Abstract
Objective(s):
Acute kidney injury (AKI), a syndrome characterized by decreased glomerular filtration, occurs in every 1 of 5 hospitalized patients. Renal ischemia-reperfusion, one of the main causes of AKI, is of particular importance in the setting of kidney transplantation.
Materials and Methods:
Sixty male rats were divided into four groups including control, nephrectomy, sham surgery and renal ischemia-reperfusion (IRI) group. The rats were anesthetized with intraperitonealketamin and xylazin. For making IRI group, right nephrectomywas performed, and after a week, the left kidney pedicle was occluded for 45 min for making ischemia that followed by 24 hr reperfusion. At the end of reperfusion phase, the lung tissues were isolated to be used in immunohistochemical and histological assays. Immunohistochemical assay was used to evaluate Bcl-2 and TNF-α, and hematoxylin-eosin staining assay was used to histopathology.
Results:
lung tissues injury after renal ischemia-reperfusion was revealed by immunohistochemistry analysis to increase TNF-α level and decrease Bcl-2 (an anti-apoptotic protein) level. Lung injury and necrosis was discovered by hematoxylin-eosin staining to be more evident in IRI group than sham and control groups.
Conclusion:
The results demonstrated that increase in TNF-α and decrease in Bcl-2 levels in lungs induces the pulmonary inflammatory damage in renal IRI model.
Keywords: Bcl-2, Inflammatory response, Lunginjury, Renal ischemia-reperfusion, TNF-α
Introduction
Acute kidney injury (AKI) is a syndrome characterized by decreased glomerular filtration. This syndrome, which happened in each 1 of 5 hospitalized patients, is associated with an over fourfold increased mortality - findings that appear to persist over the last decade (1).
AKI occurs in various clinical settings including shock, sepsis, transplantation, and vascular surgery. Despite advances in renal transplantation, the mortality of patients with Acute kidney injury has continued high over the past few decades, and renal insufficiency continues to be a sensitive marker for a poor outcome in critically ill patients (2, 3).
The etiology of AKI is various, and ischemia-reperfusion constitutes the main cause of this condition. Ischemic AKI is a dynamic procedure that often coexists with multiple organ failure and involved hemodynamic variation, inflammation, and direct damage to the tubular epithelium(4). AKI has been reported to induce acute lung injury (ALI) as well as to cause injuries to other remote organs, including the lungs (5).
ALI is a life-threatening circumstance that is frequently complicated with acute kidney injury, which is a serious condition in intensive care units.
ALI mortality has been reported to be higher than 50% and reach to over 80% when combined with ALI (6, 7). AKI leads to a systemic increase in serum chemokines and cytokines such as TNF-α that can mediate these lung alterations and characteristics of a secondary insult in lungs, AKI can change the damage response to mechanical ventilation (8-12). Acute lung inflammatory response is also associated to epithelial cytokine expression (13) as well as to the expression of the signaling cascade leading to apoptosis. Activation of epithelial proinflammatory signaling cascades is mediated by TNF-α a prototypic member of a cytokine family which regulates essential biologic functions (e.g. cell proliferation, differentiation, apoptosis, survival) and an extensive spectrum of responses to stress and injury (14).
The present study was planned to investigate the effects of unilateral renal ischemia reperfusion injury after right nephrectomy similar to kidney transplantation model on lung injury and inflammatory responses in male rat.
Materials and Methods
Animals
In this study, sixty male Wistar- Albino (200-250g) were obtained from the experimental animal research center, Faculty of Medicine, Tabriz University, Iran. The animals were housed in a room temperature (21±2°C) and humidity (60±5%) controlled room in which a 12-12 hr light-dark cycle was maintained. They had free access to standard water and food. The study was approved by the University Ethics Committee.
Surgery and experimental protocol
Rats were divided in four groups (n=15) including control group (without any procedure), nephrectomy (right kidney excision without any occlusion), sham surgery (only laparotomy), and IRI. The animals were anesthetized with intraperitoneal ketamin (50 mg/kg) and xylazin (10 mg/kg) and placed on a homeothermic table to maintain core body temperature at approximately 37°C. Right nephrectomy was performed to make IRI group and after a week, the left kidney pedicle was occluded for 45 min via micrvascular clamp for making ischemia that followed 24 hr reperfusion. At the end of reperfusion phase, after anesthesia the lungs were isolated to be used in immunohistochemical and histological assays. Immunohistochemical assay was used for evaluation of Bcl-2 and TNF-α, and Hematoxylin-Eosin staining was used for histopathological assessment.
