Abstract
Objective(s):
The present study investigated the antidiabetic and hypolipidemic properties of Dorema aucheri leave hydroalcoholic extract in nicotinamide-streptozotocin induced type 2 diabetic rats.
Materials and Methods:
nicotinamide/streptozotocin-induced diabetic rats were supplemented orally with three different doses of D. aucheri (100, 200 and 400 mg/kg BW) or glibenclamide (0.25 mg/kg) for 4 weeks. Ultimately, blood of animals has taken and glucose, insulin, lipid profiles, SGPT, alkaline phosphatase, SGOT, leptin levels were assayed.
Results:
D. aucheri has highly significant blood glucose lowering effect. Administration of the extract to diabetic rats resulted in a remarkable change in serum lipid profiles, insulin and leptin levels relative to diabetic group. Also the extract reversed back the serum levels of SGPT, alkaline phosphatase and SGOT to near normal in treated diabetic rats.
Conclusion:
D. aucheri could be useful in treatment of diabetes. Moderate dose of D. aucheri (200 mg/kg) was more effective than the others.
Keywords: Diabetes, Dorema aucheri, Insulin, Nicotinamide, Streptozotocin
Introduction
Type 2 diabetes mellitus is a major endocrine disorder and a deadly disease in human beings (1). According to the recent estimations, the prevalence of diabetes in the world, would reach to 552 million people in 2030 (2). This disease is characterized by hyperglycemia, atherosclerosis, and abnormal lipid profile and also induces several chronic complications such as retinopathy, nephropathy, and neuropathy that results in burdening heavy load on these patients andsociety (3). Dyslipidemia, a main risk factor for cardiovascular diseases as well as increase in generation of reactive oxygen species (ROS) and occurrence of oxidative stress which results in destruction of insulin producing β-cells in pancreatic langerhans islets, all have critical role in pathogenesis and progression of diabetes mellitus (4, 5).
Although chemical antidiabetic drugs such as biguanides and sulphonylureas are available at the present time, they might cause several side effects e.g. hypoglycemia, liver toxicity, dyslipidemia and increase in blood pressure (6). Since using of medicinal plants is more financial, and they have different varieties of effective compounds as well as lower side effects in comparison to synthetic drugs and also because of recommendations of World Health Organization (WHO), in recent years, there has been renewed interest in using hypoglycemic traditional plants (7). There are many medicinal herbs which have been recommended for the treatment of diabetes (8).
One of these medicinal plants is Dorema aucheri which is member of Umbelliferous family. This plant grows in the beginning of spring season in cold mountainous regions of Isfahan, Fars, Kohgiluyeh and boyer-ahmad provinces of Iran. The plant is 1-2 meter long, with thick and spindle-shaped root. Its lower leaves have three branches divisions (9). The active components of the plant extract are essence, resin, coumarins, furanocoumarins, different types of terpenes, three terpenoidal saponins, acetylene components, flavenoids and also trace amount of alkaloid (10).
Experimentally the plant has shown to have anal-gesic, anti-inflammatory and antibacterial properties (11). Sadeghi and his colleagues reported that D. aucheri has antihyperlipidaemic and antihyper-cholesterolaemic effects in rats (12). According to the available literature D. aucheri is rich in flavonoids. Flavonoids are poly phenols with several biological activities such as free radical scavenging and reduction of VLDL in plasma (13). Flavonoids are especially, recognized as antidiabetic factors and have beneficial role in treatment of diabetes (14). Furthermore because of their antioxidant properties in pancreatic tissue, they are able to protect the insulin producing β-cells (15).
The present study aims to investigate the antidiabetic and hypolipidemic as well as hepatoprotective potentials of D. aucheri on nicotinamide-streptozotocin (NA-STZ) induced type 2 diabetic rats.
Materials and Methods
Plant material
Fresh leaves of D. aucheri were collected during spring from the mountains of Semirum region in Isfahan province, Iran and confirmed scientifically by department of Botany of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Voucher specimen of the plant is deposited in the Herbarium of Shahid Beheshti University Faculty of Agriculture in number of AR337E. The leaves were air dried and then milled using mechanical grinders.
