Abstract
In response to genotoxic stress, including UVB radiation, transcription factors NF-κB and p53 inevitably influence the cellular fate. Loss of p53 function has been attributed to malignant transformation and interferes with therapeutic interventions, whereas “gain of function” mutants even enhance tumor promotion. Constitutive NF-κB activation is linked to tumor maintenance and resistance against chemotherapy. The cross talk between p53 and NF-κB, however, is still under debate. Using the non-transformed keratinocyte cell line HaCaT, we shed light on the interplay between p53 and NF-κB by providing clear evidence that chronically activated NF-κB together with designated “gain of function” mutp53 promotes apoptosis via cooperative tumor necrosis factor (TNF) production in response to UVB+IL-1. Performing chromatin immunoprecipitation analysis we demonstrate that both transcription factors bind to the TNF promoter, whereas UVB-induced inhibition of Ser-Thr-phosphatase protein phosphatase 2A facilitates prolonged phosphorylation of NF-κB and the transcriptional cofactor cAMP response element–binding protein, both being required for extended TNF transcription. Thus, two major anti-apoptotic factors, NF-κB and mutp53, in concert may generate pro-apoptotic responses. As human skin is constantly exposed to UVB, causing IL-1 production as well, we hypothesize that the remarkable amount of hotspot p53 mutations within the epidermis (4%) may serve a protective function to eliminate precancerous cells at an early stage.
Introduction
In response to cellular stresses, the cross talk between pro- and anti-apoptotic factors determines the cellular fate. Whereas activation of the tumor-suppressor p53 causes upregulation of pro-apoptotic genes following genotoxic stress (Seitz et al., 2010), activation of the transcription factor nuclear factor κB (NF-κB) is mainly associated with anti-apoptotic responses (Fujioka et al., 2012; Almeida et al., 2014). Inactivation of p53 is observed in most human cancers, with mutations in p53 occurring in about 50% of all tumors (Soussi and Wiman, 2007), reaching up to 90% in non-melanoma skin cancer (Gervin et al., 2003). Intriguingly, in addition to “loss of function” mutants that lack the tumor-suppressive function, “gain of function” mutp53 variants lose the sequence-specific DNA binding but exert complex DNA interactions instead, thereby modifying the set of target genes expressed (Goehler et al., 2005; Kim and Deppert, 2007). Accordingly, contact (p53R248W) and structural (p53R175H) mutations are enabled in order to promote a large spectrum of cancer phenotypes and contribute to drug resistance (Moll et al., 2005; Deppert, 2007; Vousden and Lane, 2007). In contrast to wtp53, both mutants were shown to confer a selective advantage during tumor development, although the individual mechanisms are still under debate (Mello and Attardi, 2013).
NF-κB is mainly triggered in response to pro-inflammatory cytokines, including IL-1. Receptor-driven activation of a downstream kinase cascade involving IκB-kinase β (IKKβ) causes proteasomal degradation of the inhibitor of κBα (IκBα), allowing for nuclear translocation of NF-κB. Because constitutive activation of NF-κB has been linked to transformation, proliferation, and anti-apoptosis (Aggarwal, 2004), limitation of its activity is warranted by negative feedback regulation involving NF-κB-dependent transcription of IκBα (Delhase et al., 1999). More recently, increasing evidence has been found that additionally attribute pro-apoptotic functions to NF-κB when exposed to DNA-damaging agents (Delhase et al., 1999; Campbell et al., 2004; Liu et al., 2006; Szoltysek et al., 2008; O'Prey et al., 2010; Narayanan et al., 2014). Accordingly, previous work from our lab showed co-stimulation with IL-1 to enhance UVB-induced apoptosis in epithelial cells by accelerated NF-κB-dependent transcription of tumor necrosis factor (TNF). Released TNF triggered TNF-R1 in an autocrine manner to additively promote apoptosis (Poppelmann et al., 2005; Strozyk et al., 2006; Barisic et al., 2008; Witt et al., 2009; Barisic et al., 2010).
A versatile cross talk between p53 and NF-κB seems to exist especially in tumor cells, whereas in most cases mutual inhibition has been proposed (Webster and Perkins, 1999; Tergaonkar et al., 2002; Bohuslav et al., 2004; Tergaonkar, 2009). Also the competition of both transcription factors for identical cofactors like CREB (cAMP response element–binding protein) was shown to limit the relative activation status of p53 versus NF-κB (Webster and Perkins, 1999; Ikeda et al., 2000; Huang et al., 2007; Zschiedrich et al., 2008; Tergaonkar, 2009; Spooren et al., 2010). In contrast, other reports attribute a cooperative role to wtp53 and NF-κB in apoptosis induction through mutual activation (Ryan et al., 2000), collaborative promoter binding (Ma et al., 2008), or direct protein–protein interaction (Schneider et al., 2010). Less, however, is known about the interplay between “gain of function” mutp53 and NF-κB in response to genotoxic stress.
Utilizing the non-transformed keratinocyte cell line HaCaT stably knocked down for p53 and/or stably inhibiting NF-κB activation, we demonstrate that mutp53R248W and mutp53R175H but not wtp53 cooperate with NF-κB through direct binding to the TNF promoter. Consequently, accelerated TNF production enhances apoptosis in response to UVB+IL-1. We further provide evidence that UVB-induced inhibition of Ser-Thr-phosphatase protein phosphatase 2A (PP2A) plays a crucial role in sustaining phosphorylation of NF-κB-p65 and CREB to maintain both factors in an active state over time. Hence, we report that two initially anti-apoptotic factors, which are commonly present in various cancers, in concert may promote pro-apoptotic responses.
