Abstract
Objective(s):
The objective of this study was to find a stable microemulsion vehicle for transdermal delivery of ibuprofen to improve the skin permeability.
Materials and Methods:
Microemulsion was prepared using different sorts of oils, surfactants and co-surfactants. Pseudo-ternary phase diagrams were used to evaluate the microemulsion domain. The effects of oleic acid and surfactant mixture on skin permeation of ibuprofen were evaluated with excised skins.
Results:
The optimum formulation F3 consisting of 6% oleic acid, 30% Cremophor RH40/Transcutol P (2:1, w/w) and 59% water phase, showed a high permeation rate of 42.98 µg/cm2/hr. The mean droplet size of microemulsion was about 43 nm and no skin irritation signs were observed on the skin of rabbits.
Conclusion:
These results indicated that this novel microemulsion is a useful formulation for the transdermal delivery of ibuprofen.
Keywords: Ibuprofen, Microemulsion, Skin permeability, Transdermal
Introduction
Microemulsion is a single optically isotropic and thermodynamically stable liquid solution with a droplet size between 10 and 100 nm. It consists of oil phase, surfactant, co-surfactant and aqueous phase which are thermodynamically stable, transparent (or translucent). Microemulsion has several advantages such as enhanced drug solubility, reduced side effects, good thermodynamic stability, and spontaneous formation (1). Recently, microemulsions have also been used for transdermal delivery to increase the absorption of ketoprofen (2), triptolide (3), apomorphine (4), lidocaine (5), estradiol (6) and cyanocobalamin (7).
Ibuprofen, a phenyl propionic acid derivative, is widely accepted as one of the best tolerated non-steroidal anti-inflammatory drugs available for the treatment of ankylosing spondylitis, acute and chronic rheumatic and rheumatoid arthritis and postoperative pain (8, 9). However, ibuprofen shows the poor gastrointestinal absorption because of the low solubility or dissolution rate to water (10-12). Therefore, the clinical use of ibuprofen was limited. Recently, increasing attention has focused on transdermal delivery of ibuprofen. Various dermal dosage forms such as patch (13) and gels (14) has been reported.
In this study, a new microemulsion system was designed for transdermal delivery of ibuprofen to improve the skin permeability, and also the effects of the excipients and ibuprofen concentration on the skin permeability of microemulsion are discussed. Based on a solubility study and pseudo ternary phase diagrams, microemulsions containing ibuprofen have been developed after screening oils, surfactants and co-surfactants. The physicochemical properties and transdermal ability of ibuprofen microemulsion in vitro were evaluated.
Materials and Methods
Ibuprofen as the model drug was obtained from Baike Pharmaceutical Co. Ltd. (Beijing, China). Excipients such as Labrafac Lipophile WL1349, Labrafil M 1944 CS, Labrasol and Transcutol P were donated by Gattefosse (Shanghai, China). Cremophor RH40 was purchased from Xietai Chemical Co. Ltd. (Shanghai, China). Tween 80, oleic acid, ethanol and propylene glycol were obtained from Meilin Industry and Trade Co. Ltd. (Tianjin, China). Acetonitrile and methanol were HPLC grade and supplied from Kemiou Chemical (Tianjin, China). Other chemicals and solvents were analytical grade.
Solubility study
Microemulsion was prepared using different sorts of oils, surfactants and co-surfactants. In order to find out appropriate oils phase and surfactants in microemulsions, the solubility of ibuprofen in various oils and surfactants was determined by adding excess amount of ibuprofen into 1ml of each vehicle (Table 1) in the centrifugal tube. Then, the mixture was vortexed and kept at 25°C in a shaking incubator to facilitate solubilization and then centrifuged at 10000 rpm for 30 min to remove the excess ibuprofen. The drug content in the supernatant was diluted with methanol and measured by HPLC.
Table 1.