Preparation of Dorema aucheri leaves hydro-alcoholic extract
Three hundred grams of the obtained powder of D. aucheri leaves was dissolved in 1200 ml of distilled water and ethanol mixture (70-30) and kept for 72 hr at room temperature. The mixture was filtered with whatman paper and then centrifuged for 20 min with speed of 3500 rpm. The supernatant was removed and dried at room temperature. Finally the obtained semisolid mass was freshly used (16).
Experimental animals
Forty-eight adult male Wistar rats (150-250 g) were purchased from animal house of Ahvaz Jundishapur University of Medical Science (AJUMS) and were kept in cages with standard laboratory conditions (temperature 22 ± 2°C with a 12/12 hr light - dark cycle) and were allowed ad libitum access to normal laboratory diet and tap water. All animals' procedures were in accordance with standards guide for the care and use of laboratory animals, established by the National Research Council of the national academic.
Induction of non-insulin dependent diabetes mellitus
For experimental induction of type 2 diabetes mellitus in adult male Wistar rats, after they were fasted overnight, firstly nicotinamide (120 mg/kg bodyweight for each rat) (Merck, Germany) dissolved in normal saline and then was administrated intraperitonealy to them. After 15 min the rats received an IP injection of STZ (60 mg/kg BW) (Sigma Aldrich, USA) dissolved in citrate buffer (pH 4.5). Development of diabetes was confirmed by the assessment of glucose level in blood before and 72 hr after STZ injection. Only those animals which their blood glucose levels were above 250 mg/dl, considered as diabetic and used for study (17).
Experimental design
All animals were randomly divided into six groups (n=8) and treated daily for 4 weeks as follows: Group I: intact control rats were administered normal saline daily for 4 weeks; Group II: diabetic control rats; Group III, IV and V: diabetic rats received D. aucheri leaves hydro-alcoholic extract orally by gastric tube in doses of 100, 200 and 400 mg/kg body weight respectively daily for 4 weeks; group VI: diabetic rats received glibenclamide (0.25 mg/kg bodyweight, Sigma Aldrich, USA) orally for 4 weeks as standard medication. By the end of the experiment, animals were deprived of food overnight (24 hr) and after mild anesthesia by ether, blood sample was directly collected from their hearts and were centrifuged at 3500 rpm for about 20 min to obtain blood serum. Serum samples were refrigerated at -70°C refrigerator until evaluation of insulin and serum biochemical parameters. (18).
The fasting blood glucose levels were measured with Elegance glucometer (CT-X10, Convergent Technologies, Germany) through lateral tail vein of rats in days 0 and 28. Also rats' body weights were analyzed at the beginning and end of the experiment.
Circulating insulin level was evaluated by radioimmunoassay (RIA) (Diosource INS-IRMA Kit). The kit has assay sensitivity of 1 mIU/ml, intra-assay coefficient of variation (CV) of 2.1% and inter-assay CV of 6.5%.
Homeostasis Model Assessment of Insulin Resistance (HOMA - IR) in this study was estimated by the formula fasting blood glucose (mg/dl) x insulin (uIU/ml) /405 (19).
Lipid profile, leptin, serum ALP, SGOT and SGPT levels measurement
The activities of pathophysiological enzymes such as serum ALP, SGOT and SGPT, as well as serum triglyceride (TG) Total Cholesterol (TC), LDL-cholesterol (LDL-c) and HDL-cholesterol (HDL-c) were estimated with Pars Azmoon kits using auto- analyser. VLDL-cholesterol (VLDL-c) concentration according to Norbert formula, equals one fifth of TG content (20). Serum Leptin concentration was measured by ELISA kit (Labor Diagnostika Nord GmbH, Germany). Inter and intra-assay coefficients of variation of it was 5.8 and 4.3 % respectively. Low-end sensitivity of this kit was 0.5 ng/ml.