Results
Co-stimulation with IL-1 enhances UVB-induced apoptosis in an NF-κB-dependent manner
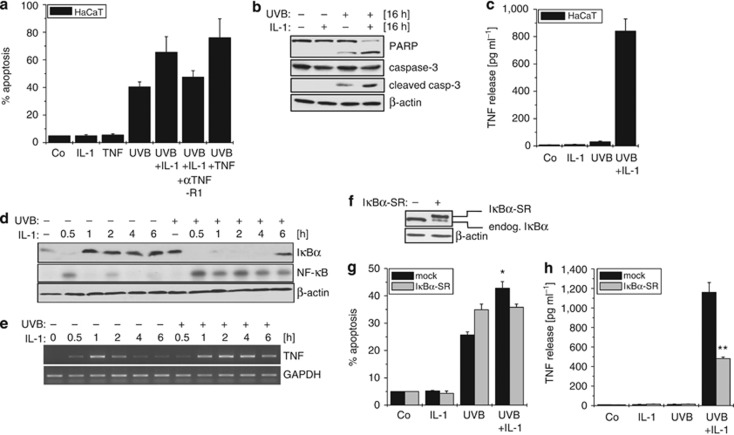
In accordance with previous findings (Poppelmann et al., 2005; Strozyk et al., 2006; Barisic et al., 2008), co-stimulation of HaCaT keratinocytes with IL-1 significantly enhanced UVB-induced apoptosis (Figure 1a). As this enhancement could be blocked with an antagonistic TNF-R1 antibody, TNF-dependent TNF-R1 activation seemed to affirm the underlying mechanism as reported before (Poppelmann et al., 2005; Barisic et al., 2008). Thus, enhancement of apoptosis was also evident by pronounced processing of caspase-3 and poly(ADP-ribose)-polymerase (PARP) (Figure 1b) and coincided with considerable TNF release (Figure 1c). Similar to epithelial cells, TNF production in keratinocytes could be attributed to disruption of the negative feedback regulation of NF-κB:
Figure 1.
IL-1 enhances UVB-induced apoptosis due to pronounced tumor necrosis factor (TNF) release. (a) Cells were stimulated with IL-1, TNF, UVB, or a combination of IL-1+UVB or TNF+UVB, or pretreated for 30 minutes with an antagonistic anti-TNF-R1 antibody. Apoptosis was determined with Cell Death Detection ELISA. (b) Cleavage of caspase-3 and poly(ADP-ribose)-polymerase (PARP) was documented by Western-blotting. (c) TNF secretion was analyzed with TNF ELISA. (d) Cells were stimulated with IL-1 or UVB+IL-1 as indicated. Cytosolic and nuclear fractions were analyzed for IκBα depletion (cyt) by Western-blotting and NF-κB activation (nuc) by electrophoretic mobility shift assay. (e) Cells were stimulated as in d and TNF expression determined by reverse transcriptase–PCR. (f) IκBα status of HaCaT-IκBα super-repressor (IκBα-SR) cells was documented by Western-blotting. (g) HaCaT-mock and -IκBα-SR cells were treated as in a, apoptosis was determined with Cell Death Detection ELISA, and (h) TNF release was documented with TNF ELISA. *P<0.05; **P<0.001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
IL-1 stimulation caused transient NF-κB activation with maximum degradation of IκBα after 30 minutes (Figure 1d). Rapid NF-κB-dependent resynthesis entailed replenishment of IκBα after 1–2 hours, which terminated nuclear NF-κB activity. In contrast, co-stimulation with UVB+IL-1 completely abrogated the reappearance of IκBα up to 6 hours, coinciding with elevated nuclear NF-κB level (Figure 1d). Consequently, prolonged transcription of TNF was facilitated upon UVB+IL-1 treatment (Figure 1e), being a prerequisite for enhanced TNF secretion (Barisic et al., 2008). The notion that inhibition of negative feedback regulation drives NF-κB-dependent TNF production was confirmed in HaCaT cells stably overexpressing an IκBα super-repressor (IκBα-SR; Figure 1f). Overall, apoptosis was slightly enhanced, but more importantly, the enhancing effect of IL-1 on UVB-induced apoptosis was abrogated (Figure 1g) because of significantly reduced TNF secretion (Figure 1h). Still, some residual TNF production remained, indicating that additional factors might contribute to elevated TNF production.
The intensity of DNA damage correlates with the apoptosis rate induced and the amount of TNF released
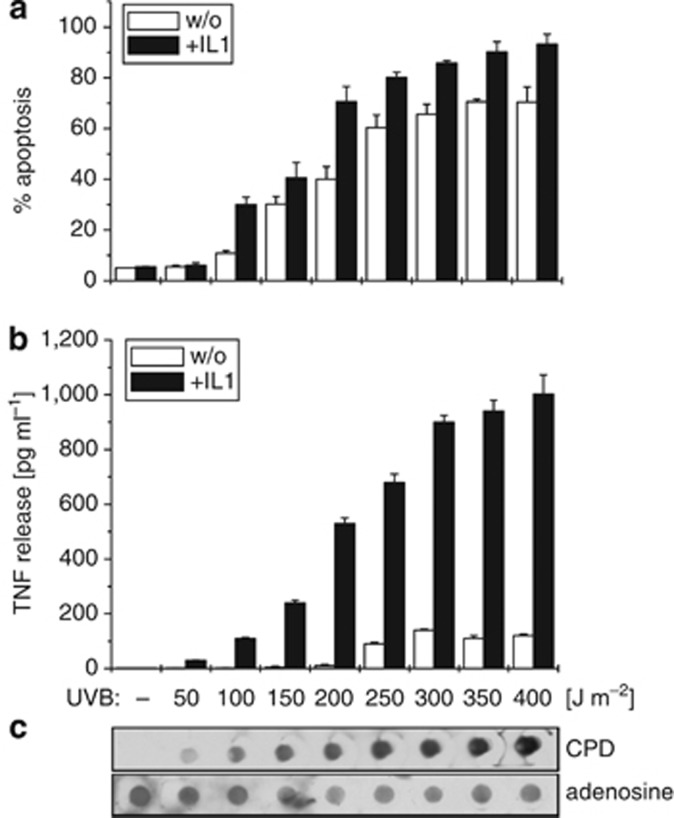
Irradiation of cells with increasing doses of UVB caused gradual enhancement of apoptosis (Figure 2a), which correlated with increasing amounts of cyclobutane-pyrimidine dimer formation (Figure 2c). At a constant IL-1 level also the amount of TNF secretion gradually increased (Figure 2b), implying a link between DNA damage and TNF production. Conclusively, we assumed an additional transcription factor to become activated in response to UVB-driven DNA damage that may cooperate with NF-κB for full TNF production in response to UVB+IL-1.
Figure 2.
