Abstract
Objective(s):
Neuropathic pain is caused by lesions or diseases affecting the somatosensory system and often responds poorly to typical medications. In this study, we evaluated anti-nociceptive effects of morphine, gabapentin and their combination on heat hyperalgesia, cold and mechanical allodynia in chronic constriction injury (CCI) model of neuropathic pain in rats.
Materials and Methods:
Morphine (2, 4 and 8 mg/kg) and gabapentin (5, 10 and 20 mg/kg) were administered either alone or in combination (morphine 2 mg/kg and gabapentin 5 mg/kg).
Results:
Our results showed that morphine and gabapentin alone produce anti-nociceptive effects at higher doses (morphine 4 and 8 mg/kg and gabapentin 10 and 20 mg/kg) whereas their combination resulted in better analgesia at lower doses as compared to other treatment groups (morphine 2 mg/kg or gabapentin 5 mg/kg).
Conclusion:
These findings suggest that gabapentin potentiates the analgesic effects of morphine in the chronic constriction injury (CCI) model of neuropathic pain and combination of these drugs may be considered as a beneficial treatment for neuropathic pain.
Keywords: Gabapentin, Hyperalgesia, Neuropathic pain, Morphine
Introduction
Neuropathic pain may occur following peripheral nerve injury or as a consequence of lesion in central nervous system (1). In this condition, excitability of neurons, often responds poorly to traditional analgesics (2). The etiology and underlying mechanisms of such pains are poorly understood and the existing treatments including anti-convulsalant agents, local anesthetics and opioids are often ineffective. Hence, it is important to increase our understanding of the mechanisms involved in the occurrence of neuropathic pain in order to develop more effective therapies (3). It has been shown that enhancement of GABA neurotransmission may diminish the level of nociception. Gabapentin is an anti-convulsant drug which acts on GABAergic system. Gabapentin has shown strong anti-nociceptive effects in some animal models of acute and chronic pain (4). But it has been reported that gabapentin induces moderate attenuation of hyperalgesia in patient with neuropathic pain (5). Over the last decades, morphine has been the most widely used opioid for treatment of acute pain (6). But there are still controversies concerning its effectiveness in the treatment of neuropathic pain (7). In clinical settings, conventional treatments are often insufficient for management of patient with neuropathic pain. Recent clinical trials support the administration of combination of drugs with synergistic effects and lower adverse effects for relief from neuropathic pain (8).
In clinical studies, co-administration of gabapentin and morphine enhances the analgesic effect of morphine and reduces its dose. In patient with diabetic neuropathy, combination of these drugs produced greater analgesic effect as compared to morphine monotherapy (9, 10). In a study, it has been shown that co-administration of gabapentin and morphine produced anti-allodynic effect in an animal model of neuropathic pain (11). However, anti-hyperalgesic effects of different combinations of gabapentin and morphine in neuropathic pain treatment have not been evaluated yet. Thus, in this study we evaluated the anti-nociceptive effect of gabapentin, morphine and their combination on heat, cold and mechanical hyperalgesia in an animal model of neuropathic pain induced by chronic constriction injury.
Materials and Methods
Animals and housing conditionsl
The experiments were performed using male Sprague-Dawley rats (200-250 g) purchased from Razi Institute (Karaj, Iran). In each cage, four rats were housed. Cages were kept in a room under controlled temperature (23±2°C), humidity (50%) and light (12/12 hr light/dark cycle), with food and water available ad libitum. All experiments were approved by the ethics committee of Kashan University of Medical sciences, Kashan, Iran and done in accordance with the European Commission Directive (86/609/EEC) for animal experiments (12).
Neuropathic pain model
Rats were anesthetized with sodium pentobarbital (65 mg/kg, intraperitoneally (IP)).The common sciatic nerve was exposed and dissected from surrounding connective tissue near the trocanter, just distal to the branching point of the posterior biceps semitendinosus nerve. Four ligatures (4.0 chromic gut) were tied loosely around the nerve with a 1-1.5 mm interval between ligatures so that the circulation through the superficial epineuria vasculature was not totally blocked (13, 14). Sham-operated rats underwent the same surgery, the left sciatic nerve was exposed but no ligation was made. Animals were housed individually in cages after the surgery. All experiments followed the guidelines of ethical standard for investigation of experimental pain in animals, Canadian Council on Animal Care guidelines and were also approved by the Research and Ethics Committee of Kashan University of Medical Sciences, Kashan, Iran.
