but no vaccine has yet been licensed (3, 4). A study of a formalin-treated hPIV-1 vaccine in the 1960s demonstrated safety but not efficacy (5).
We have pursued the development of a Jennerian (xenotropic) vaccine approach. Our previous studies showed that Sendai virus (SeV), a murine PIV, had both sequence and antigenic similarity with hPIV-1 (6–9). We found that hPIV-1 protected mice from SeV infections and that SeV safely protected nonhuman primates from hPIV-1 infections (10, 11). SeV has also proven successful as a recombinant vaccine for other paramyxovirus pathogens in animal models (12–18).
Historically, SeV has never caused disease in humans. Upon the first discovery of the virus in 1952, there was some concern that SeV was an etiological agent for human respiratory infections, but it was later determined that SeV is a pathogen of mice, not of humans (2, 19, 20). Moreover, when we tested SeV in a dose escalation phase I clinical study in human adult volunteers, we found that it was well tolerated and enhanced hPIV-1-specific antibody responses in some individuals (21). As a follow-up to the adult study, we tested SeV in a dose escalation study in 3- to 6-year-old PIV-1-seropositive children, and we describe here the early safety, tolerability, and immunogenicity data in this age group.
MATERIALS AND METHODS
Participants.
Ten healthy children between the ages of 3 and 6 years (six males, four females) were vaccinated in a phase I dose escalation study of the SeV vaccine. The protocol was reviewed and approved by the U.S. Food and Drug Administration (FDA) and the St. Jude Children's Research Hospital Institutional Review Board. The study was performed only after data from a phase I study with SeV in adults were reviewed and approved by a data safety monitoring board.
Vaccine.
The vaccine was an unmodified live SeV (Enders strain) propagated in chick egg (Spafas, Inc., Preston, CT) allantoic fluid and purified by sedimentation on a sucrose cushion and then a sucrose gradient. The vaccine was stored frozen at −80°C and was thawed and diluted in sterile saline immediately prior to intranasal administration.
Study design.
This study of SeV in healthy 3- to 6-year-old children was similar to our previous vaccine study in adults (21). Briefly, the parent/guardian of each study participant provided written informed consent. A seropositive response, indicating a previous natural exposure to hPIV-1 by the study participant, was required at the prescreen visit in order to allow the child to be vaccinated. A positive score was based on a comparison of the child's prescreen SeV-based enzyme-linked immunosorbent assay (ELISA) results (sera diluted 1:1,000) with positive- and negative-control samples. The test score was required to be ≥3 times the background (negative-control mean), and it had to exceed the mean of positive controls minus 2 standard deviations. One child did not receive the vaccine due to a seronegative test result. The time period between the screening blood draw and vaccination was 1 to 4 days.
The study evaluated three doses of intranasal live unmodified SeV-based vaccine (5 × 105, 5 × 106, and 5 × 107 50% egg infectious doses [EID50]) delivered once. A standard dose escalation design was followed, with monitoring for absence of any dose-limiting toxicity for at least 28 days in each lower-dose cohort before opening a higher-dose cohort. One child was inadvertently given a 10-fold-lower vaccine dose than anticipated (5 × 105 rather than 5 × 106 EID50); for the purposes of this report, this child's data will be considered along with data from the other children who received the 5 × 105 EID50 vaccine dose. Study participants were not to receive any other immunizations 30 days before or after the SeV vaccination.
The vaccine (0.25 ml) was delivered by dropper into each nostril (total, 0.5 ml) of the supine study participant. The primary endpoint of the study was elicited adverse events occurring within 28 days of vaccination. Safety was evaluated during clinic or at-home visits on days 2, 4, 7, 14, and 28 after vaccination. Families were also provided with a diary card to record adverse events through day 28 of the study. Blood was collected at screening and on days 14, 28, and 182 after vaccination for evaluation of binding and neutralizing antibodies. Nasal swabs obtained on days 2, 4, and 7 after vaccination were tested for the presence of replication-competent vaccine virus by serial dilutions and inoculations into the allantoic cavities of 10-day-old embryonated eggs. After incubation at 35°C for 72 h, allantoic fluid from each egg was tested for virus by hemagglutination (HA) with chicken red blood cells (RBC). This was achieved by transferring 50 μl of allantoic fluid to 96-well round-bottomed plates for mixing with 50 μl chicken RBC (0.5%) in phosphate-buffered saline (PBS). After a 45-min incubation at 4°C, HA was scored.