IL-1-induced enhancement of UVB-induced apoptosis and tumor necrosis factor (TNF) release correlates with increased DNA damage. HaCaT cells were stimulated with increasing doses of UVB (50–400 J m−2) alone or in combination with constant IL-1 doses (10 ng ml−1). (a) After 16 hours, apoptosis was determined in a Cell Death Detection ELISA and (b) TNF release was documented in a TNF ELISA. (c) In parallel, genomic DNA was extracted and UVB-induced DNA damage visualized by Southwestern dot-blot analysis using an antibody directed against cyclobutane-pyrimidine dimers (CPD). Anti-adenosine documented equal loading of genomic DNA.
p53 contributes to TNF-mediated enhancement of UVB-induced apoptosis
The transcription factor p53 plays a crucial role in mediating DNA damage responses. HaCaT cells are known to harbor mutated p53, carrying heterozygous C→T transitions (Lehman et al., 1993), changing it into a “gain of function”-like phenotype. As “gain of function” mutations of p53 are known to exert an anti-apoptotic phenotype, stable knockdown of p53 sensitized three different HaCaT-p53i clones to UVB-induced apoptosis (Figure 3a and b), whereas the IL-1-mediated upregulation (n-fold) appeared to be impaired in HaCaT-p53i cells and also yielded less TNF (Figure 3d). The protein level of other p53 family members, p63 and p73, remained unaffected by loss of p53 (Figure 3c), indicating that solely p53 might contribute to accelerated apoptosis and TNF production in response to UVB+IL-1. Accordingly, additional inhibition of NF-κB by stably overexpressing IκBα-SR in HaCaT-p53i cells (Figure 3e) minimized both, apoptosis enhancement (Figure 3f) and TNF release (Figure 3g). Conclusively, data implied that both transcription factors may collectively amplify the pro-apoptotic effect in UVB+IL-1-treated cells.
Figure 3.
Knockdown of p53 sensitizes HaCaT cells to UVB. (a) Three stable HaCaT-p53i clones were stimulated with IL-1, UVB, or both, and apoptosis was determined using Cell Death Detection ELISA. (b) Western-blot analysis documenting p53 knockdown. (c) Western-blot analysis of p63 and p73 expression level of HaCaT-mock versus -p53i cells. (d) HaCaT-mock and HaCaT-p53i (clone 2) cells were stimulated with IL-1, UVB, or both. Tumor necrosis factor (TNF) release was determined with TNF ELISA. N-fold increase of apoptosis is indicated. (e) Western blot analysis documenting IκBα and p53 status of HaCaT-p53i/IκBα super-repressor (IκBα-SR) cells. (f) HaCaT-mock, -p53i, -IκBα-SR, and -p53i/IκBα-SR cells were stimulated as in d. Apoptosis was determined with Cell Death Detection ELISA (n-fold enhancement of apoptosis is indicated) and (g) TNF release with TNF ELISA. *P<0.01; **P<0.001. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
NF-κB and p53 additively promote TNF transcription in response to UVB+IL-1
Activation of the NF-κB subunit p65 is facilitated by Ser536 phosphorylation, whereas Ser15 phosphorylation is required for p53 activation (Graham and Gibson, 2005; Ray et al., 2012). Treatment of HaCaT cells with IL-1 caused transient phosphorylation of p65, being terminated after 2 hours, the time point of IκBα resynthesis. Phosphorylation of the transcriptional coactivator CREB at Ser133 (Friedrich et al., 2010) followed the identical pattern, indicating that both proteins are required for proper IL-1-induced transcription. The DNA damage–responsive transcription factor p53 remained inactive following IL-1 stimulation, as anticipated. In response to UVB, instead, p65 stayed inactive, whereas phosphorylation of p53 rapidly occurred and lasted for at least 4 hours. Activation of p53 also coincided with CREB phosphorylation, confirming CREB to be an important cofactor for both transcription factors (Huang et al., 2007). Finally, co-stimulation with UVB+IL-1 allowed phosphorylation of all three components. Whereas the phosphorylation pattern of p53 and CREB remained unchanged compared with UVB-treated cells, p65 phosphorylation was extended (Figure 4a; (Barisic et al., 2008)). Interestingly, knockdown of p53 did not influence the phosphorylation pattern of p65 and CREB (Figure 4b), implying that NF-κB and p53 act additively but independently to enhance TNF transcription. In accordance with this assumption, co-immunoprecipitation revealed that p65 and p53 do not directly interact at the protein level (Figure 4c and d).
Figure 4.
Phosphorylation pattern of p65 and cAMP response element–binding protein (CREB) depends on PP2A. (a) HaCaT-mock and (b) HaCaT-p53i cells were treated with IL-1, UVB, or both. At the indicated time points, the status of IκBα, pSer536-p65, p65, pSer15-p53, p53, pSer133-CREB, and CREB was determined by Western-blot analysis. (c) Cells were stimulated with IL-1 or UVB+IL-1 for 2 h; p53 or (d) p65 was immunoprecipitated, and co-precipitation of p65 and p53, respectively, was documented by Western-blot analysis. (e) Catalytic subunit of PP2A (PP2Ac) was knocked down and residual activity blocked with calyculin A. After 72 hours, cells were stimulated with IL-1, UVB, or both as indicated. After 2 hours, the status of pSer536-p65, p65, pSer15-p53, p53, pSer133-CREB, and CREB was determined by Western-blot analysis. (f) Scheme displaying the processes of accelerated tumor necrosis factor expression. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IKKβ, IκB-kinase β SN, supernatant.
From previous studies, we knew that UVB-mediated inhibition of catalytic subunit of PP2A (PP2Ac) crucially contributes to abrogation of negative feedback regulation for NF-κB through chronic IKKβ activation (Barisic et al., 2008, 2010). We here discover an additional role for PP2A in tuning NF-κB activity. In control cells, only treatment with UVB+IL-1 caused p65 phosphorylation, whereas p53 and CREB were activated by UVB and UVB+IL-1, respectively. Inhibition of PP2Ac by small interfering RNA-mediated knockdown and addition of calyculin A had no effect on p53 phosphorylation but significantly stabilized phosphorylation of p65 and CREB (Figure 4e). These results disclose two ways of PP2A-mediated NF-κB regulation: indirectly by dephosphorylating IKKβ to terminate nuclear localization of NF-κB and directly by dephosphorylating p65 and CREB to terminate transcription (Figure 4f). Activation of p53, instead, is exclusively driven in a DNA damage–responsive manner and remains unaffected by PP2A.