Immunohistochemistry
Lung tissue was fixed in buffered formalin for 3 to 6 days, dehydrated in rising cycle of ethanol concentrations (60, 70, 90, 96, and 100%) and acetone, and then fixed in paraffin. Four-micrometer sections were dewaxed in xylene and rehydrated by a downward cycle of ethanol concentrations (100, 90, and 70%). Sections were then bathed in deionized water. Endogenous peroxidase was blocked by insertion sections in 0.3% peroxide diluted with methanol for 30 min and washed with PBS (phosphate buffered saline). General antibody binding was blocked by incubating sections for 1 hr in 2% rabbit blocking serum (Abcam, Cambridge, UK) diluted in PBS (1:50 dilution). Sections were incubated with the primary antibody diluted in phosphate buffered saline (1:100) in 1/2000 for TNF-α (rabbit polyclonal TNF-α, Abcam) and 1/100 for Bcl-2 (rabbit polyclonal to Bcl-2, Abcam) for 30 min to 1 hr at room temperature. The sections were washed of primary antibody with PBS and then the secondary antibody diluted in PBS and blocking serum was applied for 30 min. The sections were then stained using ABC-peroxidase substrate kit (Vector Laboratories Inc.) and washed. 3, 3′-diaminobenzidine tetrahydrochloride (Vektor DAB Peroxide Substrate; Vector Laboratories Inc) was applied to the sections for 15 min. The sections were washed with PBS, dehydrated in graded ethanol (75%, 95%, 100%), counterstained with hematoxylin (for visualization), and coverslipped. Color reaction (brown) can be seen under microscope, and the reaction can then be closed with water. Immunohistochemical staining for TNF-α and Bcl-2 was studied using light microscopy at a magnification of 40x. In each group, eight representative lung sections were investigated, 20 view fields were counted per lung section.
Histopathological assessment
For histopathological analyses, 45 min of renal ischemia followed by 24 hr of reperfusion was conducted. Isolated lungs were fixed in 10% formalin. The samples were dehydrated and embedded in paraffin. Sections (4 µm thickness) were cut and stained with hematoxylin and eosin. Histological changes were scored on a 4-point scale: (-) none, (+) mild, (++) moderate, and (+++) severe damage.
Results
The effect of renal ischemia reperfusion on lungs injury was investigated in 45 min of renal ischemia followed by 24 hr reperfusion. Via using immunohistochemistry (IHC), expression of antiapoptotic Bcl2 and inflammatory mediator TNF-α were assessed in lungs sections after IR. Also histological changes in lung were assessed via H-E after 24 hr renal reperfusion.
TNF-α expression levels in lung
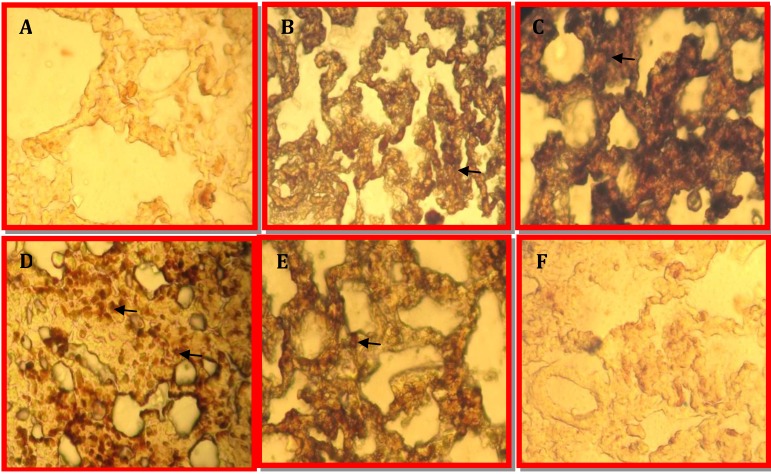
In the present study, we further defined the increases in lung TNF-α. Before ischemia and after sham surgery, TNF-α was undetectable in the lung. TNF-α levels increased by 24 hr post-ischemiareperfusion, remained elevated compared with levels of sham surgery after 24 hr reperfusion (A-C in Figure 1).
Figure 1.
Immunohistochemical assay: Expression of TNF-α level in the lungs. (A) sham, (B) nephrectomy, (C) ischemia-reperfusion group. Expression of Bcl-2 levels in the lungs. (D) sham (E) nephrectomy, (F) ischemia-reperfusion group. Arrows show expression of TNF-α and Bcl-2 levels. Magnification × 40
Bcl-2 expression levels in lung
Protein levels of Bcl-2 in lung were reduced in nephrectomia and IRI group after 24 hr reperfusion. Renal IRI decreased lung Bcl-2 at 24 hr after reperfusion. It was shown in Figure 1 (D-F). Whereas Bcl-2 levels in control group was elevated.
