Abstract
Objective(s):
The purpose of the present study was to investigate the protective effect of oxytocin on cisplatin (CP)-induced renal damage in rats.
Materials and Methods:
Fourteen adult Sprague Dawley rats, weighing 200 to 210, were administered by cisplatin (CP, 2 mg/kg/day) twice a week for five weeks. Then, they were randomly divided into two groups and treated with either saline (1 ml/kg/day) or OT (200 µg/kg/day) for five weeks. Seven rats served as control group. At the end of the treatment period, animals were sacrificed and their kidneys were assessed histologically. In addition, C-reactive protein (CRP), TGF-β and Akt expression were evaluated immunohistochemically.
Results:
Both tubules and glomeruli were found to be severely damaged with marked medullary tubulo-interstitial inflammation due to chronic cisplatin exposure, particularly in the salinetreated group (Group 1) compared to control group. Oxytocin treatment spared renal-tissue significantly by suppressing CRP and TGF-β , and enhancing Akt expression. Conclusion:
Conclusion:
We conclude that renal damage due to cisplatin toxicity was prevented to a great extent by the anti-inflammatory effect of oxytocin.
Keywords: Akt, Cisplatin toxicity, CRP, Kidney, Oxytocin TGF-β
Introduction
Cisplatin (CP) is an alkylating agent widely used in cancer treatment. It inhibits transcription-replication by covalently binding to DNA, RNA and proteins in the cell. This agent is one the major chemotherapeutics, selectively preferred in treating testis, ovary, prostate, head-neck tumors and osteosarcomas (1, 2). Being also ototoxic and neurotoxic, cisplatin is known to be primarily nephrotoxic, limiting its high dose applications (3, 4). Its tissue destructive feature operates via apoptosis and necrosis following DNA and protein damage (5). Cisplatin induces additive oxidative stress mostly owing to over-expression of the reactive oxygen species (ROS), such as superoxide anion (6-8). It also disturbs and degrades renal capillaries leading to glomerular and tubular damage (9). This agent is the major cause of inflammation triggering nuclear factor-kappa B (NF-κB) activity in the kidney, thus leading to over-expression of tumor necrosis factor-alpha (TNF-α), transforming growth factor- beta (TGF-β), monocyte chemo-attractant protein-1 (MCP-1), intercellular adhesion molecule (ICAM), hemoxygenase-1, TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2) (10). Cisplatin causes lymphocyte, TGF-β and TNF-α releasing macrophage infiltration, and resultant fibrosis in the renal medullary peritubular interstitial zone. These cytokines participate in matrix formation from myofibroblastic cells (11-13).
Consisting of nine amino acids and secreted by the hypothalamic supraoptic and paraventricular nuclei, oxytocin (OT) is a peptide hormone exerting its physiological and biological actions via its G-protein coupled receptor. OT receptors are widespread in the nervous system, vascular smooth muscle, myocard, thymus, pancreas, and adipocytes (14-16). It has anti-oxidative, anti-inflammatory, and anti-apoptotic effects (17-20). Recently, we have shown its beneficial effects against rotenone- induced Parkinson s disease and sepsis-induced polyneuropathy in rats (19, 20).
Hoping to contribute to the attempts for ameliorating the highly adverse and dose- limiting features of the most widely prescribed chemotherapeutic, cisplatin and optimizing the relevant cancer treatment protocols, we hypothesized that OT maybe of benefit in protection of the rat renal tissue during chronic exposures to this agent. We designed this preliminary experimental study to "translate" its outcomes for clinical implications in the future.
Materials and Methods
Animals
Twenty-one adult Sprague Dawley rats, weighing 200-220 g were used in this study. Animals were housed in groups of four in cages and maintained under standard conditions with 12/12 hr light/dark cycle at room temperature (22 ± 2°C). They were fed by standard pellet diet and tap water ad libitum along the study. The protocol employed in this study was approved by the Institutional Animal Care and Ethical Committee of the University of Ege.