Solubility of ibuprofen in various vehicles at 25°C (mean ± SD, n =3)
| Vehicle | Solubility of ibuprofen (mg/ml) | |
|---|---|---|
| Surfactant | Tween80 | 250.51±17.10 |
| Labrasol | 265.65±16.97 | |
| Cremophor RH40 | 259.33±13.78 | |
| Span80 | 144.70±14.15 | |
| oil | Oleic acid | 106.30±9.90 |
| LabrafacLipophile WL1349 | 98.86±15.10 | |
| Labrafil M 1944CS | 87.64±11.90 | |
| Co-surfactant | Transcutol P | 389.91±19.40 |
| Propylene Glycol | 162.68±12.25 | |
| Ethanol | 408.39±17.09 | |
Pseudo-ternary diagrams
The area of microemulsion existence was determined with the aid of previously reported pseudo-ternary phase diagrams (15). The weight ratio (Km) of surfactant to co-surfactant varied as 1:1, 2:1 and 3:1. At each ratio of surfactant to co-surfactant (S/CoS), the ratio of oil to the mixture of S/CoS was varied from 5:95 to 95:5. Distilled water was added dropwise to each oily mixture under gentle magnetic stirring at ambient temperature. Following the addition of aliquot of water phase, the mixtures were assessed visually. The points from clear to turbid and turbid to clear were investigated respectively. Based on these diagrams, selected oil, surfactant and co-surfactant were used for the preparation of ibuprofen microemulsion.
Preparation of ibuprofen microemulsions
From the screen results of pseudo-ternary phase diagrams, the ibuprofen-loaded microemulsion formulations were selected at different component ratios as described in Table 2. The ibuprofen microemulsion was prepared by adding ibuprofen in the oil phase and surfactant mixture, then water was added to them drop by drop with magnetic stirring at ambient temperature. F6 and F7 were designed to investigate the relations of drug concentration and skin permeation rate (Jss) and permeability coefficients (Kp). A 5% ibuprofen aqueous suspension was used as the control vehicle. 5% aqueous suspension of ibuprofen can obtain as following: 500 mg of ibuprofen was grinded into a fine powder with a mortar, and then 0.8% sodium carboxymethyl cellulose solution was added and continued grinding and adjusted to 10 ml.
Table 2.
Compositions of the microemulsion formulations
| Component | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
|---|---|---|---|---|---|---|---|
| Oleic acid (%) | 12 | 9 | 6 | 6 | 6 | 6 | 6 |
| Cremophor RH40 (%) | 20 | 20 | 20 | 24 | 28 | 20 | 20 |
| Transcutol P (%) | 10 | 10 | 10 | 12 | 14 | 10 | 10 |
| Water* (%) | 53 | 56 | 59 | 53 | 47 | 61 | 60 |
| Ibuprofen (%) | 5 | 5 | 5 | 5 | 5 | 3 | 4 |
Note: water was 0.8% sodium carboxymethyl cellulose solution
Transmission electron microscope
The morphology of ibuprofen microemulsion was observed using transmission electron microscopy (JEM-100SX, JEOL, Japan). One drop of diluted samples was negatively stained by 2% phosphotungstic acid (PTA) and placed on film-coated copper grids followed by drying before examination under the electron microscope.
Droplet size of microemulsion
The droplet size of microemulsion was measured with photon correlation spectroscopy (PCS) using a NICOMP particle sizing system (CW380, Santa Barbara, California, USA) at a fixed angle of 90 degrees and at a temperature of 25°C. The droplet size analysis data were evaluated using the volume distribution.
The stability of microemulsion
The microemulsion containing ibuprofen was stored at 25°C for 3 months. Then the characterization and stability were investigated. The centrifuge tests were also carried out to assess the physical stability. The ibuprofen microemulsion examples were centrifuged for 10 min at 4000 rpm and 10,000 rpm, respectively.
in vitro permeation studies
The abdominal skins were obtained from male rats. After hair was removed with clipper, the abdominal skins were excised and then the subcutaneous fat and other tissues were trimmed. The obtained skins were washed and examined for integrity. Skin samples were stored at -20°C prior to use.
The permeation experiments were performed using Franz diffusion cells with an effective diffusion area of about 1.77 cm2 and a 8 ml volume. The skins were clamped between the donor and the receptor chamber with the stratum corneum pointing to the donor chamber. The receptor compartments were filled with phosphate buffered saline (pH 7.4) and its temperature was maintained at 37°C and continuously stirred throughout the experiment. The microemulsion formulations and ibuprofen control suspension were applied on the epidermal surface of the skin, the amount of the product application on the skin was equivalent to 50 mg of ibuprofen. 200µl sample of the receiver medium was withdrawn at predetermined time and replaced immediately with an equal volume of pre-thermostated (37°C) fresh phosphate buffer. The samples were determined using HPLC after filtered through 0.22 µm filters. Each experiment was carried out in triplicate.