Immune assays. (i) Binding antibody assays.
Two different enzyme-linked immunosorbent assays (ELISAs) were performed to detect antibodies toward SeV and hPIV-1. ELISA plates (96 wells each) were coated overnight at 4°C with either purified disrupted SeV or hPIV-1 (1.0 μg/ml). After nonadsorbed virus was removed, the well surfaces were blocked (3.0% bovine serum albumin [BSA] in PBS). Sera were diluted 1:100 and then serially diluted 1:10 (3% BSA in 0.1% Tween 20), and 50 μl was applied in triplicate wells to the coated plates for a 1-h incubation at room temperature (RT). Wells were washed with PBS and 0.1% Tween 20 (PBST), and bound IgG was detected using alkaline phosphatase-conjugated goat anti-human IgG incubated for 1 h (at RT). After washing away unbound anti-human IgG with PBST, the substrate p-nitrophenyl phosphate was added, and the optical density (OD) at 405 nm was recorded. The binding titer was determined using nonlinear regression software (GraphPad Prism). The titer was defined as the highest sample dilution that scored an OD of at least 0.1 at 405 nm.
(ii) hPIV-1 neutralization assays.
All serum samples were treated by a 1:4 dilution with receptor-destroying enzyme (RDE) (SEIKEN Accurate Chemical) prior to the assay for neutralizing activity. This involved the mixing of sera with RDE overnight at 37°C. The enzyme and the serum complement were then inactivated by heating the samples to 56°C for 30 min. To detect hPIV-1-specific neutralizing antibody activity, the virus (approximately 100 PFU hPIV-1 in Eagle's minimum essential medium [EMEM]) supplemented with 2% fetal bovine serum [FBS]) was incubated in triplicate wells (96-well plates) with serial serum dilutions (1:2 dilutions starting at 1:40 in EMEM supplemented with 2% FBS) for 1 h at 37°C in humid air with 5% CO2. The mixtures were then inoculated into the wells of 96-well plates with confluent monolayers of LLC-MK2 cells. The media or viruses without antibodies were plated in wells to serve as controls. Following an overnight incubation at 37°C in humid air with 5% CO2, the inoculum was removed and replaced with 150 μl Dulbecco's modified Eagle's medium, 0.1% bovine serum albumin (BSA), and acetylated trypsin (2 μg/ml) and was incubated at 37°C in humid air with 5% CO2. After 4 days, the virus was scored for HA with chicken RBC. The neutralization titer was defined as the highest sample dilution for which the majority of wells scored negatively for HA.
RESULTS AND DISCUSSION
Ten study participants, including males and females between the ages of 3 and 6 years, were vaccinated in a phase I study of live unmodified SeV vaccine. A preexisting antibody response to SeV reflective of a previous natural exposure to hPIV-1 was required as a prerequisite for vaccination. Each eligible participant received a single intranasal dose of the SeV vaccine, at either a low (5 × 105 EID50), medium (5 × 106 EID50), or high (5 × 107 EID50) dose. Participant characteristics and clinical adverse events are summarized in Table 1, and safety laboratory parameters measured before and 14 days after vaccination are shown in Table 2. As shown, the SeV vaccine was uniformly well tolerated, with no participant experiencing adverse events greater than grade 1 or 2. SeV was not detected in any nasal swab sample, as tested by egg inoculation.
TABLE 1.