Only mutp53 but not wtp53 binds to the TNF promoter to enhance NF-κB-driven TNF transcription
According to our findings so far, we assumed that p65 and p53 may cooperatively bind to the TNF promoter in response to UVB+IL-1. This idea was strengthened by the finding that, besides p65 and CREB consensus elements, a putative (partial) p53 binding motive also exists within the TNF promoter sequence ((Kuo and Leiden, 1999), Supplementary Figure S1 online). As expected, chromatin immunoprecipitation analysis of p65 consolidated the prolonged association of NF-κB with the TNF promoter in response to UVB+IL-1 in semiquantitative (Figure 5a) and quantitative PCR analyses (Figure 5b). In order to dissect the binding properties of different p53 variants, we overexpressed wtp53 as well as mutp53R248W and mutp53R175H in HaCaT-p53i cells (Figure 5c). As assumed, endogenous mutp53 bound to the TNF promoter for at least 2 hours following UVB+IL-1 treatment (Figure 5d semiquantitative and 5e quantitative). Interestingly, wtp53 was shown to hardly associate with the TNF promoter compared to mutp53R248W and mutp53R175H (Figure 5d and e). Taken together, binding of NF-κB and only “gain of function” mutp53 was significantly intensified over time, whereas binding of wtp53 was neglectable. Concordantly, we could identify different effects of wtp53 versus mutp53 expression on apoptosis as well as TNF release (Figure 5f and g). IL-1-mediated enhancement of UVB-induced apoptosis was stronger in cells harboring mutp53— i.e. normal HaCaT cells as well as mutp53R248W and mutp53R175H expressing HaCaT-p53i cells, and remained only marginal in HaCaT-p53i- and -wtp53-expressing cells (Figure 5f). Of note, the apoptotic responses correlated well with the amount of TNF released by the individual cell lines. Data confirmed accelerated TNF release to closely correlate with pronounced enhancement of apoptosis and strengthens the view that this effect is most pronounced in mutp53-expressing cells (Figure 5g).
Figure 5.
p53 and p65 cooperate to enhance transcription of tumor necrosis factor (TNF). (a) HaCaT cells were treated with IL-1 or UVB+IL-1 as indicated. Reverse transcriptase–PCR (RT-PCR) analysis of NF-κB-p65 chromatin immunoprecipitation (ChIP) is shown. (b) N-fold expression calculated by quantitative PCR (qPCR) of three independent experiments. (c) Western-blot analysis documenting expression of wtp53, mutp53R175H, and mutp53R248W in HaCaT-p53i cells. (d) HaCaT-mock, -p53i, -p53i-wtp53, -p53i-mutp53R248W, and -p53i-mutp53R175H cells were treated as in a. RT-PCR analysis of p53 ChIP is shown. (e) N-fold expression calculated by qPCR of three independent experiments. (f) HaCaT-mock, -p53i, -p53i-wtp53, -p53i-mutp53R248W, and -p53i-mutp53R175H cells were treated with IL-1, UVB, or both. After 16 hours, apoptosis was determined with Cell Death Detection ELISA (n-fold enhancement of apoptosis is indicated) and (g) TNF release with TNF ELISA. *P<0.05; **P<0.01. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
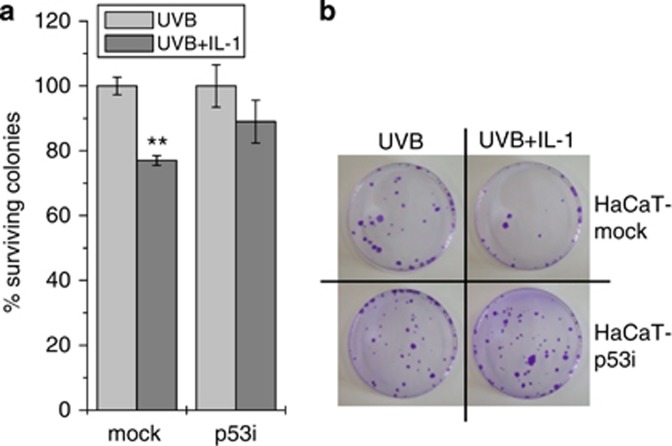
Mutp53 prevents clonogenic outgrowth of UVB+IL-1-treated cells
Three weeks following UVB or UVB+IL-1 stimulation, clonogenic outgrowth of HaCaT-mock compared with HaCaT-p53i cells was determined. Whereas cell density of UVB+IL-1-treated HaCaT-p53i cells reached an average 89% compared with UVB-treated cells, only 77% cell density was achieved for HaCaT-mock cells (Figure 6a and b). This supported the concept that cell survival might be impaired following UVB+IL-1 treatment if p53 is mutated.
Figure 6.
Mutp53 prevents clonogenic outgrowth of UVB+IL-1-treated cells. (a) Percentage of clonogenic outgrowth of HaCaT-mock versus -p53i cells 3 weeks after UVB and UVB+IL-1 stimulation, respectively. Outgrowth of UVB-only treated cells is set as 100% and the cell density of UVB+IL-1-treated cells calculated accordingly. **P<0.01. (b) Display of one representative experiment.
Taken together, we here disclose the cooperation of “gain of function” mutp53 and NF-κB at the TNF promoter and provide evidence that two initially anti-apoptotic proteins in concert may generate pro-apoptotic responses.
Discussion
Dysregulation of both p53 and NF-κB confers a selective advantage during tumor development and conveys therapy resistance. A versatile cross talk between NF-κB and p53 exists at multiple levels; however, the balance between synergistic and antagonistic processes may rely on the cellular context. In the present study, we confirm the molecular mechanism by which chronic NF-κB activation contributes to enhancement of UVB-induced apoptosis via upregulation of TNF in keratinocytes (Poppelmann et al., 2005; Barisic et al., 2008).
Intriguingly, at a constant IL-1 concentration required for NF-κB activation, increasing UVB exposure linked TNF release to the intensity of DNA damage induced, pointing at p53 to support TNF transcription. Hence, TNF release and thereby IL-1-mediated enhancement of UVB-induced apoptosis was minimized in HaCaT-p53i-IκBα-SR cells.