HPLC analysis of samples
The samples were analyzed using a P3000A pump and a UV3000 UV-Vis detector with a Venusil XBP C18 column (5 µm, 250 4.6 mm). The mobile phase composed of 0.02mol/l potassium dihydrogen phosphate buffer and acetonitrile (6:4 v/v) at a flow rate of 1 ml/min, and the detection wavelength was 223 nm. All operations were carried out at ambient temperature.
Data analysis of skin permeation
The cumulative amount of ibuprofen permeated (Qn, µg/cm2) through skins was determined based on the following equation:
| (1) |
Where Cn stands for the drug concentration of the receiver solution at each sampling time, Ci for the drug concentration of the ith sample, V0 and Vi for the volumes of the receiver solution and the sample, respectively, and S stands for the effective diffusion area. The cumulative amount of ibuprofen permeated plotted as a function of time. The slope and intercept was acquired by regression method from the linear portion of the plot. The skin permeation rate at steady-state (Jss, µg/cm2/hr) was calculated from the slope of the linear portion of the plots of Qn against time.
Apparent permeability coefficients (Kp) were calculated according to the equation:
| (2) |
Where Kp was the permeability coefficient, and C0 represents the drug concentration which remains constant in the vehicle.
Skin irritation studies
Skin irritation tests were conducted in rabbits to determine whether microemulsion containing ibuprofen produced irritation. The irritation test of each healthy male rabbits (2.0-2.5kg) was divided into four regions for ibuprofen control suspension, microemulsion without ibuprofen, F3 and F6, respectively. Each formulation weighing 0.5 g was applied on the hair-free skin of rabbits by uniform spreading within the area of 4 cm2. The experiment was carried out for 7 days and the application sites were graded according to a visual scoring scale. Skin irritation was scored following the Draize method (16, 17), and the grading scales are summarized in Table 3.
Table 3.
Grading scale of the Draize method
| Grade | Formation of erythema and edema |
|---|---|
| 0 | None |
| 1 | Slight |
| 2 | Well defined |
| 3 | Moderate |
| 4 | Severe erythema and edema |
Statistical analysis
Results were expressed as the means ± SD. Statistical data were analyzed by the Student's t-test with P<0.05 as minimal level of significance.
Results
Solubility Studies
Microemulsion has been shown to be specific to the nature and ratio of the oil/surfactant, the surfactant concentration. It is not arbitrary, but very specific pharmaceutical excipient combinations could form good emulsifying systems. In general, the excipients (oils phase, Co-surfactant and surfactants) chosen in the study includes all products the author can buy. These components are oily phase, surfactant and co-surfactant which are natural, semi-synthetic and synthetic. Then drug-excipient screening for solubility, compatibility, and stability are determined. After assessment of the solubility of the drug in the individual components, the pseudo-ternary phase diagrams were used to evaluate the overall solubilizing power of the system. Base on the rules such as to achieve maximal drug loading; achieve minimal self-emulsification time and droplet size. In our study, in order to find out appropriate oils phase and surfactants in microemulsions, the solubility of ibuprofen in various oils and surfactants was determined.
The results were shown in Table 1. Among the oils, the solubility of ibuprofen was highest in oleic acid, followed by Labrafac Lipophile WL1349 and Labrafil M 1944CS. In addition, ibuprofen had a higher solubility in Cremophor RH40, Labrasol and Tween 80, but had a less solubility in Span 80.