Participant characteristics (3- to 6-year age group) and adverse reactions to Sendai virus vaccine
| Patient no. | Gender | Age at vaccination | Dose of vaccine (EID50)a | Adverse reactionb | Study day of adverse reaction | Grade of adverse reaction | Relationship of adverse reaction to vaccination |
|---|---|---|---|---|---|---|---|
| S702 | Female | 5 yr 4 mo | 5 × 105 | None | |||
| S703 | Female | 3 yr 2 mo | 5 × 105 | Cough | 1 | 1 | Possible |
| Cough | 22 | 1 | Unrelated | ||||
| Rhinorrhea | 26 | 1 | Unrelated | ||||
| Otitis media | 156 | 1 | Unrelated | ||||
| S704 | Female | 3 yr 1 mo | 5 × 105 | Irritability | 0 | 1 | Possible |
| Eczema | 14 | 1 | Unrelated | ||||
| S707 | Male | 5 yr 5 mo | 5 × 105 | None | |||
| S706 | Male | 3 yr 9 mo | 5 × 106 | Cough | 13 | 1 | Unrelated |
| Cough | 15 | 2 | Unrelated | ||||
| Cough | 16 | 1 | Unrelated | ||||
| Nasal congestion | 16 | 1 | Unrelated | ||||
| S708 | Male | 5 yr 10 mo | 5 × 106 | None | |||
| S709 | Male | 4 yr 4 mo | 5 × 106 | Eczema | Screen | 1 | Unrelated |
| Rhinorrhea | 27 | 1 | Unrelated | ||||
| Cough | 27 | 1 | Unrelated | ||||
| S710 | Male | 5 yr | 5 × 107 | None | |||
| S711 | Female | 3 yr | 5 × 107 | Fever | 7 | 2 | Possible |
| S713 | Male | 3 yr 10 mo | 5 × 107 | Hyperactivity | 0 | 1 | Unlikely |
| Nasal congestion | 5 | 1 | Possible | ||||
| Rhinorrhea | 7 | 1 | Possible | ||||
| Irritability | 12 | 1 | Unlikely | ||||
| Rhinorrhea | 15 | 1 | Unlikely | ||||
| Nasal congestion | 17 | 1 | Unlikely |
EID50, 50% egg infectious dose.
Sendai virus vaccine was well tolerated in the 3- to 6-year age group.
TABLE 2.
Chemistry and cell count results before and 14 days after Sendai virus vaccination
| Testa | Result (median [range]) |
|
|---|---|---|
| Prevaccination | 14 days postvaccination | |
| ALT (U/liter) | 17.5 (12–35) | 17 (12–29) |
| Amylase (U/liter) | 70.5 (21–83) | 67.5 (28–88) |
| AST (U/liter) | 35 (25–43) | 34 (27–43) |
| Bilirubin (mg/dl) | 0.3 (0.2–0.4) | 0.2 (0.1–0.5) |
| Creatinine (mg/dl) | 0.31 (0.3–0.5) | 0.3 (0.29–0.4) |
| Hgb (g/dl) | 12.7 (11.5–14.2) | 12.6 (11.7–14.5) |
| ANC (cells/mm3) | 2,950 (2,100–5,800) | 3,400 (2,200–7,500) |
| Platelets (103 cells/mm3) | 283 (232–352) | 274 (252–607) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; Hgb, hemoglobin; ANC, absolute neutrophil count.
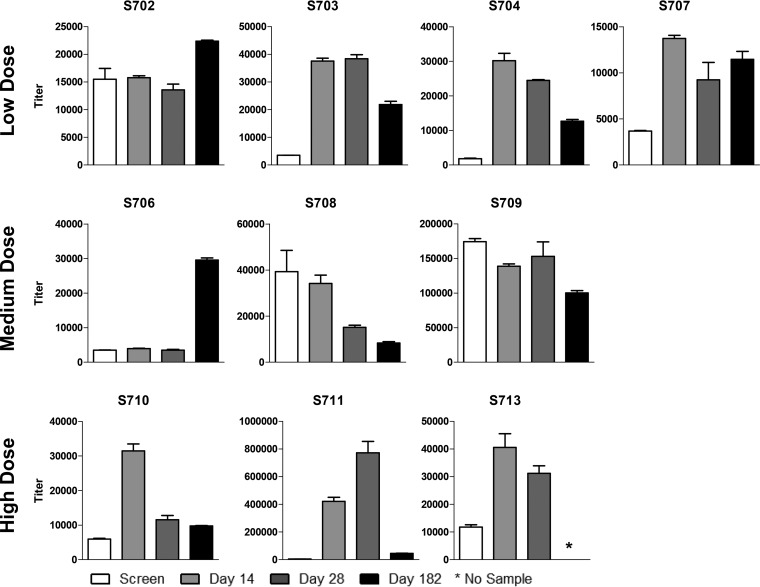
Despite the presence of SeV-specific binding antibodies prior to vaccination in all the study participants and the lack of vaccine amplification, there was a moderate or substantial increase in SeV-specific antibody titers after vaccination in 8 of the 10 children (Fig. 1). Improvements in antibody titers were achieved in children who received low, medium, and high vaccine doses and were usually recognized within 14 days after vaccination. There was some waning for participants S703, S704, S707, S710, S711, and S713, while the titers in participants S702 and S706 were enhanced at the 6-month time point. A gradual increase in immune responses can typify immune reactivities toward live viral vaccines, but a late response can also result from a coincident natural exposure to hPIV-1.