Behavioral tests of neuropathic pain
Hyperalgesia to noxious thermal stimulus and allodynia to cold and mechanical stimuli were determined as behavioral score of neuropathic pain by using the radiant heat plantar, acetone and von Frey test, respectively (15, 16). These tests were performed during the day part of the circadian cycle (09:00-16:00 hr). When cage exploration and major grooming activities were ceased, the behavioral tests were started. The behavioral scores of neuropathic pain were determined 1 day before the surgery as the baseline value and also 30 min after the injections on days 3, 7, 14 and 21 post-surgery.
Thermal hyperalgesia (plantar test)
Thermal hyperalgesia was assessed as previously reported (39). Paw withdrawal latency in response to radiant heat was measured by using plantar test apparatus (Ugo Basile, Varese, Italy). Rats were placed within a Plexiglass enclosure (but not restrained) on a transparent glass floor. A source of infrared beam (as the heat source) was placed beneath the mid-plantar surface of the hind paw. Thermal withdrawal latency was defined as the time period (seconds) between the onset of exposure to heat stimulus and paw withdrawal using a feedback-controlled shut-down unit. A cut-off time of 22 sec was used to avoid tissue damage. Each paw was tested three times alternatively at minimum intervals of 5 min between each exposure to avoid sensitization of the hind paw (17, 18). Mean latency of the withdrawal responses for ipsilateral (operated) and contralateral (non-operated) paws were calculated separately.
Cold allodynia (acetone test)
Cold allodynia was measured using the acetone spray test (evaporation-evoked cooling) as described previously (19). Rats were placed on a wire mesh floor. Acetone bubbles which were produced at the end of a tube connected to a syringe were applied 5 times (at 5 min intervals) to the plantar surface of the hind paw. The frequency of paw withdrawal was expressed as percentage (the number of paw withdrawals/number of trials×100).
Mechanical allodynia (von Frey filament stimulation)
Mechanical allodynia was quantified by measuring the hind paw withdrawal response to von Frey filament. We studied the effect of von Frey filament stimulation (with bending forces ranging from 2 to 60 g, Stolting Inc., Wood Dale, IL). Rats were placed on a mesh (0.8×0.8 cm cell) floor, covered by an inverted transparent plastic box (18×18×25 cm) and allowed to adapt for approximately 15 min, or until exploratory behavior ceased. A series of von Frey filament stimuli were delivered in an ascending order of forces to the central region of the plantar surface of the hind paw. The stimulation was applied three times consecutively by pushing down on the hind paw until the rat withdrew its paw or the fiber bowed. Lifting the paw due to normal locomotor behavior was ignored. The smallest filament size which evoked at least two withdrawal responses during three consecutive applications was considered as withdrawal threshold (20). Each filament was applied for approximately 1 sec and the inter-stimulus intervals were about 5 sec.
Treatment
Gabapentin (GP) (5, 10 and 20 mg/kg), morphine (Mor) (2, 4 and 8 mg/kg) and their combination (Mor 2 mg/kg and GP 5 mg/kg) were administered IP on days 3,7,14 and 21 post-surgery and behavioral tests were done 30 min after the treatment.
Statistical analysis
All data are presented as Mean±SEM and differences were considered significant if the P-value was less than 0.05. Values for behavioral response were analyzed using analysis of variance (ANOVA) with repeated measures followed by Tukey's honest squares difference (HSD) test. Drug treatment was considered as the between-subjects and day as within-subjects.