Experimental protocol
Seven rats were grouped as naive control (NC), and randomly chosen 14 rats were injected IP. by 2 mg/kg/day cisplatin (Sisplatin Flakon, Kocak Farma), twice a week for 5 weeks. Total cisplatin dose was 20 mg/kg/rat. Seven randomly chosen cisplatin (CP) animals were also injected IP every day by 200 µg/kg/day OT (oxytocin, Pituisan®, Ege Vet, Alfasan International B.V., Holland) for five weeks (CP+OT) while the other seven cisplatin (CP) animals received 1 ml/kg/day, IP saline (%0.9 NaCl) in the same regime (CP+S). During the treatment period, all animals monitored daily for health conditions. Then, they were sacrificed and their kidneys were resected for histological and immunohistochemical evaluations.
Histopathological examination of kidney
For histological and immunohistochemical studies, all animals were anesthetized by ketamin (40 mg/kg, Alfamine®, Alfasan International BV, Holland) and xylazine (4 mg/kg, Alfazyne®, Alfasan International B.V., Holland) IP and perfused with 200 ml of 4% formaldehyde in 0.1 M phosphate-buffered saline (PBS). Formalin-fixed kidney sections (4 µm) were stained with hematoxylene and eosine. All sections were photographed with Olympus C-5050 digital camera mounted on Olympus BX51 microscope.
Morphological evaluation was performed by computerized image analysis system (Image- Pro Express 1.4.5, Media Cybernetics, Inc. USA) on 10 microscopic fields per section examined at a magnification of ×20, by the observer blind to the study group.
Kidney sections from every rat in all groups were evaluated semi-quantitatively in terms of the extent of tubular necrosis/atrophy, glomerular damage, and interstitial inflammation by being rated as follows: 0-5% = score 0; 6-20% = score 1; 21-40% = score 2; 41-60% = score 3; 61-80% = score 4; and 81-100% = score 5 (21, 22).
Akt, CRP and TGF-β immunohistochemistry