FIG 1.
Analysis of Sendai virus (SeV)-specific antibody among recipients of intranasal SeV vaccine. Serum samples obtained from ten 3- to 6-year-old participants at indicated time points prevaccination and postvaccination were examined for SeV-specific antibody binding. Results are reported as antibody titers defined by nonlinear regression calculations. Antibody binding titer was defined as the highest sample dilution that scored an OD of at least 0.1 at 405 nm on the ELISA. All study participants had SeV-specific binding antibody titers of at least 1,000 prior to vaccination. Means and standard errors are shown. *, assays were conducted prior to collection of the 6-month sample from participant S713.
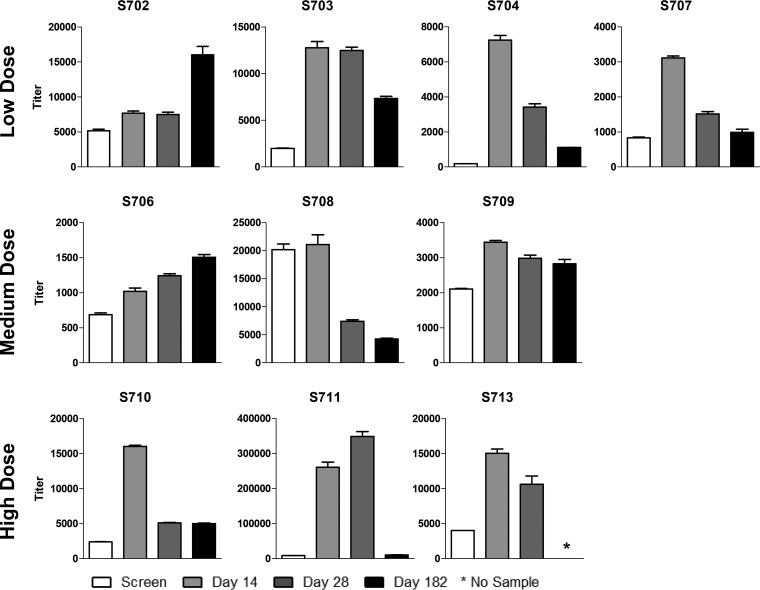
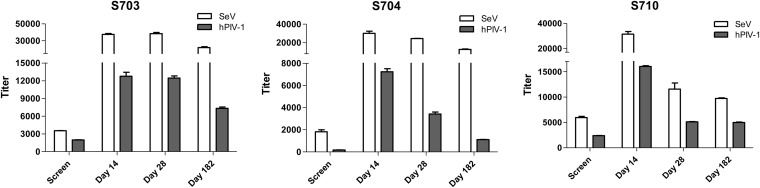
hPIV-1-specific binding antibodies were improved in the majority of the study participants after vaccination, as expected due to the known sharing of antigenic determinants between hPIV-1 and SeV (7, 8) (Fig. 2). Increases in these antibodies by day 14 were observed among 9 of the 10 individuals (Student's t test, P < 0.05). SeV-specific and hPIV-1-specific antibodies often exhibited similar temporal trends (Fig. 3; note that the SeV and hPIV-1 assays were not designed to be compared quantitatively). Like SeV-specific binding antibodies, hPIV-1-specific antibodies often waned by 6 months but remained higher than prevaccination levels.
FIG 2.