Histological changes
We examined histopathologically the effects of renal ischemia reperfusion on rat lungs. Routine H-E staining revealed that Severe alveolar collapse and loss of alveolar geometry and deposition of eosinophilic material in the alveoli (Figure 2). As well as intense mononuclear leukocyte infiltration and intra alveolar hemorrhage, leukocyte infiltration, intravascular perfusion, severs infiltration of mononuclear leukocyte and peribronchiolar polymorphonuclear was observed. Also accumulation of leukocytes, hyperplasia and Focal necrosis were present in lungs tissue compared to the sham-operated and control group after 24 hr renal reperfusion (Table 1).
Figure 2.
Histological assay: sham group (A-C) via normal alveolar and bronchial geometry. Magnification × 20. Ischemia-reperfusion group: (D) severe alveolar collapse and loss of alveolar geometry and deposition of eosinophilic material in the alveoli. Magnification × 20. (E) Intense mononuclear leukocyte infiltration and intraalveolar hemorrhage. Magnification×40. (F) Leukocyte infiltration and intravascular perfusion. Magnification×40. (G) Sever infiltration of mononuclear leukocyte and peribronchiolar polymorphonuclear. Magnification × 40. (H) Accumulation of leukocytes and hyperplasia. Magnification × 40. (I) Foci necrosis. Magnification × 40
Table 1.
Histological changes in the lungs after 24 hr renal ischemia reperfusion (H&E)
| Groups | Alveolar collapse | Intra alveolar mononuclear leukocyte infiltration | Intra alveolar hemorrhage | Intravascular leukocyte infiltration and perfusion | Infiltration of peribronchiolarpolymorphonuclear | Foci necrosis and hyperplasia |
|---|---|---|---|---|---|---|
| Control | - | - | - | - | - | - |
| Sham | - | - | - | - | - | - |
| Nephrectomy | + | + | ++ | ++ | + | + |
| IR | +++ | +++ | +++ | +++ | +++ | +++ |
A minimum of 10 fields for each kidney slide were examined and assigned for severity of changes using scores on a scale of: (–) none, (+) mild, (++) moderate, and (+++) severe damage. (n = 7 for each group)
Discussion
AKI is associated with decreased allograft survival in transplanted kidney recipients and with high mortality in patients with native kidneys (15). The major cause of AKI is ischemia and reperfusion injury. The pathogenesis of renal IRI is complex and still not entirely understood, however inflammation is presently accepted as an important pathogenic component (16). IRI results in endothelial and leukocyte activation, production of reactive oxygen species, tubular cell death and release of inflammatory mediators, such as cytokines and chemokines (16). IR is initiated by production of reactive oxygen species, which initially seem to be responsible for the generation of chemotactic activity for neutrophils. In reperfusion injury, a variety of cytokines and mediators may be responsible for priming neutrophils (17, 18). Besides the local damage caused by IRI, distant organs can also be affected (19-21). Although many studies have been performed to demonstrate the systemic effect of IRI, the mechanism is not well established. To study the systemic effects of inflammatory mediators released from renal ischemia reperfusion injury, we used a rat model of unilateral renal ischemia injury after right nephrectomy similar to kidney transplanation. TNF- α, Bcl-2 levels and injury in lung tissue in mal rat were subjected to 45 min of renal ischemia injury, and lungs were studied at 24 hr after reperfusion.
One of the goals of this project was determining the amount of TNF-α in the lungs following renal IRI. It was observed that renal IR causes increase in the expression of TNF-α in the lungs of ischemia group compared with the control group and increases its expression, which causes necrosis and injury in the lungs. These proinflammatory molecules can induce direct tissue damage and are also potent activators of leukocytes and thereby promote their sequestration in organs susceptible to leukocyte mediating injury such as the lung alveolar capillary bed, leading to endothelial cell injury, the development of pulmonary hypertension and increased vascular permeability (17, 18).