For immunohistochemistry, sections were incubated with H2O2 (10%) for 30 min to eliminate endogenous peroxidase activity and blocked with 10% normal goat serum (Invitrogen) for 1 hr at room temperature. Subsequently, sections were incubated in primary antibodies against Akt, CRP and TGF-β (Bioss, Inc.; 1/100) for 24 hr at 4°C. Antibody detection was performed with the Histostain-Plus Bulk kit (Invitrogen, Inc.) against rabbit IgG, and 3,3' diaminobenzidine (DAB) was used to visualise the final product. All sections were washed in PBS and photographed with an Olympus C-5050 digital camera mounted on Olympus BX51 microscope. Brown cytoplasmic staining was scored positive for immune-expression. The number of immune-expression positive cells was assessed systematically, scoring at least 50 glomeruli and tubuler cells per field in 10 fields of tissue sections at a magnification of 100x.
Statistical analysis
All data were analyzed by non-parametric (Mann-Whitney U) test. Data are presented as mean values ± standard error of the mean (SEM). P-values of 0.05 or less were regarded as statistically significant.
Results
Histopathological evaluation
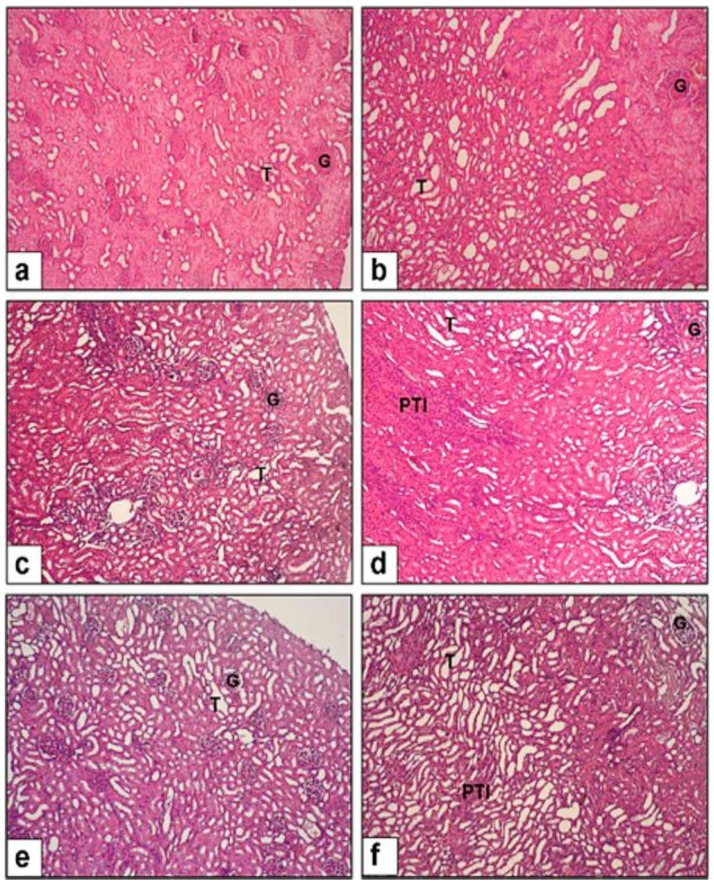
Figure 1 demonstrates the histopathological alterations in renal sections. Glomerular structure shrinkage was prominent in all CP rats, with the CP+S group showing the highest damage possibly owing to apoptosis and necrosis. Moreover, the CP+S group's slides displayed intensive and extensive tubular and peritubular mononuclear cell infiltration. Peritubular fibrotic areas and tubular atrophy could also be traced in the same animals (Figure 1). CP+OT group rats' sections displayed significantly much less glomerular and tubular damage compared to the saline group (P<0.005 and P<0.05, respectively; Table 1). The most striking difference was observed in the tubulo-interstitial areas that expressed significantly much less inflammation than those of the saline treated animals (P<0.005, Table 1).