Analysis of human parainfluenza virus type 1 (hPIV-1)-specific antibody among recipients of intranasal SeV vaccine. Serum samples obtained from ten 3- to 6-year-old participants at indicated time points prevaccination and postvaccination were examined for hPIV-1-specific antibody binding. Results are reported as antibody titers defined by nonlinear regression calculations. Antibody binding titer was defined as the highest sample dilution that scored an OD of at least 0.1 at 405 nm on the ELISA. Means and standard errors are shown. *, assays were conducted prior to collection of the 6-month sample from participant S713. Student's t tests were performed to compare hPIV-1-specific binding antibody responses before and after vaccinations. Except for one participant (S708), significant improvements in hPIV-1-specific antibody binding titers occurred on day 14 after vaccinations compared to prevaccination titers for all individuals (Student's t test [GraphPad Prism], P < 0.05).
FIG 3.
Similar temporal trends for Sendai virus (SeV)-specific and human parainfluenza virus type 1 (hPIV-1)-specific binding antibody titers. SeV and hPIV-1 binding titers were plotted side by side for participants S703, S704, and S710.
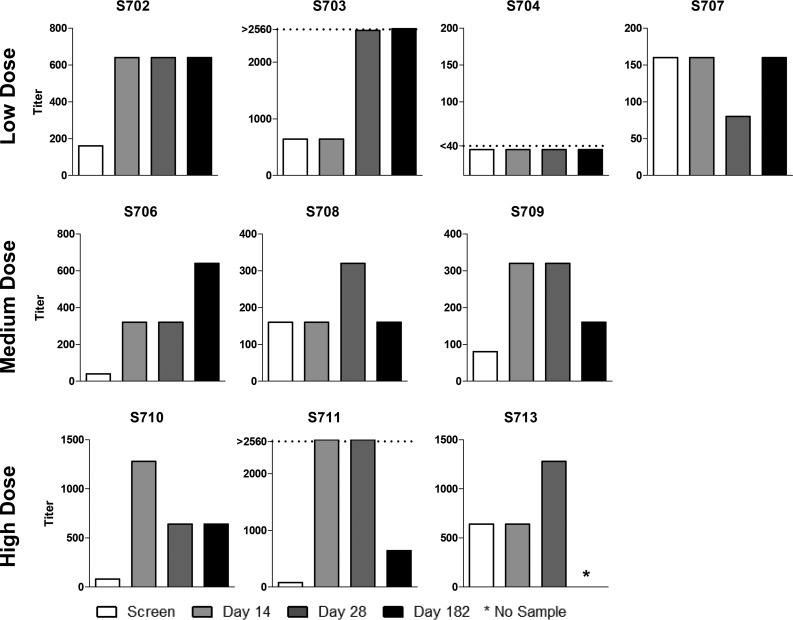
Neutralizing antibody titers toward hPIV-1 are shown in Fig. 4. Again, the majority of the participants exhibited increases in titers after vaccination, and increases were observed among participants who received the low, medium, and high doses of vaccines. The changes in binding activity predicted changes in neutralizing activity in some, but not all, cases. For participants S704 and S707, for example, improvements in binding antibody titers toward SeV and hPIV-1 were not matched by improvements in neutralizing function. It is possible that antibodies in these participants bound viral epitopes that failed to inhibit viral growth. Alternatively, the results may have been affected by nonspecific serum factors that enhanced or inhibited viral growth, thus obscuring an accurate readout of neutralizing antibody activities.
FIG 4.
Analysis of human parainfluenza virus type 1 (hPIV-1)-specific neutralizing antibody among recipients of intranasal SeV vaccine. Serum samples obtained from ten 3- to 6-year-old participants at indicated time points prevaccination and postvaccination were examined for hPIV-1-specific neutralizing antibody activities. Results are reported as antibody titers defined by nonlinear regression calculations. The neutralization titer was defined as the highest sample dilution for which the majority of wells in the assay scored negatively for hemagglutination (HA). *, assays were conducted prior to collection of the 6-month sample from participant S713; dotted lines, results were either too high or too low for quantification.