Pseudo ternary phase diagram study
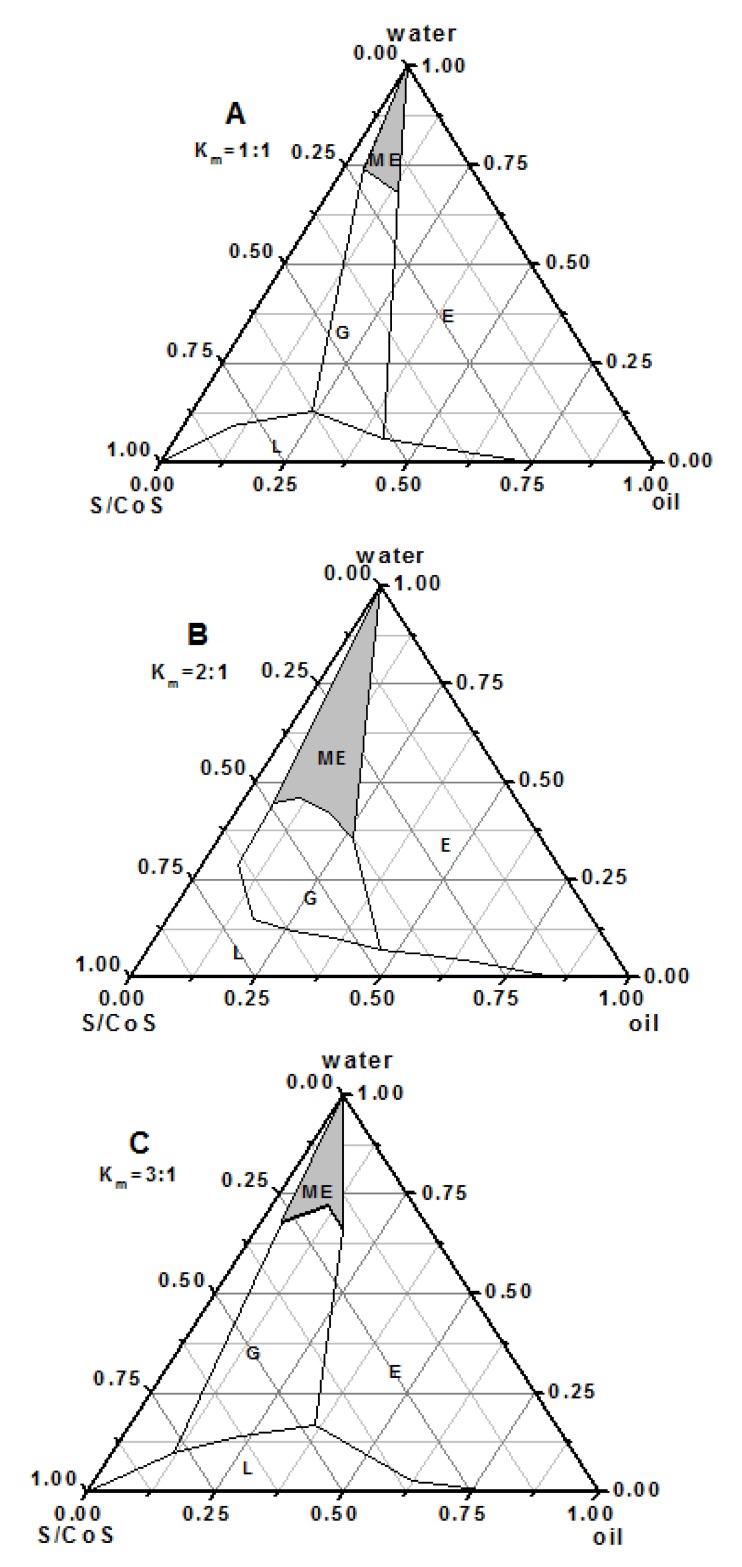
Pseudo-ternary phase diagrams were constructed in the absence of ibuprofen to obtain appropriate concentration ranges of components in the areas of microemulsions. The phase diagrams containing Cremophor RH40 as a surfactant and oleic acid as an oil and Transcutol P as a co-surfactant with various Km values (1:1, 2:1, and 3:1) are described in Figure 1. The addition of Transcutol P increased the area of microemulsion at all Km values than that with Cremophor RH40 alone (data not given). It was also found that microemulsion region was increased gradually with increase in Km, reaching a maximum at Km of 2:1. In brief, system at Km 2:1 formed a larger single phase region than the systems at other Km. Thus, the ratio of Cremophor RH40 and Transcutol P was fixed at 2:1. After the overall assessment of the solubilizing ability of the drug in the individual systems, oleic acid, Cremophor RH40 and Transcutol P were selected as oil phase, surfactant and co-surfactant, respectively. The optimum formulation F3 consisted 6% oleic acid, 30% Cremophor RH40/ Transcutol P (2:1, w/w) and 59% water phase (Table 2).
Figure 1.