Figure 1.
Evaluation of renal damage in cisplatin-induced toxicity. All images 10X magnification. (a-b) NC group displayed normal glomerular (G) and tubular (T) structure,(c-d) CP+S group developed severe glomerular (G) damage and peritubular infiltration (PTI), (e-f) CP+OT group displayed much less peritubular mononuclear cell infiltration with relatively spared tubular structure and much less apparent peritubular fibrosis
Table 1.
Comparison of the group average scores in tubular necrosis /atrophy, glomerular damage, and interstitial inflammation
| Tubular necrosis /atrophy | Glomerular damage | Interstitial inflammation | |
|---|---|---|---|
| NC | ND | ND | ND |
| CP+S | 4.16 ± 0.30 | 4.5 ± 0.22 | 4.33 ± 0.21 |
| CP+OT | 2.33 ± 0.21 ** | 3.16 ± 0.30 * | 1.66 ± 0.42 ** |
Data were expressed as mean±SEM. NC: naive control, ND: not detected, CP: Cisplatin, S: Saline, OT: Oxytocin
P<0.005, CP+OT vs. CP+S group
P<0.05, CP+OT vs. CP+S group
Immunoexpression of Akt, TGF- β and CRP
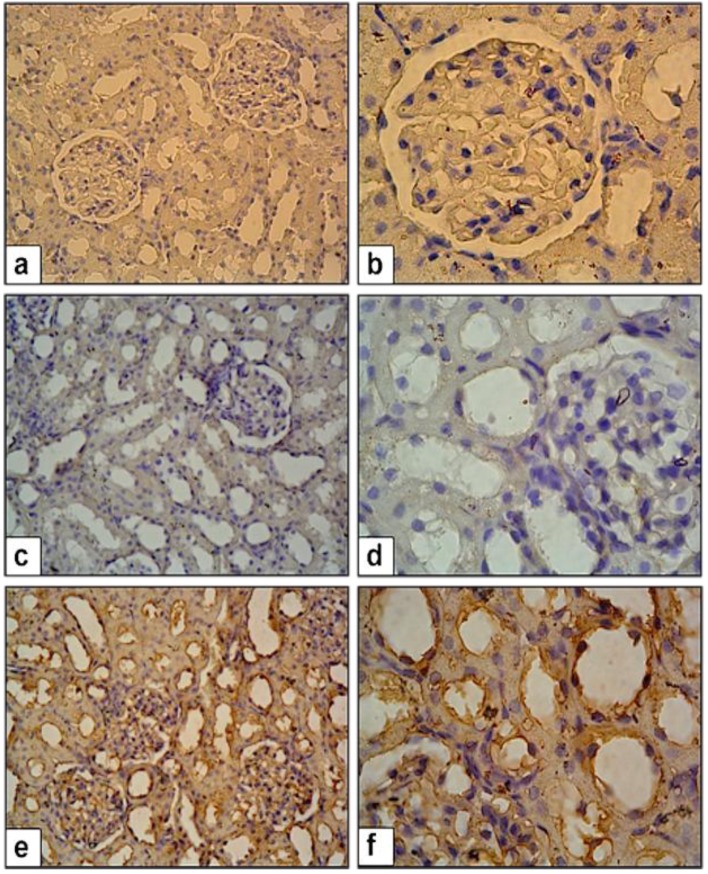
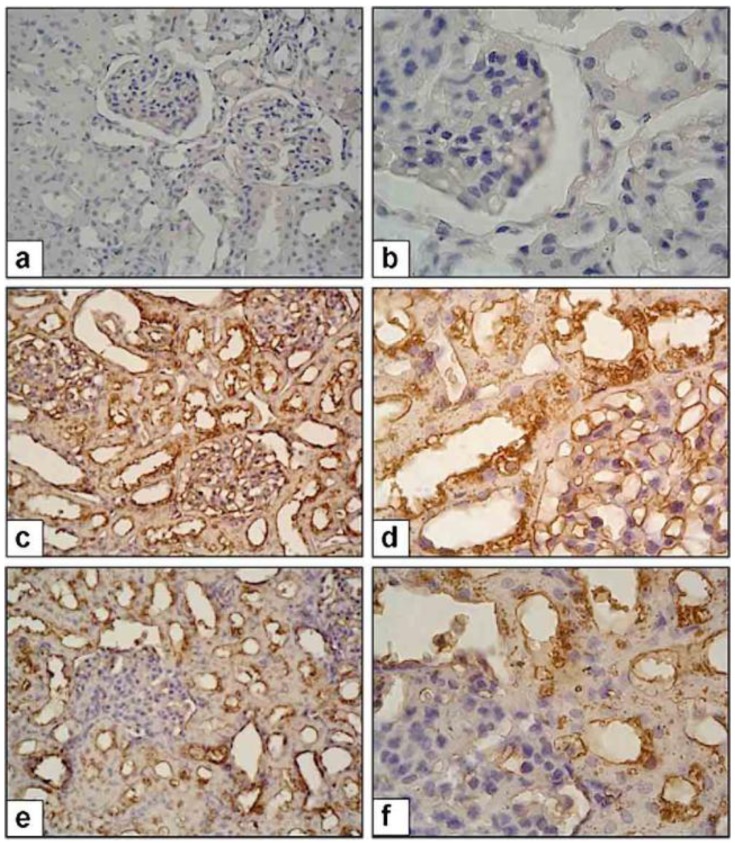
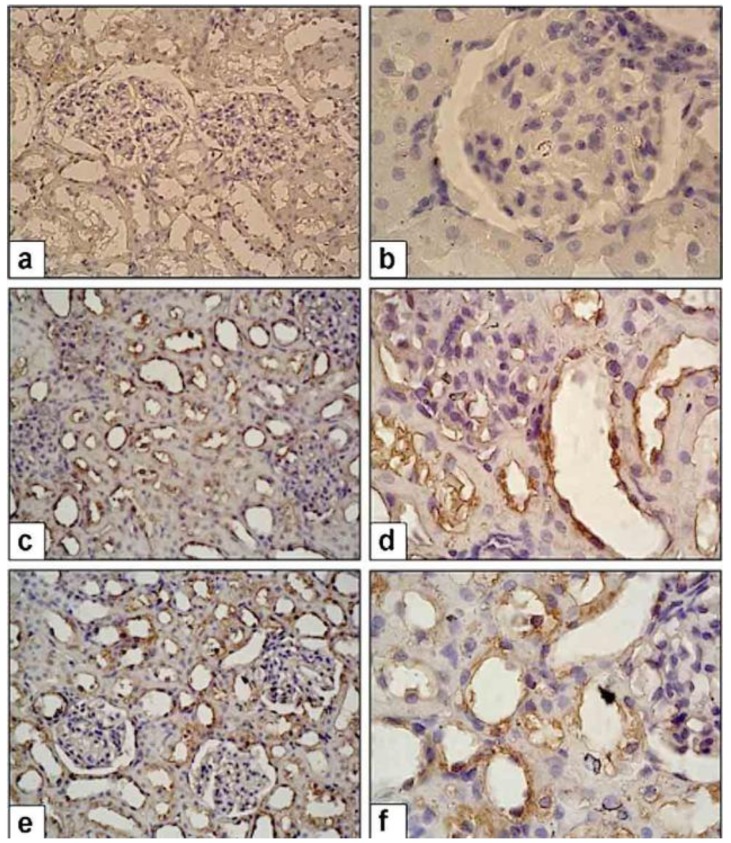
Figure 2 shows the Akt immunostaining in renal sections. Also, the ratios of Akt (+) cells in renal sections for all groups are given in Table 2. CP+OT rats had significantly higher Akt (+) cells than CP+S group (P<0.01, Table 2). Figure 3 and 4 illustrate the immunoexpression of CRP and TGF-β in renal sections of all groups. As summarized in Table 2, CP+S rats had significantly higher TGF-β (+) and CRP (+) cells than in the naïve control group (P<0.01, Table 2). Treatment of CP with OT (CP+OT group) significantly diminished TGF- β and CRP immunexpression when compared with saline treated (CP+S) group. (P<0.01 and P<0.05, repectively; Table 2).