This study of healthy 3- to 6-year-old children showed the safety and immunogenicity of live unmodified SeV vaccine and demonstrated increases in antibody responses, even when children were seropositive for PIV-1 at study entry. The results reinforced the shared antigenicity of murine and human PIV-1 and the fact that, despite abundant contact between mice (the natural host of SeV) and humans, there has never been a confirmed case of SeV-associated human disease. A safe intranasal paramyxovirus vaccine holds great appeal, in part because needles and syringes are not required. SeV is also attractive because of its natural host range restriction (22) partnered with its ability to elicit rapid and durable B cell and T cell responses in systemic and mucosal tissues (12, 23–25). Results in this report encourage the progression of SeV vaccine testing to younger seronegative children. The success of SeV in future studies may ultimately provide a means for preventing croup and other forms of serious paramyxovirus disease in children.
ACKNOWLEDGMENTS
These studies were supported in part by funding from NIH NIAID grants P01 AI054955 and R01 AI088729, NIH NCI grant P30 CA21765, and the American Lebanese Syrian Associated Charities (ALSAC).
We thank the late John Coleman and Jerry Shenep for assistance, respectively, with GMP production to support the initial stages of the pediatric vaccine study and with the clinical development of the Sendai virus vaccine.
REFERENCES
- 1.Counihan ME, Shay DK, Holman RC, Lowther SA, Anderson LJ. 2001. Human parainfluenza virus-associated hospitalizations among children less than five years of age in the United States. Pediatr Infect Dis J 20:646–653. doi: 10.1097/00006454-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Karron RA, Collins PL. 2007. Parainfluenza viruses, p 1497–1526. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 3.Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. 1969. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol 89:435–448. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett EJ, maro-Carambot E, Surman SR, Newman JT, Collins PL, Murphy BR, Skiadopoulos MH. 2005. Human parainfluenza virus type I (HPIV1) vaccine candidates designed by reverse genetics are attenuated and efficacious in African green monkeys. Vaccine 23:4631–4646. doi: 10.1016/j.vaccine.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 5.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. 1969. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol 89:449–463. [DOI] [PubMed] [Google Scholar]
- 6.Lyn D, Gill DS, Scroggs RA, Portner A. 1991. The nucleoproteins of human parainfluenza virus type 1 and Sendai virus share amino acid sequences and antigenic and structural determinants. J Gen Virol 72:983–987. doi: 10.1099/0022-1317-72-4-983. [DOI] [PubMed] [Google Scholar]
- 7.Smith FS, Portner A, Leggiadro RJ, Turner EV, Hurwitz JL. 1994. Age-related development of human memory T-helper and B-cell responses toward parainfluenza virus type-1. Virology 205:453–461. doi: 10.1006/viro.1994.1665. [DOI] [PubMed] [Google Scholar]
- 8.Dave VP, Allan JE, Slobod KS, Smith SF, Ryan K, Powell U, Portner A, Hurwitz JL. 1994. Viral cross-reactivity and antigenic determinants recognized by human parainfluenza virus type 1-specific cytotoxic T-cells. Virology 199:376–383. doi: 10.1006/viro.1994.1135. [DOI] [PubMed] [Google Scholar]
- 9.Gorman WL, Gill DS, Scroggs RA, Portner A. 1990. The hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus type 1 and Sendai virus have high structure-function similarity with limited antigenic cross-reactivity. Virology 175:211–223. doi: 10.1016/0042-6822(90)90201-2. [DOI] [PubMed] [Google Scholar]
- 10.Sangster M, Smith FS, Coleclough C, Hurwitz JL. 1995. Human parainfluenza virus-type 1 immunization of infant mice protects from subsequent Sendai virus infection. Virology 212:13–19. doi: 10.1006/viro.1995.1448. [DOI] [PubMed] [Google Scholar]