The pseudo-ternary phase diagrams of the oilsurfactant- water system at 1:1(A), 2:1(B), and 3:1(C) weight ratios of Cremophor RH40 to Transcutol P. E: emulsion; G: gel; L: isotropic region; shaded region: microemulsion
Characterization of microemulsion
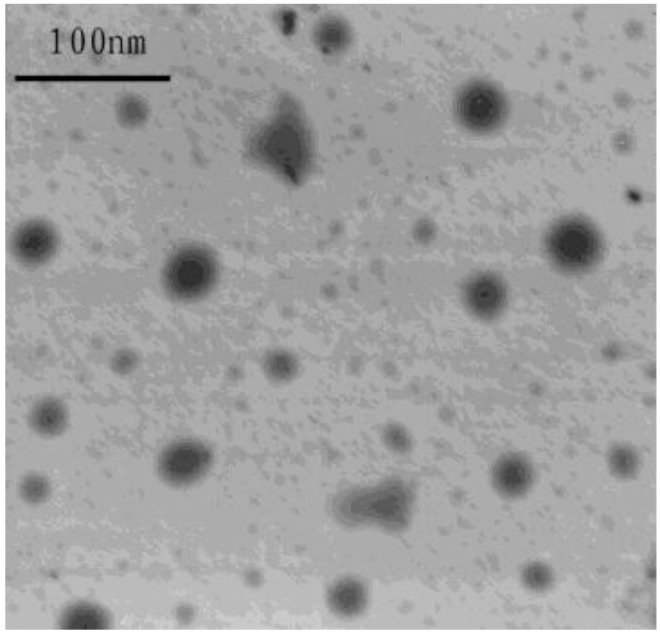
Physicochemical characteristics of microemulsion appear in Table 4. Morphology of the ibuprofen microemulsion was characterized using TEM (Figure 2). The average size of all microemulsions was showed in Table 4. It was obvious that the average droplet size of microemulsion with more oil increase significantly, while with more surfactant, no significant change in average droplet size of microemulsion was observed. Statistical data show that there were no statistically significant difference between 0 day and 3 months storage (P>0.05) for the same formulation. There was no significant difference in droplet size, appearance, and drug content after 3 months storage during the stability study. After centrifugation ibuprofen microemulsion still remain clear and transparent, neither stratification nor precipitation were found. This indicates that ibuprofen microemulsion is chemically and physically stable.
Table 4.
The droplet size (volume distribution) of the selected formulations at 0 day and after 3 months storage (mean ± SD, n =3)
| Formulation | time | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
|---|---|---|---|---|---|---|---|---|
| Droplet size (nm) | 0 day | 62.81±5.71 | 53.10±4.30 | 43.33±3.13 | 45.43±4.95 | 49.37±5.97 | 39.57±3.10 | 42.61±3.92 |
| Droplet size (nm) | 3 months storage | 67.35±4.96 | 55.73±5.02 | 42.12±2.97 | 46.86±5.31 | 47.84±4.69 | 40.62±2.64 | 41.38±4.57 |
Figure 2.
Transmission electron microphotography of ibuprofen microemulsion (F3)
in vitro permeation studies
The permeation parameters of the tested microemulsions and ibuprofen control suspension were shown in Table 5. The Jss of ibuprofen was increased 1.31 to 2.64 times higher with different microemulsions when compared with control. The optimum formulation F3 had the highest permeation rate of 42.98 4.98 µg cm-2 hr-1.
Table 5.
Percutaneous permeation parameters of the selected vehicles (mean ± SD, n =3)
| Formulation | Jss (μg/cm2/hr) | Kp(×l03 cm/hr) |
|---|---|---|
| F1 | 32.09±3.42 | 0.64±0.07 |
| F2 | 35.83±3.44 | 0.72±0.07 |
| F3 | 42.98±4.98 | 0.86±0.10 |
| F4 | 34.88±3.55 | 0.70±0.07 |
| F5 | 25.41±3.23 | 0.51±0.06 |
| F6 | 21.39±3.82 | 0.43±0.08 |
| F7 | 31.92±2.52 | 0.64±0.05 |
| control | 16.24±2.07 | 0.32±0.04 |
Skin irritation studies
In the skin irritation studies, the irritation score was depicted in Table 6. Score of less than 2 is considered no skin irritation. The four formulations did not cause any noticeable signs of erythema or edema on rabbit skin throughout the study. Very slight erythema of rabbit's skin was observed from skin irritation studies. So, there was no further histopathological study.
Table 6.