Results
Behavioral tests of neuropathic pain
The majority of the animals appeared healthy and well-groomed. Rats did not show any sign of autotomy after the sciatic nerve ligation. Paw gesture of the ipsilateral paw was slightly altered; but did not interfere with the normal activity of the rats. There was no evidence of the occurrence of contralateral hyperalgesia and allodynia in none of the test groups. Morphine and gabapentin had no effect on behavioral responses in sham-operated animals.
Cold allodynia
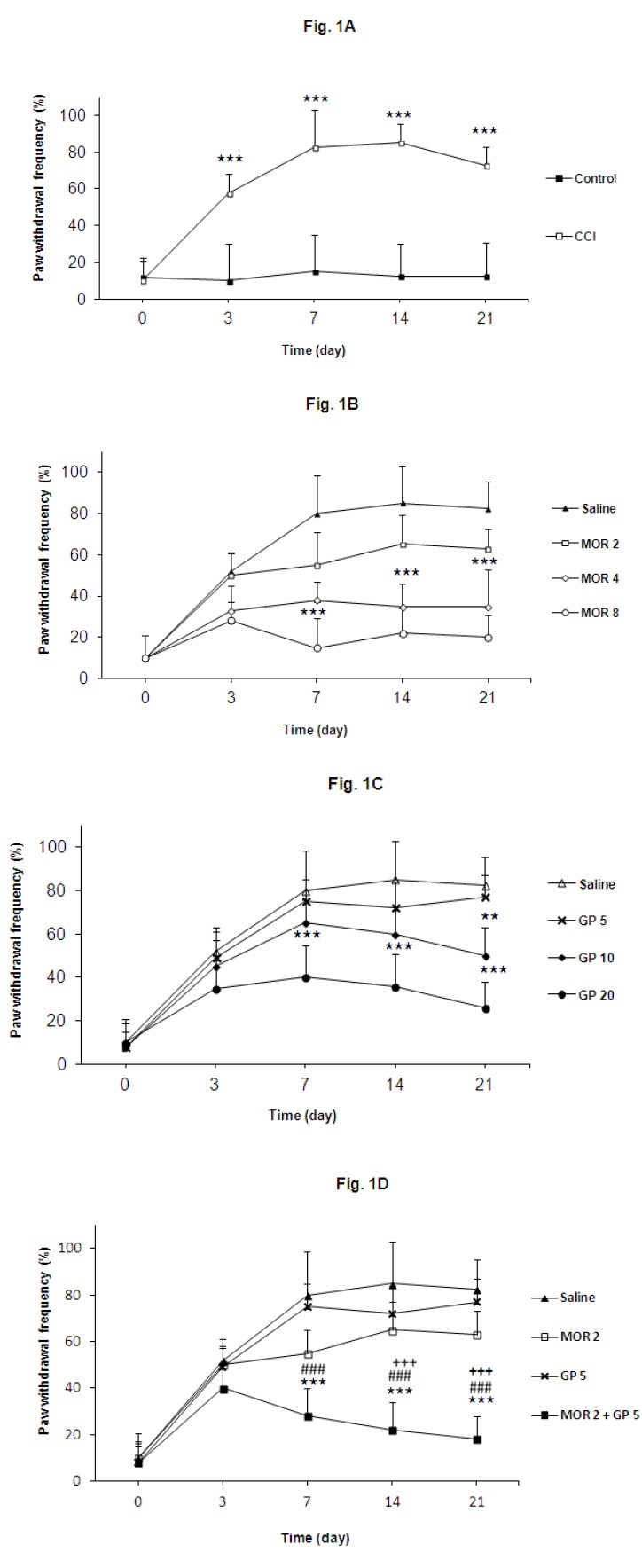
The results of the behavioral tests for cold allodynia are shown in Figure 1A. The ipsilateral paw of nerve ligated animals became much more sensitive to acetone application (P<0.001). Sham operation did not produce any modification of the nociceptive response. Morphine (4 and 8 mg/kg), gabapentin (10 and 20 mg/kg) and their combination (Mor 2 mg/kg and GP 5 mg/kg) significantly reduced the withdrawal frequency in comparison with control group (P<0.001). Co-administration of both drugs (Mor 2 mg/kg and GP 5 mg/kg) seems to be more effective than gabapentin or morphine alone on cold allodynia score in acetone test (Figures 1B, 1C and 1D).