In another study it was shown that blocking the lymphatic thoracic duct after ischemia-reperfusion in small intestine of rats, decreased TNF-α levels in the lungs of rats compared to the ones with open lymph duct and injury is reduced (22). Inflammatory mediator such as TNF-α is released by renal ischemia and can reach the lungs and can induce inflammation and neutrophil accumulation and activity. It is also shown that the pattern of cytokine and chemokine expression in the lung, similar to its expression in the kidney after ischemic injury of the kidney (23). TNF has been demonstrated to play a pivotal role in developing pulmonary edema in ALI via activation of p55-mediated death signaling, rather than activation of previously well-characterized p55-mediated proinflammatory signaling (24). Our study revealed that severe mononuclear leukocyte infiltration, intra alveolar hemorrhage, leukocyte infiltration, infiltration of mononuclear leukocyte as well as peribronchiolar polymorphonuclear were observed in lungs tissue compared to the sham-operated and control groups after 24 hr renal ischemia-reperfusion and subsequently causes injury and necrosis in lungs tissue. Bilateral nephrectomy has been demonstrated to enhance blood urea nitrogen levels, infiltrate neutrophil into the lung, increase pulmonary inflammatory cytokine expression [neutrophil, interleukin-6, keratinocyte-derived chemokine, chemokine, and TNF-α], and protein leakage in addition to primary increase in both systemic and pulmonary neutrophil elastase activity (5).
Bcl-2 proteins family is another group of proteins that are involved in apoptosis. In one study it has been found that the expression of Bcl-2 was reduced after initiation of reperfusion in the gastrointestinal tract (25). In this study, Bcl-2 protein expressions increased significantly in ischemia-reperfusion of the gastrointestinal tract compared to the controls. Status of the Bcl-2 family proteins regulates the release of cytochrome c from the mitochondria. Bcl-2 protein inhibits apoptosis and may facilitate survival and cell differentiation (25).
In the present study, Bcl-2 expression in lungs ischemia group was decreased compared to sham group and the findings of previous studies showed that one of the factors causing this increases in injury and apoptosis in the lungs is decrease of antiapoptotic Bcl-2 protein expression in these tissuesprobably.
The results of the present study, the renal ischemia-reperfusion on lung development represents injury in lung inrenal ischemia and nephrectomy groups compared to the control. There is a large amount of evidence suggesting that pulmonary cell apoptosis may play a direct role in the pathophysiology of acute lung injury (26). Apoptosis is a mechanism of cell death that increases the risk of infection or injury that is associated with the activation of death signaling pathway. Although excessive cell apoptosis, is observed in a number of diseases, as well as increased apoptosis in epithelial and endothelial cells of lung, and apoptosis in acute lung injury is associated with inflammation (27).
The results of this investigation agree with similar studies that ischemia has been established bilaterally. Campanholle et al showed that expression of TNF-α after bilateral renal ischemiareperfusion has been increased in both lungs and kidneys. The study reported that proinflammatory mediators such as TNF-α and IL-1β in serum significantly increased after renal ischemia reperfusion. Although it appears that IL-6 levels increased after IRI, there is no difference in the level of the sham group which can lead to injury of distant organs such as lungs. In this study we suggested that TNF-α via the thoracic lymph duct of the kidney was transferred to distant organs (23).
Conclusion
Renal ischemia results in distant effects and alterations in lung which are important in morbidity and mortality in clinical point of view. Renal ischemia-reperfusion leads to lung damage via inflammation and necrosis which may be caused by an increase in TNF-α and a decrease in Bcl-2 level.
Acknowledgment
This study was part of the MSc student thesis entitled "effects of renal ischemia reperfusion (IRI) on inflammatory response and apoptosis of lung and heart and cardiac function of the male rat " which has been kindly supported by Tuberculosis & Lung Research Center of Tabriz University of Medical Sciences, Tabriz, Iran.
References
- 1.Wang HE, Muntner P, Chertow GM, Warnock DG. Acute Kidney Injury and Mortality in Hospitalized Patients. Am J Nephrol . 2012;35:349–355. doi: 10.1159/000337487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275:1489–1494. [PubMed] [Google Scholar]
- 4.Li L, Okusa MD. Blocking the Immune respone in ischemic acute kidney injury: the role of adenosine 2A agonists. Nat Clin Pract Nephrol. 2006;2:432–444. doi: 10.1038/ncpneph0238. [DOI] [PubMed] [Google Scholar]