Figure 2.
Akt expression in control and study groups. (a-b) NC group. (c-d) CP+S group, (e-f) CP+OT group enhanced Akt expression. 40X and 100X magnification
Table 2.
Quantitative comparison of Akt, C-reactive protein expression and Transforming growth factor-beta immunoexspressions
| Akt expression (%) | CRP expression (%) | TGF-β expression (%) | |
|---|---|---|---|
| NC | 10.4 ± 2.3 | 6.2 ± 3.5 | 5.8 ± 3.2 |
| CP+S | 12.6 ± 3.4 | 38.6 ± 5.6 * | 43.2 ± 5.4 * |
| CP+OT | 56.8 ± 7.2 †† | 20.8 ± 4.8 † | 22.6 ± 2.1 †† |
Data were expressed as mean ± SEM. NC: naive control, ND: not detected, CP: Cisplatin, S: Saline, OT: Oxytocin, TGF: Transforming growth factor, CRP: C-reactive protein
P<0.001, CP+S vs. NC group
P<0.05, CP+OT vs. CP+S group
P<0.01, CP+OT vs. CP+S group
Figure 3.
CRP expression in control and study groups. (a-b) NC Group, (c-d) CP+S group, CRP expression is significantly higher than NC group, (e-f) CP+OT group expression is significantly lower than CP+S group. 40X and 100X magnification
Figure 4.
TGF-β expression in control and study groups. (a-b) NC group, (c-d) CP+S group, TGF-β expression is higher than NC group, (e-f) P+OT group, TGF-β expression is lower than CP+S Group. 40X and 100X magnification
Discussion
The present study demonstrated that OT attenuated the renal tubulo-interstitial fibrosis-induced by chronic cisplatin treatment in the rat. Additionally, OT also ameliorated tubular injury by decreasing the cisplatin associated renal over-expression of TGF-β1 and CRP, meantime, activating Akt signaling. OT treatment significantly reduced interstitial macrophage infiltration thus leading to suppression of inflammation and cytotoxicity induced by cisplatin. These findings suggest that the nephro-protective effects of OT are likely to be achieved, at least partly, via suppression of TGF-β1/Smad2 signaling pathways and the resultant relative anti-apoptotic activity in these rats.
Cisplatin, a major anti-neoplastic drug of solid tumors, has multiple intracellular effects, such as direct toxicity with reactive oxygen species, enhancement of mitogen activated protein kinases (MAPK), triggering apoptosis, and stimulating inflammation and fibrogenesis (23, 18). Cisplatin- induced renal injury is characterized by a series of ultra-structural changes, including tubular necrosis, loss of microvilli, alterations in number and size of lysosomes, and mitochondrial vacuolization (23).