Figure 1.
(A) Effects of chronic constriction injury (CCI) of sciatic nerve on cold allodynia. (B) Effect of morphine (2, 4 and 8 mg/kg) on cold allodynia. (C)Effect of gabapentin (5, 10 and 20 mg/kg) on cold allodynia. (D) Effect of morphine and gabapentin combination (Mor 2 mg/kg and GP 5 mg/kg), morphine (2 mg/kg) and gabapentin (5 mg/kg) on cold allodynia. The behavioral responses were determined prior to surgery (day 0) and 3, 7, 14 and 21 days post-surgery. Results are expressed as Mean±SEM, n=8 in all groups. ***P<0.001 vs control group, ###P<0.001 vs morphine (2 mg/kg) treatment group, +++P<0.001 vs gabapentin (5 mg/kg) treatment group
Thermal hyperalgesia
Partial sciatic nerve ligation decreased paw withdrawal latency to the thermal stimulus in ipsilateral (P<0.001), but sham operation did not produce any significant change in withdrawal latency (Figure 2A).
Figure 2.
(A) Effects of chronic constriction injury (CCI) of sciatic nerve on heat hyperalgesia. (B) Effect of morphine (2, 4 and 8 mg/kg) on heat hyperalgesia. (C) Effect of gabapentin (5, 10 and 20 mg/kg) on heat hyperalgesia. (D) Effect of morphine and gabapentin combination (Mor 2 mg/kg and GP 5 mg/kg), morphine (2 mg/kg) and gabapentin (5 mg/kg) on heat hyperalgesia. The behavioral responses were determined prior to surgery (day 0) and 3, 7, 14 and 21 days post-surgery. The results are expressed as Mean ± S.E.M., n=8 in all groups. ***P<0.001 vs control group, ###P<0.001 vs morphine (2 mg/kg) treatment group, +++P<0.001 vs gabapentin (5 mg/kg) treatment group
Morphine (4 and 8 mg/kg) and gabapentin (10 and 20 mg/kg) blocked thermal hyperalgesia in ipsilateral paw when they were administered alone (Figures 2B, 2C) whereas their combination (Mor 2 mg/kg and Gbp 5 mg/kg) produced significant anti-hyperalgesia as compared to sham group or either separate groups of morphine (2 mg/kg) and gabapentin (5 mg/kg) (Figure 2D).
Mechanical allodynia
Partial sciatic nerve ligation led to a significant decrease of withdrawal threshold of ipsilateral paw in comparison with sham operated group (P<0.001) (Figure 3A). Morphine (4 and 8 mg/kg), gabapentin (10 and 20 mg/kg) and the combination of morphine and gabapentin (Mor 2 mg/kg and Gbp 5 mg/kg), significantly increased withdrawal threshold of ipsilateral paw (P<0.001) (Figures 3B and 3C). As shown in Figure 3D, statistically significant differences were observed between the animals treated with combination of morphine and gabapentin (Mor 2 mg/kg and Gbp 5 mg/kg) and the animals treated with morphine or gabapentin.
Figure 3.