Previous studies have described mutp53 to augment TNF-induced NF-κB activity (Weisz et al., 2007) or have shown that NF-κB-p65 is required for TNF-induced upregulation of p53 target genes in different mouse tissues (Wang et al., 2009; O'Prey et al., 2010). However, a collaboration of the two transcription factors at the human TNF promoter has never been reported. Even though NF-κB-p53 protein–protein interactions have been proposed at responsive promoter elements (Gurzov et al., 2010) or at NF-κB-responsive reporter constructs (Kawauchi et al., 2008), we could not co-immunoprecipitate p53 and p65 in UVB+IL-1-exposed cells. This indicates that both factors independently but additively trigger the TNF promoter. The activation kinetics for p65, p53, and the joint coactivator CREB clearly showed UVB to activate p53 and also to extend IL-1-induced activation for p65 and CREB. Although p53 phosphorylation follows an established ataxia telangiectasia mutated-dependent (ATM) mechanism (Zhang et al., 2011), we here deciphered a PP2A-dependent mechanism that drives prolonged p65 and CREB phosphorylation. On the basis of current and former data, we propose the following scenario to underlie activation of p53 and hyperactivation of NF-κB at the TNF promoter.
IL-1 causes canonical activation of NF-κB, whereas UVB activates p53. In parallel, UVB-induced inhibition of PP2Ac allows for nuclear persistence of NF-κB (Barisic et al., 2008; Witt et al., 2009; Barisic et al., 2010; Zhang et al., 2011; Konrath et al., 2014) and warrants prolonged activation of p65 and CREB. The fact that NF-κB activity still vanishes at later times point can be attributed to other IκB members, such as IκBɛ, that take over NF-κB inhibition in a delayed and more linear manner (Hoffmann et al., 2002).
Thus, the present study is of high patho-/physiological relevance, because IL-1 is constantly produced from UVB-exposed keratinocytes within human skin (Feldmeyer et al., 2007). UVB radiation may serve as a carcinogen by activation of skin oncogenes, inactivation of tumor suppressors, and repression of cell-based immune responses that are generally able to eliminate highly antigenic skin tumors (Yarosh and Kripke, 1996). At the same time, UVB-induced DNA damage is a prerequisite for execution of apoptosis, leading to elimination of cancer-prone cells (Kulms and Schwarz, 2000; Murphy et al., 2001). The molecular switch, however, has not yet been identified. As p53 carries hotspots for UVB-induced mutations, numerous studies exist postulating 4% of all keratinocytes to bare p53 mutations; however, less squamous cell carcinoma develop (Murphy et al., 2001; Klein et al., 2010). It is further common knowledge that up to 50% of all tumors and up to 90% of non-melanoma skin cancers bear p53 mutations (Murphy et al., 2001; Gervin et al., 2003), clearly supporting that mutp53 selectively promotes tumor progression once the oncogenic pressure exceeds a certain threshold. Still, we hypothesize that at sub-oncogenic stresses and concomitant IL-1 production, provided e.g. by keratinocytes from the microenvironment, expression of mutp53 may even be beneficial for mildly damaged cancer-prone cells to enforce their elimination via TNF-dependent apoptosis.
In addition, the present study sheds light on the ongoing discussions about the p53–NF-κB interplay in cancer and may have even broader implications regarding cancer treatment, where currently a lot of effort is made to reconstitute wtp53 and/or inhibit NF-κB in cancer cells (Lin and Karin, 2003; Muller and Vousden, 2013).
Materials and Methods
Results of quantitative PCR, Cell Death Detection-, and TNF-ELISA are presented as mean±SD of three independently performed experiments. Reverse transcriptase–PCR, electrophoretic mobility shift assay, and western blot analyses represent one out of three independently performed experiments. For statistical analysis, Student's t-test was performed.
Cells and reagents
The human keratinocyte cell line HaCaT (Fusenig et al., 1990) was cultured in DMEM, 10% fetal calf serum, and 1% PenStrep (Thermo Scientific, Waltham, MA) and irradiated with UVB (300 J m−2) using TL12 fluorescent bulbs (290–320 nm, Philips; Eindthoven, the Netherlands) or stimulated with recombinant human IL-1β (10 ng ml−1) or TNF (100 ng ml−1; both R&D Systems, Minneapolis, MN). TNF-R1 was blocked with an antagonistic antibody H-398 (40 μg ml−1) kindly provided by Dr Peter Scheurich, University of Stuttgart, Germany. TNF release was measured in a TNF ELISA (R&D Systems) and apoptosis in a Cell Death Detection-ELISA (Roche, Mannheim, Germany). For PP2Ac knockdown, 1.6 × 105 cells were transfected with 120 pmol scrambled 5′-gCggCUgCCggAAUUUACCTT-3′ or PP2Ac 5′-gAggUUCgAUgUCCAgUUATT-3′ small interfering RNA (MWG, Ebersberg, Germany) using Lipofectamin 2000 (Thermo Scientific). Calyculin A (Cell Signaling, Beverley, MA) was added at 5 μM. Transfectants were generated by electroporating 6.5 × 106 cells in DMEM, 10% fetal calf serum, and 1.5% DMSO with 25 μg of plasmids encoding pRetroSuper-blasto-p53 (Zalcenstein et al., 2003), IκBα-S32/36A (pBKCMV-IκB-SR), or mock (pcDNA3; EasyjecT-plus, Peqlab, Erlangen, Germany) and stable clones selected with blasticidine (5 μg ml−1) or G418 (1,7 mg ml−1, PAA, Cölbe, Germany). Silent mutations were introduced into pCMV-neo-Bam-based expression constructs (p53wt, p53R175H, p53R248W) using a Quick Change Site-directed Mutagenesis Kit (Stratagene, Kirkland, WA) and the following primers: F: 5′-ATCACACTggAAgATAgCTCCggCAACCTTCTAggACggAACAgC-3′ R: 5′-gCTgTTCCgTCCTAgAAggTTgCCggAgCTATCTTCCAgTgTgAT-3′.