Many potential therapeutic approaches targeting these steps for the prevention of cisplatin-induced renal injury has been extensively studied (23, 18). In particular, recent evidence indicates that inflammation has an important role in the pathogenesis of cisplatin induced renal injury. In the present study, we observed that oxytocin significantly decreased intra-renal CRP and TGF-β1 expression in accordance with the dosage administered, which was paralleled by a significant attenuation of tubulo-interstitial fibrosis at 4 wk. Our results suggest that oxytocin suppresses the progression of tubulo-interstitial inflammation and tubulo-interstitial fibrosis through a mechanism involving decreased CRP and TGF-β1 expression in cisplatin-induced nephropathy.
Excessive deposition of extracellular matrix (ECM) in the tubulo-interstitium causes decline in renal function in progressive renal disease (24). Epithelial-mesenchymal transition (EMT) of tubular epithelial cells, characterized by loss of epithelial cell characteristics and gain of ECM-producing myofibroblast characteristics, is associated with tubulointerstitial fibrosis. Tubular EMT can be stimulated by TGF-β1 (25-27) advanced glycation end products (28, 29), and angiotensin II (30). However, TGF-β1 is probably the key inducer of EMT because TGF-β1 signaling is sufficient to induce EMT in cultured epithelial cells (26, 27). In a normal rat tubular epithelial cell line, NRK-52E; Li et al (31) found that TGF-β1-induced Smad 2 phosphorylation resulted in stimulation of EMT with the loss of E-cadherin, and de novo expression of α-SMA and collagens I, III, and IV. Moreover, they observed that over-expression of Smad 7 resulted in inhibi-tion of TGF-β1-induced Smad 2 activation with the prevention of EMT and collagen synthesis. TGF-β1 induced EMT in renal tubular epithelial cells, directly through activation of MAPK and indirectly through extra-cellular signal regulated kinases (ERKs), directed Smad 2 phosphorylation (31, 32).
Stuveling et al demonstrated that CRP is an inflammatory biomarker and a strong predictor of renal function abnormalities in humans (33). The production of CRP is presumably restricted to the liver, but a recent study has suggested that the kidney may be a second site of CRP formation (34).
Padilla et al showed that rat CRP, similarly to human CRP, can activate autologous complement (35). This finding suggests that biological functions of CRP are conserved among species. In this study, we evaluated intrarenal CRP expression to test anti-inflammatory effects of OT on cisplatin-induced renal injury. The results of this study revealed that OT treatment decreased cisplatin-induced over-expression of CRP expression. On the basis of present and previous studies, we propose that the attenuation of tubulo-interstitial inflammation by OT treatment in cisplatin treated rat kidneys may be related, in part, to the decrease in intrarenal CRP expression.
In this study, we showed that OT has cytoprotective effects against cisplatin-induced tubular toxicity and these effects are mediated by the anti-apoptotic effects of OT and involve Akt activation. Cell fate also depends on the activation of anti-apoptotic pathways these results from loss of equilibrium between apoptotic and anti-apoptotic mediators. Of note, among the signaling pathways that participate in the inhibition of apoptosis, the phosphatidylinositol 3 phosphate kinase (PI3K)/Akt axis is considered a master regulator of cell survival and activating mutations of this pathway are widely implicated in numerous cancer types (36). PI3K activation causes Akt phosphorylation, and then anti-apoptotic mediators such as Bcl-xl and X-linked inhibitor of apoptosis protein (XIAP) are activated (37, 38).
Our study neatly showed that OT has been protective during chronic cisplatin-induced nephro-toxicity and interstitial fibrosis. For the first time in literature, we thereby identified this peptide's anti-apoptotic activity specifically attributing its operating mechanism via Akt signaling. We believe that this is of importance because of being strongly suggestive that OT could directly activate anti-apoptotic signaling at the cellular level. We reckon that these present findings referring to a receptor mediated activity could be an important additive actor involved in the other previously proposed/ defined potent tissue protection mechanisms of OT.
Conclusion
In summary, OT treatment decreased renal TGF-β1 expression and reduced tubulo-interstitial fibrosis in the rats. CRP and macrophage infiltration was strongly attenuated by OT in this animal model of cisplatin induced renal damage. Moreover, the anti-inflammatory and anti-fibrotic effects of OT were accompanied by suppression of TGF-β1/Smad2 in the cisplatin-injured kidneys. Our data suggest that OT protects rat kidneys against cisplatin nephro-toxicity through anti-apoptotic mechanisms. Therefore, we conclude that our results further imply that administration of OT may slow the progression of tubulo-interstitial nephritis by inhibiting the inflammatory and fibrotic processes.