A) Effects of chronic constriction injury (CCI) of sciatic nerve on mechanical allodynia. (B) Effect of morphine (2, 4 and 8 mg/kg) on mechanical allodynia. (C) Effect of gabapentin (5, 10 and 20 mg/kg) on mechanical allodynia. (D) Effect of morphine and gabapentin combination (Mor 2 mg/kg and GP 5 mg/kg), morphine (2 mg/kg) and gabapentin (5 mg/kg) on mechanical allodynia. The behavioral responses were determined prior to surgery (day 0) and 3, 7, 14 and 21 days post-surgery. The results are expressed as Mean ± S.E.M., n=8 in all groups. ***P<0.001 vs control group, ###P<0.001 vs morphine (2 mg/kg) treatment group, +++P<0.001 vs gabapentin (5 mg/kg) treatment group
Discussion
Our results demonstrate that treatment of neuropathic pain with gabapentin and morphine results in anti-allodynic and anti-hyperalgesic effects and their combination possesses synergistic anti-nociceptive properties. In this study, 3 days after chronic constriction injury of the sciatic nerve, as a model of neuropathic pain, rats showed a relatively high degree of hyperalgesia against thermal and mechanical stimulus. This model, as one of the most frequently used models of the study of neuropathic pain and its treatment, is based on a unilateral loose ligation of the sciatic nerve (21). This model also shows many pathophysiological properties of chronic neuropathic pain in human subjects, such as allodynia and hyperalgesia (22). Morphine (2, 4 and 8 mg/kg),gabapentin (5, 10 and 20 mg/kg) or their combination (Mor 2 mg/kg and GP 5 mg/kg) were administered on days 3,7,14 and 21 after surgery. Based on our results, administration of gabapentin (10 and 20 mg/kg), morphine (4 and 8 mg/kg) and their combination (Mor 2 mg/kg and GP 5 mg/kg) produce significant anti-antinociceptive effects and the analgesic effects of co-administration of the drugs seemed to be more effective than gabapentin or morphine alone in CCI model of neuropathic pain. This is in accordance with another report in which it was demonstrated that acute treatment of rats with gabapentin and morphine alone or in combination produced anti-allodynic effect in an animal model of neuropathic pain (11). In this study, our results showed that these drugs and their combination not only reduce allodynia but also diminish cold and heat hyperalgesia due to chronic constriction injury sciatic nerve. Interestingly, we demonstrated that co-administration of gabapentin and morphine achieved better analgesia at lower doses as compared to sham or either morphine (2 mg/kg) or gabapentin (5 mg/kg).
Neuropathic pain as a common complication of many disorders such as cancer, diabetes mellitus, degenerative spine disease and some infections, affects the quality of life (23). Gabapentin and opioids have been used for treatments of neuropathic pain (24). However, these drugs which have been administered as single agents reduce pain by only 26 to 38 percent, owing to dose limiting adverse effects. It has been proposed that combination of mechanistically distinct analgesic agents may result in additive effects or synergism at lower doses, with fewer side effects than use of either drug separately (25-28). Gabapentin is a3-alkylated analogue of gama-aminobutyric acid, which modulates a2d calcium-channel subunits, a mechanism which is supposed to be important in neuropathic pain (29). Naloxone as an opioid antagonist did not change gabapentin analgesia, and repeated administration of gabapentin does not lead to analgesic tolerance (30). Furthermore, preclinical studies suggest that opioid tolerance can be prevented by using gabapentin (31-33). Common adverse effects of morphine intake are respiratory depression, sedation, nausea and vomiting, constipation, and pruritus (34, 35). The most common adverse effects of gabapentin are sedation, ataxia, and dizziness (36). These adverse effects rarely occur with gabapentin, if the drug is used in combination with morphine. Although sedation is an effect of both drugs but it is mediated only supraspinally, whereas both these drugs have been shown to have analgesic effects at supraspinal, spinal, and even peripheral sites of action (37, 38). As we demonstrated in this study, combination of gabapentin and morphine may provide more potentiating analgesia than sedation.
Conclusion
Here, we showed that morphine and gabapentin alone (Mor 4 and 8 mg/kg and GP 10 and 20 mg/kg) or in combination produced anti-nociceptive effects. Interestingly, gabapentin and morphine combination produces better analgesia at lower doses (Mor 2 mg/kg and GP 5 mg/kg) as compared to sham or either morphine (2 mg/kg) or gabapentin (5 mg/kg). Our findings suggest that combination of these drugs may offer a beneficial treatment for improving symptoms associated with different types of pain, especially neuropathic pain.
Acknowledgment
This publication is based on the data from an MD degree thesis which was financially supported by Vice Chancellor of Research, Kashan University of Medical Sciences, Kashan, Iran.