Results of skin irritation studies on rabbits (mean ± SD, n =4)
| Visual observation | ||
|---|---|---|
| Formulation | Erythema | Edema |
| Control | 1.25±0.50 | 0.75±0.50 |
| Blank microemulsion | 0.50±0.58 | 1.25±0.50 |
| F3 | 1.50±0.58 | 1.00±0.82 |
| F6 | 1.50±0.58 | 1.50±0.58 |
Discussion
The solubilitiy of ibuprofen in various oils, surfactants and co-surfactants was analyzed to screen components for microemulsions. Previous reports indicated that the superior dermal flux appeared mainly due to the large solubilizing capacity of the microemulsions, which led to larger concentration gradient towards the skin (5, 18). Previous reports indicated that oleic acid could increase fluidity of lipid portion of the stratum corneum due to the large solubilizing capacity (19). Thus, oleic acid was chosen as oily phase due to its good drug solubility and emulsion forming ability. Therefore, three surfactants (Cremophor RH40, Labrasol and Tween 80) and three co-surfactants (Transcutol P, Propylene Glycol and Ethanol) were chosen for further evaluation. When Tween 80 or Labrasol was used as surfactant, the microemulsion area was very small regardless of what co-surfactant was used. The systems containing Cremophor RH40 as surfactant and Transcutol P as co-surfactants formed a stable and broad microemulsion area. In the case of Cremophor RH40, Ethanol as a co-surfactant also formed broad microemulsion area but the system was not stable, thus only Transcutol P was chosen as a co-surfactant.
in vitro permeation studies indicated that the high permeation rate of microemulsions might attribute to the high concentration of surfactants in microemulsions as permeation enhancers. The surfactants may increase permeation into the intercellular regions of stratum corneum, enhancing the penetrate effects of skins.
Chen et al (20) reports 3% gel to promote the absorption of ibuprofen. They used the full-thickness porcine ear skin for the permeation experiments. But in our paper we used the abdominal skin from male rats for the permeation experiments. In that paper the Jss value for the permeation parameters of microemulsion-based ibuprofen hydrogel formulation were between 9.76±0.66 µg/cm2/hr and 38.06±1.04 µg/cm2/hr and in our experiments the Jss value was between 16.24±2.07 µg/cm2/hr and 42.98±4.98 µg/cm2/hr. There are differences between the different species of the skin, resulting in different experimental results.
To our knowledge, most commercial topical ibuprofen products commonly contain 5% ibuprofen. For example (21): IbusprayTM, IbumousseTM, Proflex CreamTM, Fenbid GelTM and Deep Relief GelTM. So the content of microemulsion-based hydrogel formulation of ibuprofen in the article (20) is lower than the content of some commercially available formulations. In our study, the ibuprofen content was 5%, consistent with commercially available products. Moreover, skin irritation tests were conducted in rabbits to determine whether microemulsion containing ibuprofen produced irritation in our study. But it had not been reported in the article mentioned above.
Then drug-excipient screening for solubility, compatibility, and stability are determined. After assessment of the solubility of the drug in the individual components, the pseudo-ternary phase diagrams were used to evaluate the overall solubilizing power of the system.
In our study, in order to find out appropriate oils phase and surfactants in microemulsions, the solubility of ibuprofen in various oils and surfactants was determined. Various vehicles that we can acquired included surfactants (Tween80, Labrasol, Cremophor RH40), oil (Span80, Oleic acid, Labrafac, Lipophile WL1349), Co-surfactant (Labrafil M 1944CS, Transcutol P, Propylene Glycol, Ethanol). After the overall assessment of the solubilizing ability of the drug in the individual systems and we choose the optimum formulation F3 consisting of 6% oleic acid, 30% Cremophor RH40/Transcutol P (2:1, w/w) and 59% water phase, showed a high permeation rate of 42.98 µg/cm2/hr.
In this study, the effect of amount of oleic acid on the permeation of ibuprofen was investigated. With the reduction of the amount of oleic acid (F1~F3), Jss and Kp are increased. Oleic acid can be used as the oil phase microemulsion, it also increases lipid fluidity between horny; however, an excess of oleic acid in the formation, can inhibit in vitro skin permeation. But, too small amount of the oil phase, microemulsion formation instability. Considering the above factors, the final formulation contains 6% oleic acid.
Formulation amount of RH40 and TP also play an important role on the skin permeation of ibuprofen. The results showed RH40 and TP could inhibit the skin's permeability. With the increase in the amount of RH40 and TP, Jss and Kp is reduced. Therefore, the final formulation contained RH40 amount of 20%, and TP amount of 10%."
As can be seen, the amount of ibuprofen permeated was found increasing with an increase in the concentration of the drug. The Jss of ibuprofen from F6 and F7 was 21.39±3.82 µg/cm2/hr and 31.92±2.52 µg/cm2/hr; whereas that Jss from F3 was 42.98±4.98 µg/cm2/hr. F3 was 42.98±4.98 µg/cm2/hr. The Kp of ibuprofen using F6 and F7 was 0.43±0.08×l0-3 cm/hr and 0.64±0.05×l0-3 cm/hr whereas that obtained from F3 was 0.86±0.10×l0-3 cm/hr.