Electromobility shift analysis
Cells were lysed in low-salt buffer (10 mM HEPES, pH 7.9; 10 mM KCl; 0.1 mM EDTA; 0.1 mM EGTA; 1 mM dithiotreitol; 0.1 mM phenylmethylsulfonylfluoride, Complete; Roche) on ice for 20 minutes. Nuclear pellets were lysed in high-salt buffer (20 mM HEPES, pH 7.9; 400 mM NaCl; 1 mM EDTA; 1 mM EGTA; 1 mM dithiotreitol; 0.1 mM phenylmethylsulfonylfluoride, Complete; Roche). The NF-κB consensus oligo-nucleotide (sc-2505; Santa Cruz, Heidelberg, Germany) was end-labeled using [γ32P] ATP and T4 polynucleotide kinase (Thermo Scientific), and purified using a QIAquick Nucleotide Removal Kit (Qiagen, Minden, Germany). Binding reactions comprising 15 μg protein extract, 4 μl of 5 × binding buffer (20 mM HEPES, pH 7.5; 50 mM KCl; 2.5 mM MgCl2; 20% (w/v) ficoll; 1 mM dithiotreitol), 2 μg poly[dIdC], 2 μg BSA, and 70,000 c.p.m. 32P-labeled NF-κB consensus nucleotide were incubated for 20 minutes at room temperature, separated on 4% native PAGE, and detected autoradiographically (Strozyk et al., 2006).
Immunoprecipitation and western blot analysis
Cells were lysed in lysis buffer (50 mM HEPES, pH 7.5; 150 mM NaCl; 10% glycerol; 1% Triton X-100; 1.5 mM MgCl2; 1 mM EGTA; 100 mM NaF; 10 mM pyrophosphate; 0.01% NaN3, Complete, PhosSTOP, Roche), subjected to 7.5–15% SDS-PAGE, blotted, and incubated with antibodies (β-actin, #4970; caspase-3, #9665; CREB, #9197; phospho-CREB, #9198; GAPDH, #2118; IκBα #4814; phospho-p53, #9284; phospho-p65, #3033; PP2Ac, #2038—Cell Signaling; p53, 554293; poly(ADP-ribose)-polymerase, 551025—BD Biosciences, Heidelberg, Germany; p63, sc-843; p65, sc-8008—Santa Cruz; p73, PC385—Calbiochem, Darmstadt, Germany) and visualized by chemiluminescent detection (SuperSignal, Thermo Scientific). For immunoprecipitation, protein extracts were incubated with anti-p53 or -p65 antibody (#9282; #8242, Cell Signaling) and protein-A-Agarose (KPL, Gaithersburg, ML) beads overnight at 4 °C. Proteins were eluted and subjected to western blot analysis.
Southwestern dot-blot analysis
Genomic DNA was extracted according to the manufacturer's protocol (Qiagen). A volume of 2 μg DNA in denaturation buffer (1.5 M NaCl; 0.5 M NaOH) was vacuum dot-blotted onto a nylon+ membrane, neutralized (1 M Tris; 2 M NaCl), and fixed for 15 minutes at 80 °C. Cyclobutane-pyrimidine dimers were detected with KTM53 antibody (Kamiya Biomedical, Thousand Oaks, CA) and equal loading was monitored with anti-adenosine (Research Plus Inc., Manasquan, NJ).
Semiquanititative reverse transcriptase–PCR
RNA was extracted using GIT buffer and reverse-transcribed with an AMV Reverse Transcriptase kit (Thermo Scientific). Primers were used in a 20-μl reaction utilizing the RedTaq polymerase system (Sigma, St Louis, MO): GAPDH: F: 5′-gCCTCCTgCACCACCAACTgC-3′ R: 5′-CCCTCCgACgCCTgCTTCAC-3′ TNF: F: 5′-TgCTTgTTCCTCAgCCTCTT-3′ R: 5′ATCCCAAAgTAgACCTgCCC-3′.
Chromatin Immunoprecipitation
Cells were crosslinked in 1% formaldehyde, 125 mM Glycine added, and lysed in lysis buffer (10 mM Tris, pH 7.5; 10 mM NaCl; 3 mM MgCl2; 2.5% NP40; 10 μM MG-132; PhosSTOP®, Complete®, Roche). Pelleted nuclei were resuspended in chromatin immunoprecipitation-buffer (10 mM Tris, pH 8.0; 1 mM EDTA pH 8.0; 0.5 mM EGTA pH 8.0; 10 μM MG-132; PhosSTOP® and Complete®, Roche), chromatin sheared (Covaris S2, Woburn, MA) at 20dc 8i 200 cps fs 600s, diluted in buffer (16.7 mM Tris, pH 8.0; 1.2 mM EDTA pH 8.0; 167 mM NaCl; 1.1% Triton X-100; 0.01% SDS; 10 μM MG-132; PhosSTOP®, Complete®, Roche), and precleared by adding Sepharose® CL-4B (Sigma). Complexes were precipitated with anti-p-p53 (#9284, Cell Signaling) or p65 (sc-372, Santa Cruz) antibody and Protein-A-Sepharose 4 Fast Flow (Thermo Scientific) overnight at 4 °C. Precipitates were washed in low-salt buffer (20 mM Tris, pH 8.1; 150 mM NaCl; 2 mM EDTA; 1% Triton X-100; 0.1% SDS), high-salt buffer (20 mM Tris, pH 8.1; 500 mM NaCl; 2 mM EDTA; 1% Triton X-100; 0.1% SDS), wash buffer (10 mM Tris, pH 8.0; 1 mM EDTA; 1% deoxycholate; 1% NP40; 0.25 M LiCl), and Tris-EDTA buffer (10 mM Tris, pH 8.0, 1 mM EDTA). Complexes were eluted (0.1 M NaHCO3; 1% SDS) and crosslinking reversed in 0.2 M NaCl overnight at 65 °C. After proteinase K treatment (55 °C, 3 hours) and phenol–chloroform extraction, DNA fragments were analyzed using the DyNAmo ColorFlash SYBR Green qPCR kit in a PikoReal24 cycler with PikoReal 2.2 software (Thermo Scientific): p65: F: 5′-CCACAgCAATgggTAggAgAATg-3′ R: 5′-TTCATgAAgCTCTCACTTCTCAg-3′ p53: F: 5′-CgggTCAgAATgAAAgAAgAAg-3′ R: 5′-gAgggAAAAgCTgTgTTgAg-3′.
Clonogenic assay
A total of 50,000 cells were seeded in a 10-cm dish, stimulated, and medium changed every other day. After 3 weeks, cells were stained with crystal violet and dissolved in KH2PO4–ethanol, and absorption measured at 595 nm.
Acknowledgments
The work was funded by the German Research Foundation (DFG, KU 1981/5-1).