References
- 1.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology . 2008; 70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 2.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain . 1988; 33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 4.Rose MA, Kam PC. Gabapentine : pharmacology and its use in pain management. Anethesia . 2002; 57:451–462. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- 5.Turan A, Karamanlioglu B, Memis D, Hamamcioglu MK, Tukenmez B, Pamukcu Z, et al. Analgesic effects of gabapentin after spinal surgery. Anesthesiology . 2004; 100:935–938. doi: 10.1097/00000542-200404000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, et al. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev . 2013; 65:223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dworkin RH, Oconor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS. Pharmacologic management of neuropathic pain: evidence-based recommendation. Pain . 2007; 132:237–51. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 8.Gilron I, Max MB. Combination pharmacotherapy for neuropathic pain: current evidence and further directions. Expert Rev Nerother . 2005; 5:823–830. doi: 10.1586/14737175.5.6.823. [DOI] [PubMed] [Google Scholar]
- 9.Meymandi MS, Sepehri GR, Mobasher M. Gabapentin enhances analgesic response to morphine in acute model of pain in male rats. Pharmacol Biochem Behav . 2006; 85:185–889. doi: 10.1016/j.pbb.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Matthews EA, Dickenson AH. A combination of gabapentin and morphine mediates enhanced inhibitory effects on dorsal horn neuronal responses in a rat model of neuropathy. Anesthesiology . 2002; 96:633–640. doi: 10.1097/00000542-200203000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Arciniega MD, Diaz-Reval MI, Cortes-Arroyo, Ramirez AM, Munoz FJ. Anti-nociceptive synergism of morphine and gabapentin in neuropathic pain induced by chronic constriction injury. Pharmacol Biochem Behav . 2009; 92:457–464. doi: 10.1016/j.pbb.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Banafshe HR, Mesdaghinia A, Arani MN, Ramezani MH, Heydari A, Hamidi GA. Lithium attenuates pain-related behavior in a rat model of neuropathic pain: possible involvement of opioid system. Pharmacol Biochem Behav . 2012; 100:425–430. doi: 10.1016/j.pbb.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Tal M, Bennett GJ. Extra territorial pain in rat with a peripheral mononeuropathy: mechano hyperalgesia and mechano allodynia in the territory of an uninjerd nerve. Pain . 1994; 57:375–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 14.Pahlavan Y, Sepehri G, Sheibani V, Afarinesh Khaki M, Gojazadeh M, Pahlavan B, et al. Study the antinociceptive effect of intracerebroventricular injection of aqueous extract of origanum vulgare leaves in rat: possible involvement of opioid system. Iran J Basic Med Sci . 2013; 16:1109–1113. [PMC free article] [PubMed] [Google Scholar]
- 15.Hamidi GA, Ramezani MH, Arani MN, Talaei SA, Mesdaghinia A, Banafshe HR. Ethosuximide reduces allodynia and hyperalgesia and potentiates morphine effects in the chronic constriction injury model of neuropathic pain. Eur J Pharmacol . 2012; 674:260–264. doi: 10.1016/j.ejphar.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Karimi G, Hosseinzadeh H, Rassoulzadeh M, Razavi B, Taghiabadi E. Antinociceptive Effect of Elaeagnus angustifolia Fruits on Sciatic, Nerve Ligated Mice. Iran J Basic Med Sci. 2010; 13:97–101. [Google Scholar]
- 17.Verdi J, Jafari-Sabet M, Mokhtari R, Mesdaghinia A, Banafshe HR. The effect of progesterone on expression and development of neuropathic pain in a rat model of peripheral neuropathy. Eur J Pharmacol . 2013; 699:207–212. doi: 10.1016/j.ejphar.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Babaei-Balderlou F, Zare S, Heidari R, Farrokhi F. Effects of melatonin and vitamin E on peripheral neuropathic pain in streptozotocin-induced diabetic rats. Iran J Basic Med Sci . 2010; 13:1–8. [Google Scholar]