Declaration of interest
The authors report no declaration of interest.
References
- 1.Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986; 8:368–379. doi: 10.1016/s0272-6386(86)80112-3. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005; 4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 3.Atessahin A, Yilmaz S, Karahan I, Ceribasi AO, Karaoglu A. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology . 2005; 212:116–123. doi: 10.1016/j.tox.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Tikoo K, Bhatt DK, Gaikwad AB, Sharma V, Kabra DG. Differential effects of tannic acid on cisplatin induced nephrotoxicity in rats. FEBS Lett . 2007;581:2027–2035. doi: 10.1016/j.febslet.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 5.Jordan P, Carma-Fonseca M. Molecular mechanism involved in cisplatin cytotoxicity . Cell Mol Life Sci . 2000; 57:1229–1235. doi: 10.1007/PL00000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadzaku Y, Shoji T, Takino Y. Mechanism of the increase in lipid peroxide induced by cisplatin in the kidney rats. Toxicol Lett. 1992; 62:293–300. doi: 10.1016/0378-4274(92)90033-g. [DOI] [PubMed] [Google Scholar]
- 7.Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol . 2009; 61:223–242. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates Cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol. 2001; 12:2683–2690. doi: 10.1681/ASN.V12122683. [DOI] [PubMed] [Google Scholar]
- 9.Pabla N, Dong Z. Cispaltin nephrotoxicity: mechanism and renoprotective strategies . Kidney Int . 2008; 73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 10.Ramesh G, Reeves WB. Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney Int . 2004; 65:490–499. doi: 10.1111/j.1523-1755.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamate J, Sato K, Ide M, Nakanishi M, Kuwamura M, Sakuma S, et al. Participation of different macrophage populations and myofibroblastic cells in chronically developed renal interstitial fibrosis after cisplatin-induced renal injury in rats. Vet Pathol . 2002; 39:322–333. doi: 10.1354/vp.39-3-322. [DOI] [PubMed] [Google Scholar]
- 12.Yamate J, Machida Y, Ide M, Kuwamura M, Sawamoto O, La Marre J. Effects of lipopolysaccharide on the appearance of macrophage populations and fibrogenesis in cisplatin-induced rat renal injury. Exp Toxicol Pathol. 2004; 56:13–24. doi: 10.1016/j.etp.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Olson BR, Hoffman GE, Sved AF, Stricker EM, Verbalis JG. Cholecystokinin induces c-fos expression in hypothalamic oxytocinergic neurons projecting to the dorsal vagal complex. Brain Res . 1992; 569:238–248. doi: 10.1016/0006-8993(92)90635-m. [DOI] [PubMed] [Google Scholar]
- 14.Yazawa H, Hirasawa A, Horie K, Saita Y, Iida E, Honda K, et al. Oxytocin receptors expressed and coupled to Ca2+ signalling in a human vascular smooth muscle cell line. Br J Pharmacol. 1996; 117:799–804. doi: 10.1111/j.1476-5381.1996.tb15263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jankowski M, Wang D, Hajjar F, Mukaddam-Daher S, McCann SM, Gutkowska J. Oxytocin and its receptors are synthesized in the rat vasculature. Proc Natl Acad Sci U S A. 2000; 97:6207–6211. doi: 10.1073/pnas.110137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutkowska J, Jankowski M. Oxytocin: old hormone, new drug. Pharmaceuticals. 2009; 2:168–183. doi: 10.3390/ph2030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegen BC. Oxytocin protects 376 against sepsis-induced multiple organ damage: role of neutrophils. J Surg Res . 2005; 126:73–81. doi: 10.1016/j.jss.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Rashed LA, Hashem RM, Soliman HM. Oxytocin inhibits NADPH oxidase and P38 MAPK in cisplatin-induced nephrotoxicity. Biomed Pharmacother . 2011; 65:474–480. doi: 10.1016/j.biopha.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Erbas O, Ergenoglu AM, Akdemir A, Yeniel AO, Taskiran D. Comparison of melatonin and oxytocin in the prevention of critical illness polyneuropathy in rats with experimentally induced sepsis. J Surg Res. 2012; 11:043. doi: 10.1016/j.jss.