Glossary
- CREB
cAMP response element–binding protein
- IκBα
inhibitor of κBα
- IκBα-SR
IκBα super-repressor
- PP2A
protein phosphatase 2A
- TNF
tumor necrosis factor
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Almeida LO, Abrahao AC, Rosselli-Murai LK, et al. NFkappaB mediates cisplatin resistance through histone modifications in head and neck squamous cell carcinoma (HNSCC) FEBS Open Bio. 2014;4:96–104. doi: 10.1016/j.fob.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisic S, Schmidt C, Walczak H, et al. Tyrosine phosphatase inhibition triggers sustained canonical serine-dependent NFkappaB activation via Src-dependent blockade of PP2A. Biochem Pharmacol. 2010;80:439–447. doi: 10.1016/j.bcp.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Barisic S, Strozyk E, Peters N, et al. Identification of PP2A as a crucial regulator of the NF-kappaB feedback loop: its inhibition by UVB turns NF-kappaB into a pro-apoptotic factor. Cell Death Differ. 2008;15:1681–1690. doi: 10.1038/cdd.2008.98. [DOI] [PubMed] [Google Scholar]
- Bohuslav J, Chen LF, Kwon H, et al. p53 induces NF-kappaB activation by an IkappaB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J Biol Chem. 2004;279:26115–26125. doi: 10.1074/jbc.M313509200. [DOI] [PubMed] [Google Scholar]
- Campbell KJ, Rocha S, Perkins ND. Active repression of antiapoptotic gene expression by RelA(p65) NF-kappa B. Mol Cell. 2004;13:853–865. doi: 10.1016/s1097-2765(04)00131-5. [DOI] [PubMed] [Google Scholar]
- Delhase M, Hayakawa M, Chen Y, et al. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- Deppert W. Mutant p53: from guardian to fallen angel. Oncogene. 2007;26:2142–2144. doi: 10.1038/sj.onc.1210276. [DOI] [PubMed] [Google Scholar]
- Feldmeyer L, Keller M, Niklaus G, et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- Friedrich MW, Aramuni G, Mank M, et al. Imaging CREB activation in living cells. J Biol Chem. 2010;285:23285–23295. doi: 10.1074/jbc.M110.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Son K, Onda S, et al. Desensitization of NFkappaB for overcoming chemoresistance of pancreatic cancer cells to TNF-alpha or paclitaxel. Anticancer Res. 2012;32:4813–4821. [PubMed] [Google Scholar]
- Fusenig NE, Boukamp P, Breitkreutz D, et al. In vitro transformation of human skin epithelial cells: role of RAS oncogene in malignant progression. Toxicol In Vitro. 1990;4:627–634. doi: 10.1016/0887-2333(90)90132-d. [DOI] [PubMed] [Google Scholar]
- Gervin CM, McCulla A, Williams M, et al. Dysfunction of p53 in photocarcinogenesis. Front Biosci. 2003;8:s715–s717. doi: 10.2741/1136. [DOI] [PubMed] [Google Scholar]
- Goehler T, Jaeger S, Warnecke G, et al. Mutant p53 proteins bind DNA in a DNA structure-selective mode. Nucleic Acids Res. 2005;33:1087–1100. doi: 10.1093/nar/gki252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B, Gibson SB. The two faces of NFkappaB in cell survival responses. Cell Cycle. 2005;4:1342–1345. doi: 10.4161/cc.4.10.2047. [DOI] [PubMed] [Google Scholar]
- Gurzov EN, Germano CM, Cunha DA, et al. p53 up-regulated modulator of apoptosis (PUMA) activation contributes to pancreatic beta-cell apoptosis induced by proinflammatory cytokines and endoplasmic reticulum stress. J Biol Chem. 2010;285:19910–19920. doi: 10.1074/jbc.M110.122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Levchenko A, Scott ML, et al. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- Huang WC, Ju TK, Hung MC, et al. Phosphorylation of CBP by IKKalpha promotes cell growth by switching the binding preference of CBP from p53 to NF-kappaB. Mol Cell. 2007;26:75–87. doi: 10.1016/j.molcel.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Sun X, Li Y, et al. p300/CBP-dependent and -independent transcriptional interference between NF-kappaB RelA and p53. Biochem Biophys Res Commun. 2000;272:375–379. doi: 10.1006/bbrc.2000.2786. [DOI] [PubMed] [Google Scholar]
- Kawauchi K, Araki K, Tobiume K, et al. Activated p53 induces NF-kappaB DNA binding but suppresses its transcriptional activation. Biochem Biophys Res Commun. 2008;372:137–141. doi: 10.1016/j.bbrc.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Kim E, Deppert W. Interactions of mutant p53 with DNA: guilt by association. Oncogene. 2007;26:2185–2190. doi: 10.1038/sj.onc.1210312. [DOI] [PubMed] [Google Scholar]
- Klein AM, Brash DE, Jones PH, et al. Stochastic fate of p53-mutant epidermal progenitor cells is tilted toward proliferation by UV B during preneoplasia. Proc Natl Acad Sci USA. 2010;107:270–275. doi: 10.1073/pnas.0909738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrath F, Witt J, Sauter T, et al. Identification of new IkappaBalpha complexes by an iterative experimental and mathematical modeling approach. PLoS Comput Biol. 2014;10:e1003528. doi: 10.1371/journal.pcbi.1003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulms D, Schwarz T. Molecular mechanisms of UV-induced apoptosis. Photodermatol Photoimmunol Photomed. 2000;16:195–201. doi: 10.1034/j.1600-0781.2000.160501.x. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Leiden JM. Transcriptional regulation of T lymphocyte development and function. Annu Rev Immunol. 1999;17:149–187. doi: 10.1146/annurev.immunol.17.1.149. [DOI] [PubMed] [Google Scholar]
- Lehman TA, Modali R, Boukamp P, et al. p53 mutations in human immortalized epithelial cell lines. Carcinogenesis. 1993;14:833–839. doi: 10.1093/carcin/14.5.833. [DOI] [PubMed] [Google Scholar]