Eun-Seok Park et al (22) prepared a series of 17 polyoxyethylene (POE) alkyl ethers containing 5% ibuprofen and investigated the influence of polyethoxylated non-ionic surfactants on the transport of ibuprofen across rat skin. in vitro Jss through excised rat skin was found 21.05 µg/cm2/hr for the control (5% ibuprofen aqueous suspension) and were 66.19 µg/cm2/hr and 110.24 µg/cm2/hr containing different POE alkyl ethers-water vehicles. The experiments has used the same experimental rabbits as ours. The Jss were higher than those of our study.
Al-Saidan et al (23) prepared saturated solutions of ibuprofen, of different concentrations, and investigated their effect on permeation of ibuprofen across rat epidermis. The amount of ibuprofen permeated across rat epidermis was found increasing with an increase in the concentration of the drug in these saturated solutions. The corresponding Jss of ibuprofen are from 77.4±8.3 to 3592.9±724.2 µg/cm2/hr, respectively. The Kp of ibuprofen are from 7.42±0.8×l0-3 cm to 95.8±19.3×l0-3 cm/hr. The Jss of ibuprofen and Kp are much higher than those of our research. The rats used in that paper were newborn, within 24 hr of birth. We think that the skin permeability of newborn rats is much higher than that of adult rats. The difference of skin contributed to the difference of permeability.
In our study, skin irritation was evaluated in accordance with the method reported by Draize and co-workers (16). The test was used to determine dermal irritation potential of the formulation on the rabbit skin. According to Draize et al, compounds producing scores of 2 or less are considered no skin irritation. The total skin irritation score of erythema and edema on application of the test formulation was showed in Tables 5. The irritation scores revealed that no erythema and edema was observed on the site of skin after application of microemulsion in the irritation studies. Based on above observations, it was concluded that the microemulsion was safe, less irritant and well tolerable formulation for transdermal delivery.
Conclusion
In this study, a microemulsion system was constructed for transdermal delivery of ibuprofen. Various formulation factors were evaluated to find out an optimum microemulsion vehicle that had high skin permeation rate. The optimum formulation F3 which consisted of oleic acid 6%, Cremophor RH40 20%, Transcutol P 10%, water phase 59% (w/w) and ibuprofen 5% was obtained by in vitro permeation studies. The microemulsion system is physical stable for 3 months and no skin irritation was observed. So the employment of microemulsion was found to be a suitable carrier system for the transdermal administration of ibuprofen.
Acknowledgment
This work was supported by the Talent Introduction Program of Hebei University (No. y2005064), the Medical and Engineering Science Research Center of Hebei University (No. BM201109). Hebei Provincial Natural Science Foundation of China-Shijiazhuang Pharmaceutical Group (CSPC) Foundation (No. H2013201274) and Program for the Top Young Talents of Hebei Province.
References
- 1.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev . 2000;45:89–121. doi: 10.1016/s0169-409x(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 2.Rhee YS, Choi JG, Park ES, Chi SC. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm . 2001;228:161–170. doi: 10.1016/s0378-5173(01)00827-4. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Chang X, Weng T, Zhao X, Gao Z, Yang Y, et al. A study of microemulsion systems for transdermal delivery of triptolide. J Control Release . 2004;98:427–436. doi: 10.1016/j.jconrel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Peira E, Scolari P, Gasco MR. Transdermal permeation of apomorphine through hairless mouse skin from microemulsions. Int J Pharm. 2001;226:47–51. doi: 10.1016/s0378-5173(01)00759-1. [DOI] [PubMed] [Google Scholar]
- 5.Kreilgaard M, Pedersen EJ, Jaroszewski JW. NMR characterisation and transdermal drug delivery potential of microemulsion systems. J Control Release. 2000;69:421–433. doi: 10.1016/s0168-3659(00)00325-4. [DOI] [PubMed] [Google Scholar]