- 11.Hurwitz JL, Soike KF, Sangster MY, Portner A, Sealy RE, Dawson DH, Coleclough C. 1997. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine 15:533–540. doi: 10.1016/S0264-410X(97)00217-X. [DOI] [PubMed] [Google Scholar]
- 12.Jones B, Zhan X, Mishin V, Slobod KS, Surman S, Russell CJ, Portner A, Hurwitz JL. 2009. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine 27:1848–1857. doi: 10.1016/j.vaccine.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takimoto T, Hurwitz JL, Zhan X, Krishnamurthy S, Prouser C, Brown B, Coleclough C, Boyd K, Scroggs RA, Portner A, Slobod KS. 2005. Recombinant Sendai virus as a novel vaccine candidate for respiratory syncytial virus. Viral Immunol 18:255–266. doi: 10.1089/vim.2005.18.255. [DOI] [PubMed] [Google Scholar]
- 14.Takimoto T, Hurwitz JL, Coleclough C, Prouser C, Krishnamurthy S, Zhan X, Boyd K, Scroggs RA, Brown B, Nagai Y, Portner A, Slobod KS. 2004. Recombinant Sendai virus expressing the G glycoprotein of respiratory syncytial virus (RSV) elicits immune protection against RSV. J Virol 78:6043–6047. doi: 10.1128/JVI.78.11.6043-6047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhan X, Hurwitz JL, Krishnamurthy S, Takimoto T, Boyd K, Scroggs RA, Surman S, Portner A, Slobod KS. 2007. Respiratory syncytial virus (RSV) fusion protein expressed by recombinant Sendai virus elicits B-cell and T-cell responses in cotton rats and confers protection against RSV subtypes A and B. Vaccine 25:8782–8793. doi: 10.1016/j.vaccine.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhan X, Slobod KS, Krishnamurthy S, Luque LE, Takimoto T, Jones B, Surman S, Russell CJ, Portner A, Hurwitz JL. 2008. Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections. Vaccine 26:3480–3488. doi: 10.1016/j.vaccine.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones BG, Sealy RE, Rudraraju R, Traina-Dorge VL, Finneyfrock B, Cook A, Takimoto T, Portner A, Hurwitz JL. 2012. Sendai virus-based RSV vaccine protects African green monkeys from RSV infection. Vaccine 30:959–968. doi: 10.1016/j.vaccine.2011.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones BG, Sealy RE, Surman SL, Portner A, Russell CJ, Slobod KS, Dormitzer PR, DeVincenzo J, Hurwitz JL. 2014. Sendai virus-based RSV vaccine protects against RSV challenge in an in vivo maternal antibody model. Vaccine 32:3264–3273. doi: 10.1016/j.vaccine.2014.03.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tai FH, Chiu S, Ma CC. 1967. Seroepidemiologic studies of Sendai virus infection in Taiwan. Taiwan I Hsueh Hui Tsa Chih 66:312–318. [PubMed] [Google Scholar]
- 20.Stark JE, Heath RB. 1967. The development of antibodies against Sendai virus in childhood. Arch Gesamte Virusforsch 20:438–444. doi: 10.1007/BF01275224. [DOI] [PubMed] [Google Scholar]
- 21.Slobod KS, Shenep JL, Lujan-Zilbermann J, Allison K, Brown B, Scroggs RA, Portner A, Coleclough C, Hurwitz JL. 2004. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine 22:3182–3186. doi: 10.1016/j.vaccine.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 22.Bousse T, Chambers RL, Scroggs RA, Portner A, Takimoto T. 2006. Human parainfluenza virus type 1 but not Sendai virus replicates in human respiratory cells despite IFN treatment. Virus Res 121:23–32. doi: 10.1016/j.virusres.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Rudraraju R, Surman S, Jones B, Sealy R, Woodland DL, Hurwitz JL. 2011. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology 410:429–436. doi: 10.1016/j.virol.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ely KH, Roberts AD, Woodland DL. 2003. Cutting edge: effector memory CD8+ T cells in the lung airways retain the potential to mediate recall responses. J Immunol 171:3338–3342. doi: 10.4049/jimmunol.171.7.3338. [DOI] [PubMed] [Google Scholar]
- 25.Sealy R, Jones BG, Surman SL, Hurwitz JL. 2010. Robust IgA and IgG-producing antibody forming cells in the diffuse-NALT and lungs of Sendai virus-vaccinated cotton rats associate with rapid protection against human parainfluenza virus-type 1. Vaccine 28:6749–6756. doi: 10.1016/j.vaccine.2010.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]