- 5.Ishii T, Doi K, Okamoto K, Imamura M, Dohi M, Yamamoto K, et al. Neutrophil elastase contributes to acute lung injury induced by bilateral nephrectomy. Am J Pathol . 2010;177:1665–1673. doi: 10.2353/ajpath.2010.090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu KD, Matthay MA. Advances in critical care for the nephrologist: acute lung injury/ARDS. Clin J Am Soc Nephrol . 2008;3:578–586. doi: 10.2215/CJN.01630407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus JM. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med . 1995;155:1505–1511. [PubMed] [Google Scholar]
- 8.Dodd-O JM, Hristopoulos M, Scharfstein D, Brower R, Hassoun P, King LS, et al. Interactive effects of mechanical ventilation and kidney health on lung function in an in vivo mouse model. Am J Physiol Lung Cell Mol Physiol . 2009;296:L3–L11. doi: 10.1152/ajplung.00030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18:155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 10.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003; 14:1549–1558. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 11.Molls RR, Savransky V, Liu M, Bevans S, Mehta T, Tuder RM, et al. Keratinocyte-derived chemokine is an early biomarker of ischemic acute kidney injury. Am J Physiol Renal Physiol . 2006;290:F1187–F1193. doi: 10.1152/ajprenal.00342.2005. [DOI] [PubMed] [Google Scholar]
- 12.Zarbock A, Schmolke M, Spieker T, Jurk K, Van Aken H, Singbartl K. Acute uremia but not renal inflammation attenuates aseptic acute lung injury: a critical role for uremic neutrophils. J Am Soc Nephrol. 2006;17:3124–3131. doi: 10.1681/ASN.2006040358. [DOI] [PubMed] [Google Scholar]
- 13.DiMango E, Zar HJ, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal BB. Tumour necrosis factors receptor associated signalling molecules and their role in activation of apoptosis, JNK and NF-kappaB. Ann Rheum Dis . 2000;59 Suppl 1:i6–16. doi: 10.1136/ard.59.suppl_1.i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilney NL, Guttmann RD. Effects of initial ischemia/reperfusion injury on the transplanted kidney. Transplantation . 1997;64:945–947. doi: 10.1097/00007890-199710150-00001. [DOI] [PubMed] [Google Scholar]
- 16.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 17.Baue AE. The horror of autotoxicus and multipleorgan failure. Arch Surg . 1992;127:1451–1462. doi: 10.1001/archsurg.1992.01420120085016. [DOI] [PubMed] [Google Scholar]
- 18.Koike K, Moore EE, Moore FA, Kim FJ, Carl VS, Banerjee A. Gut phospholipase A2 mediates neutrophil priming and lung injury after mesenteric ischemia reperfusion. Am J Physiol . 1995;268:G397–403. doi: 10.1152/ajpgi.1995.268.3.G397. [DOI] [PubMed] [Google Scholar]
- 19.Deng J, Hu X, Yuen PS, Star RA. Alpha-melanocytestimulating hormone inhibits lung injury after renal ischemia/reperfusion. Am J Respir Crit Care Med. 2004;169:749–756. doi: 10.1164/rccm.200303-372OC. [DOI] [PubMed] [Google Scholar]
- 20.Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol . 2003; 14:1549–1558. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 21.K ksoy C, Kuzu MA, Kuzu I, Erg n H, G rhan I. Role of tumour necrosis factor in lung injury caused by intestinal ischaemiareperfusion. Br J Surg . 2001;88:464–468. doi: 10.1046/j.1365-2168.2001.01737.x. [DOI] [PubMed] [Google Scholar]
- 22.Cavriani G, Domingos HV, Soares AL, Trezena AG, Ligeiro-Oliveira AP, Oliveira-Filho RM, et al. Lymphatic system as a path underlying the spread of lung and gut injury after intestinal ischemia/reperfusion in rats. Shock. 2005;23:330–336. doi: 10.1097/01.shk.0000157303.76749.9b. [DOI] [PubMed] [Google Scholar]
- 23.Campanholle G, Landgraf RG, Gon alves GM, Paiva VN, Martins JO, Wang PH, et al. Lung inflammation is induced by renal ischemia and reperfusion injury as part of the systemic inflammatory syndrome. Inflamm Res . 2010;59:861–869. doi: 10.1007/s00011-010-0198-0. [DOI] [PubMed] [Google Scholar]
- 24.Patel BV, Wilson MR, O’Dea K, Takata M. TNFInduced Death Signaling Triggers Alveolar Epithelial Dysfunction in Acute Lung Injury. J Immunol. 2013;190:4274–4282. doi: 10.4049/jimmunol.1202437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao WL, Wang GM, Shi Y, Wu JX, Qi YJ, Zhang JF, et al. Differential expression of Bcl-2 and Bax during gastric ischemia-reperfusion of rats. World J Gastroenterol . 2011;17:1718–1724. doi: 10.3748/wjg.v17.i13.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neff TA, Guo RF, Neff SB, Sarma JV, Speyer CL, Gao H, et al. Relationship of acute lung inflammatory injury to Fas/FasL system. Am J Pathol. 2005;166:685–694. doi: 10.1016/S0002-9440(10)62290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matute-Bello G, Liles WC, Frevert CW, Nakamura M, Ballman K, Vathanaprida C, et al. Recombinant human Fas ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits. Am J Physiol Lung Cell Mol Physiol. 2001;281:L328–L335. doi: 10.1152/ajplung.2001.281.2.L328. [DOI] [PubMed] [Google Scholar]