- 6.Peltola S, Saarinen-Savolainen P, Kiesvaara J, Suhonen TM, Urtti A. Microemulsions for topical delivery of estradiol. Int J Pharm. 2003;254: 99–107. doi: 10.1016/s0378-5173(02)00632-4. [DOI] [PubMed] [Google Scholar]
- 7.Salimi A, Sharif Makhmal Zadeh B, Moghimipour E. Preparation and characterization of cyanocobalamin (vit B12) microemulsion properties and structure for topical and transdermal application. Iran J Basic Med Sci . 2013;16:865–872. [PMC free article] [PubMed] [Google Scholar]
- 8.Yong CS, Oh YK, Jung SH, Rhee JD, Kim HD, Kim CK, et al. Preparation of ibuprofen-loaded liquid suppository using eutectic mixture system with menthol. Eur J Pharm Sci . 2004;23:347–353. doi: 10.1016/j.ejps.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Newa M, Bhandari KH, Li DX, Kwon TH, Kim JA, Yoo BK, et al. Preparation, characterization and in vivo evaluation of ibuprofen binary solid dispersions with poloxamer 188. Int J Pharm . 2007;343:228–237. doi: 10.1016/j.ijpharm.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Glowka FK. Stereoselective pharmacokinetics of ibuprofen and its lysinate from suppositories in rabbits. Int J Pharm . 2000;199:159–166. doi: 10.1016/s0378-5173(00)00377-x. [DOI] [PubMed] [Google Scholar]
- 11.Ghorab MK, Adeyeye MC. Enhancement of ibuprofen dissolution via wet granulation with betacyclodextrin. Pharm Dev Technol . 2001;6:305–314. doi: 10.1081/pdt-100002611. [DOI] [PubMed] [Google Scholar]
- 12.Maghsoodi M, Kiafar F. Co-precipitation with PVP and Agar to Improve Physicomechanical Properties of Ibuprofen. Iran J Basic Med Sci . 2013;16:635–642. [PMC free article] [PubMed] [Google Scholar]
- 13.Ji HY, Lee HW, Kim YH, Jeong DW, Lee HS. Simultaneous determination of piroxicam, Meloxicam and tenoxicam in human plasma by liquid chromatography with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005; 826: 214–219. doi: 10.1016/j.jchromb.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Gupta SK, Bansal P, Bhardwaj RK, Jaiswal J, Velpandian T. Comparison of analgesic and antiinflammatory activity of meloxicam gel with diclofenac and piroxicam gels in animal models: pharmacokinetic parameters after topical application. Skin Pharmacol Appl Skin Physiol . 2002;15:105–111. doi: 10.1159/000049397. [DOI] [PubMed] [Google Scholar]
- 15.Zhu W, Yu A, Wang W, Dong R, Wu J, Zhai GX. Formulation design of microemulsion for dermal delivery of penciclovir. Int J Pharm . 2008;360:184–190. doi: 10.1016/j.ijpharm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Draize J, Woodard G, Calvery H. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377–390. [Google Scholar]
- 17.Joshi M, Pathak S, Sharma S, Patravale V. Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: Nanoject. Int J Pharm . 2008;364:119–126. doi: 10.1016/j.ijpharm.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 18.Sintov AC, Shapiro L. New microemulsion vehicle facilitates percutaneous penetration in vitro and cutaneous drug bioavailability in vivo. J Control Release . 2004;95:173–183. doi: 10.1016/j.jconrel.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 19. Kanikkannan N, Kandimalla K, Lamba SS, Singh M. Structure-activity relationship of chemical penetration enhancers in transdermal drug delivery. Curr Med Chem . 2000; 7:593–608. doi: 10.2174/0929867003374840. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Chang X, Du D, Li J, Xu H, Yang X. Microemulsion-based hydrogel formulation of ibuprofen for topical delivery. Int J Pharm. 2006;315:52–58. doi: 10.1016/j.ijpharm.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Hadgraft J, Whitefield M, Rosher PH. Skin penetration of topical formulations of ibuprofen 5%: an in vitro comparative study. Skin Pharmacol Appl Skin Physiol . 2003;16:137–142. doi: 10.1159/000069759. [DOI] [PubMed] [Google Scholar]
- 22.Park ES, Chang SY, Hahn M, Chi SC. Enhancing effect of polyoxyethylene alkyl ethers on the skin permeation of ibuprofen. Int J Pharm. 2000;209:109–119. doi: 10.1016/s0378-5173(00)00559-7. [DOI] [PubMed] [Google Scholar]
- 23.Al-Saidan SM. Transdermal self-permeation enhancement of ibuprofen. J Control Release . 2004;100:199–209. doi: 10.1016/j.jconrel.2004.08.011. [DOI] [PubMed] [Google Scholar]