- Lin A, Karin M. NF-kappaB in cancer: a marked target. Semin Cancer Biol. 2003;13:107–114. doi: 10.1016/s1044-579x(02)00128-1. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang D, Minemoto Y, et al. NF-kappaB is required for UV-induced JNK activation via induction of PKCdelta. Mol Cell. 2006;21:467–480. doi: 10.1016/j.molcel.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Ma S, Tang J, Feng J, et al. Induction of p21 by p65 in p53 null cells treated with Doxorubicin. Biochim Biophys Acta. 2008;1783:935–940. doi: 10.1016/j.bbamcr.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Mello SS, Attardi LD. Not all p53 gain-of-function mutants are created equal. Cell Death Differ. 2013;20:855–857. doi: 10.1038/cdd.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll UM, Wolff S, Speidel D, et al. Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol. 2005;17:631–636. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- Murphy G, Young AR, Wulf HC, et al. The molecular determinants of sunburn cell formation. Exp Dermatol. 2001;10:155–160. doi: 10.1034/j.1600-0625.2001.010003155.x. [DOI] [PubMed] [Google Scholar]
- Narayanan A, Amaya M, Voss K, et al. Reactive oxygen species activate NFkappaB (p65) and p53 and induce apoptosis in RVFV infected liver cells. Virology. 2014;449:270–286. doi: 10.1016/j.virol.2013.11.023. [DOI] [PubMed] [Google Scholar]
- O'Prey J, Crighton D, Martin AG, et al. p53-mediated induction of Noxa and p53AIP1 requires NFkappaB. Cell Cycle. 2010;9:947–952. doi: 10.4161/cc.9.5.10872. [DOI] [PubMed] [Google Scholar]
- Poppelmann B, Klimmek K, Strozyk E, et al. NFκB-dependent down-regulation of tumor necrosis factor receptor-associated proteins contributes to interleukin-1-mediated enhancement of ultraviolet B-induced apoptosis. J Biol Chem. 2005;280:15635–15643. doi: 10.1074/jbc.M413006200. [DOI] [PubMed] [Google Scholar]
- Ray D, Murphy KR, Gal S. The DNA binding and accumulation of p53 from breast cancer cell lines and the link with serine 15 phosphorylation. Cancer Biol Ther. 2012;13:848–857. doi: 10.4161/cbt.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KM, Ernst MK, Rice NR, et al. Role of NF-kappaB in p53-mediated programmed cell death. Nature. 2000;404:892–897. doi: 10.1038/35009130. [DOI] [PubMed] [Google Scholar]
- Schneider G, Henrich A, Greiner G, et al. Cross talk between stimulated NF-kappaB and the tumor suppressor p53. Oncogene. 2010;29:2795–2806. doi: 10.1038/onc.2010.46. [DOI] [PubMed] [Google Scholar]
- Seitz SJ, Schleithoff ES, Koch A, et al. Chemotherapy-induced apoptosis in hepatocellular carcinoma involves the p53 family and is mediated via the extrinsic and the intrinsic pathway. Int J Cancer. 2010;126:2049–2066. doi: 10.1002/ijc.24861. [DOI] [PubMed] [Google Scholar]
- Soussi T, Wiman KG. Shaping genetic alterations in human cancer: the p53 mutation paradigm. Cancer Cell. 2007;12:303–312. doi: 10.1016/j.ccr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Spooren A, Kooijman R, Lintermans B, et al. Cooperation of NFkappaB and CREB to induce synergistic IL-6 expression in astrocytes. Cell Signal. 2010;22:871–881. doi: 10.1016/j.cellsig.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Strozyk E, Poppelmann B, Schwarz T, et al. Differential effects of NF-kappaB on apoptosis induced by DNA-damaging agents: the type of DNA damage determines the final outcome. Oncogene. 2006;25:6239–6251. doi: 10.1038/sj.onc.1209655. [DOI] [PubMed] [Google Scholar]
- Szoltysek K, Pietranek K, Kalinowska-Herok M, et al. TNFalpha-induced activation of NFkappaB protects against UV-induced apoptosis specifically in p53-proficient cells. Acta Biochim Pol. 2008;55:741–748. [PubMed] [Google Scholar]
- Tergaonkar V. p53 and NFkappaB: fresh breath in the cross talk. Cell Res. 2009;19:1313–1315. doi: 10.1038/cr.2009.132. [DOI] [PubMed] [Google Scholar]
- Tergaonkar V, Pando M, Vafa O, et al. p53 stabilization is decreased upon NFkappaB activation: a role for NFkappaB in acquisition of resistance to chemotherapy. Cancer Cell. 2002;1:493–503. doi: 10.1016/s1535-6108(02)00068-5. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- Wang P, Qiu W, Dudgeon C, et al. PUMA is directly activated by NF-kappaB and contributes to TNF-alpha-induced apoptosis. Cell Death Differ. 2009;16:1192–1202. doi: 10.1038/cdd.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GA, Perkins ND. Transcriptional cross talk between NF-kappaB and p53. Mol Cell Biol. 1999;19:3485–3495. doi: 10.1128/mcb.19.5.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz L, Damalas A, Liontos M, et al. Mutant p53 enhances nuclear factor kappaB activation by tumor necrosis factor alpha in cancer cells. Cancer Res. 2007;67:2396–2401. doi: 10.1158/0008-5472.CAN-06-2425. [DOI] [PubMed] [Google Scholar]
- Witt J, Barisic S, Schumann E, et al. Mechanism of PP2A-mediated IKKbeta dephosphorylation: a systems biological approach. BMC Syst Biol. 2009;3:71. doi: 10.1186/1752-0509-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarosh DB, Kripke ML. DNA repair and cytokines in antimutagenesis and anticarcinogenesis. Mutat Res. 1996;350:255–260. doi: 10.1016/0027-5107(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Zalcenstein A, Stambolsky P, Weisz L, et al. Mutant p53 gain of function: repression of CD95(Fas/APO-1) gene expression by tumor-associated p53 mutants. Oncogene. 2003;22:5667–5676. doi: 10.1038/sj.onc.1206724. [DOI] [PubMed] [Google Scholar]
- Zhang XP, Liu F, Wang W. Two-phase dynamics of p53 in the DNA damage response. Proc Natl Acad Sci USA. 2011;108:8990–8995. doi: 10.1073/pnas.1100600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zschiedrich I, Hardeland U, Krones-Herzig A, et al. Coactivator function of RIP140 for NFkappaB/RelA-dependent cytokine gene expression. Blood. 2008;112:264–276. doi: 10.1182/blood-2007-11-121699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.