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Erbas O, Oltulu F, Taskiran D. Amelioration of rotenone-induced dopaminergic cell death in the striatum by oxytocin treatment. Peptides . 2012; 38: 312–317. doi: 10.1016/j.peptides.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Erdogan H, Fadillioğlu E, Kotuk M, Iraz M, Tasdemir S, Oztas Y, et al. Effects of Ginkgo biloba on plasma oxidant injury induced by bleomycin in rats. Toxicol Ind Health . 2006; 22:47–52. doi: 10.1191/0748233706th245oa. [DOI] [PubMed] [Google Scholar]
- 22.Ashrafi F, Nematbakhsh M, Safari T, Talebi A, Nasri H, Khazaei M, et al. A combination of vitamin C and losartan for cisplatin-induced nephrotoxicity in rats. Iran J Kidney Dis . 2012; 6:361–365. [PubMed] [Google Scholar]
- 23.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci . 2007; 334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 24.Lan HY. Tubular epithelial-myofibroblast transdifferentiation mechanism in proximal tubule cell. Curr Opin Nephrol Hypertens . 2003; 12:25–29. doi: 10.1097/00041552-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol . 2001; 159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pick E, Moustakas A, Kurisaki A, Heldin CH, ten Dijke P. TGF-alpha 1 receptor/ALK-5 and Smad proteins mediate epithelial to mesenchymal transdifferentiation in Nmu MG breast epithelial cells. J Cell Sci. 1999; 112:4557–4568. doi: 10.1242/jcs.112.24.4557. [DOI] [PubMed] [Google Scholar]
- 27.Bottinger EP, Bitzer M. article-titleTGF- alpha signaling in renal disease. J Am Soc Nephrol. 2002; 13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 28.Oldfield MD, Bach LS, Forbes JM, Nikolic-Pateerson D, McRobert A, Thallas V, et al. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin Invest . 2001; 108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li JH, Wang W, Huang XR, Oldfield M, Schmidt AM, Cooper ME, et al. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol . 2004; 164:1389–1397. doi: 10.1016/S0002-9440(10)63225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang XR, Li JH, Chen YX, Johnson RJ, Lan HY. SMAD signaling, a novel pathway of angiotensin II-induced renal fibrosis. J Am Soc Nephrol . 2003; 14:1535–1548. doi: 10.1097/01.asn.0000067632.04658.b8. [DOI] [PubMed] [Google Scholar]
- 31.Li JH, Zhu HJ, Huang XR. Smad7 inhibits fibrotic effect of TGF-_ on renal tubular epithelial cells by blocking Smad2 activation. J Am Soc Nephrol. 2002; 13:1464–1472. doi: 10.1097/01.asn.0000014252.37680.e4. [DOI] [PubMed] [Google Scholar]
- 32.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin -1 signaling is necessary for transforming growth factor-_ activation of p38 MAPK and epithelial plasticity. J Biol Chem . 2001; 276: 46707–46713. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- 33. Stuveling EM, Hillege HL, Bakker SJ, Gans RO, De Jong PE, De Zeeuw D. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int . 2003; 63:654–661. doi: 10.1046/j.1523-1755.2003.00762.x. [DOI] [PubMed] [Google Scholar]
- 34. Jabs WJ, Logering BA, Gerke P, Kreft B, Wolber EM, Klinger MH, et al. The kidney as a second site of human C-reactive protein formation in vivo. Eur J Immunol . 2003; 33:152–161. doi: 10.1002/immu.200390018. [DOI] [PubMed] [Google Scholar]
- 35. Padilla ND, Bleeker WK, Lubbers Y, Rigter GM, Van Mierlo GJ, Daha MR, et al. Rat C-reactive protein activates the autologous complement system. Immunology . 2003; 109:564–571. doi: 10.1046/j.1365-2567.2003.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol . 2001; 2:760–768. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- 37.Sanz AB, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. Mechanisms of renal apoptosis in health and disease. J Am Soc Nephrol . 2008; 19:1634–1642. doi: 10.1681/ASN.2007121336. [DOI] [PubMed] [Google Scholar]
- 38.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol . 2008; 9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]