- 19.Seltzer Z, Dubner R, Shir Y. A novel behavioural model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain . 1990; 43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 20.Palecek J, Dougherty PM, Kim SH, Paleckova V, Lekan H, Chung JM, et al. Responses of spinothalamic tract neurons to mechanical and thermal stimuli in an experimental model of peripheral neuropathy in primates . J Neurophysiol . 1992; 68:1951–1966. doi: 10.1152/jn.1992.68.6.1951. [DOI] [PubMed] [Google Scholar]
- 21. Ro LS, Jacobs JM. The role of the saphenous nerve in experimental sciatic nerve mononeuropathy produced by loose ligatures: a behavioral study. Pain . 1993; 52:359–369. doi: 10.1016/0304-3959(93)90170-T. [DOI] [PubMed] [Google Scholar]
- 22.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain . 1994; 59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 23.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002; 18:350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol . 2003; 60:1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 25.Raja SN, Haythornthwaite JA, Pappagallo M, Clark MR, Travison TG, Sabeen S, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo- controlled trial. Neurology . 2002; 59:1015–1021. doi: 10.1212/wnl.59.7.1015. [DOI] [PubMed] [Google Scholar]
- 26.Watson CP, Moulin D, Watt-Watson J, Gordon A, Eisenhoffer J. Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain . 2003; 105:71–78. doi: 10.1016/s0304-3959(03)00160-x. [DOI] [PubMed] [Google Scholar]
- 27.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA . 1998; 280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 28.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998; 280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 29.Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, et al. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002; 303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- 30.Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol . 1997; 121:1513–1522. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimoyama M, Shimoyama N, Inturrisi CE, Elliott KJ. Gabapentin enhances the antinociceptive effects of spinal morphine in the rat tail-flick test. Pain . 1997; 72:375–382. doi: 10.1016/s0304-3959(97)00065-1. [DOI] [PubMed] [Google Scholar]
- 32.Eckhardt K, Ammon S, Hofmann U, Riebe A, Gugeler N, Mikus G. Gabapentin enhances the analgesic effect of morphine in healthy volunteers. Anesth Analg . 2000; 91:185–191. doi: 10.1097/00000539-200007000-00035. [DOI] [PubMed] [Google Scholar]
- 33.Gilron I, Biederman J, Jhamandas K, Hong M. Gabapentin blocks and reverses antinociceptive morphine tolerance in the rat paw-pressure and tail-flick tests. Anesthesiology. 2003; 98:1288–1292. doi: 10.1097/00000542-200305000-00037. [DOI] [PubMed] [Google Scholar]
- 34.Babaei R, Javadi-Paydar M, Sharifian M, Mahdavian S, Almasi-Nasrabadi M, Norouzi A, et al. Involvement of nitric oxide in pioglitazone memory improvement in morphine-induced memory impaired mice. Pharmacol Biochem Behav . 2012; 103:313–321. doi: 10.1016/j.pbb.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Ezzatabadipour M, Majidi M, Malekpour-Afshar R, Eftekharvaghefi SH, Nematollahi-Mahani SN. The effects of morphine on tissue structure of the growth plate in male rats. Iran J Basic Med Sci . 2011; 14:514–520. [PMC free article] [PubMed] [Google Scholar]
- 36.Marson AG, Kadir ZA, Hutton JL, Chadwick DW. The new antiepileptic drugs: a systematic review of their efficacy and tolerability. Epilepsia . 1997; 38:859–880. doi: 10.1111/j.1528-1157.1997.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 37.Singh L, Field MJ, Ferris P, Hunter JC, Oles RJ, Williams RG, et al. The antiepileptic agent gabapentin (Neurontin) possesses anxiolytic-like and antinociceptive actions that are reversed by D-serine. Psychopharmacology (Berl) . 1996; 127:1–9. doi: 10.1007/BF02805968. [DOI] [PubMed] [Google Scholar]
- 38.Carlton SM, Zhou S. Attenuation of formalin-induced nociceptive behaviors following local peripheral injection of gabapentin. Pain . 1998; 76:201–207. doi: 10.1016/s0304-3959(98)00043-8. [DOI] [PubMed] [Google Scholar]
- 39.Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol. 2014; 723:202–206. doi: 10.1016/j.ejphar.2013.11.033. [DOI] [PubMed] [Google Scholar]