Statistical analysis
Obtained data were expressed by SPSS software version 16 by the equation mean ± SEM. One-way ANOVA test was used for statistical analysis followed by significant difference (LSD) test. Values of P< 0.05 were considered to be statistically significant.
Results
Effect of D. aucheri on blood glucose and insulin level
After induction of diabetes in wistar rats, the blood glucose concentrations increased and insulin levels decreased significantly (P< 0.001 and P< 0.05 respectively) compared with control group (Table 1). In this study, daily administration of hydro-alcoholic extract of D. aucheri for 4 weeks in all doses, decreased blood glucose concentration (P< 0.001) and increased insulin levels significantly compared with diabetic control group as shown in Table 1. Moreover, the dose of 200 mg/kg body weight of the extract, showed to be able to remarkably decrease in the insulin resistance in comparison with diabetic control group (P< 0.01).
Table 1.
Effect of hydroalcoholic extract of Dorema aucheri leave on insulin, fasting plasma glucose levels and HOMA-IR in nicotinamide/streptozotocin-induced diabetic rats
| Groups | Fasting blood glucose level (mg/dl) | Insulin level (µIU/ml) | HOMA-IR |
|---|---|---|---|
| Control | 114.25 ±3.59 (P< 0.001) | 22.647±6.87 (P< 0.05) | 6.295±2.10 |
| Diabetic | 167.5±8.60 | 11.916±1.21 | 4.875±.4.4 |
| Diabetic + D. aucheri(100 mg/kg) | 121.429±9.13 (P< 0.001) | 25.857±1.63 (P< 0.05) | 7.896±.88 |
| Diabetic + D. aucheri(200m g/kg) | 128.5±3.46 (P< 0.001) | 30.071±1.92 (P< 0.001) | 9.705±0.79 (P< 0.01) |
| Diabetic + D. aucheri(400 mg/kg) | 122.375±5.61 (P< 0.001) | 24.9±3.89 (P< 0.05) | 7.217±0.93 |
| Diabetic + GLB (0.25 mg/kg) | 110.5±5.13 (P< 0.001) | 19.071±1.76 | 5.117±0.36 |
Results are expressed as mean ± SEM. GLB: Glibenclamide
Significant differences are versus diabetic control group
Effect of D. aucheri on lipid profile
As shown in Table 2, after nicotinamid/ strepto-zotocin-induction of diabetes in animals, serum triglyceride, VLDL and LDL levels increaseed significantly (P< 0.05 and P< 0.01) compared to control group. Treatment of diabetic animals with D. aucheri extract reduced TG, VLDL and LDL levels compared to diabetic control group after 28 days of experimental period. Mentioned reductions were significant in doses of 100 and 200 mg/kg body weight D. aucheri extract (Table 2). Also HDL level decreased and total cholesterol level increased in diabetic group compared to control group. Although these variations were not remarkable, but administration of D. aucheri extract in doses of 100 mg/kg bodyweight caused significant decrease in cholesterol level (P< 0.05) and the dose of 200 mg/kg bodyweight significantly increased HDL level compared to diabetic group (P< 0.01). Glibenclamide-treated diabetic rats didn't show any significant statistical difference in lipid profile compared with diabetic group.
Table 2.
Effect of hydroalcoholic extract of Dorema aucheri leave on lipid profile (mg/dl) in nicotinamide/streptozotocin-induced diabetic rats
| Parameters Groups | Total cholesterol | Triglycerides | HDL | VLDL | LDL |
|---|---|---|---|---|---|
| Control | 58.625 ± 3.94 | 91.25 ± 10.04 (P< 0.05) | 55.375 ± 4.64 | 18.25 ± 2 (P<0.05) | 2.387 ± 1.09 (P< 0.01) |
| Diabetic | 67.875 ± 1.99 | 126.125 ± 13.04 | 47 ± 1.70 | 25.22 ± 2.6 | 8.8 ± 1.58 |
| Diabetic + D. aucheri (100 mg/kg) | 57 ± 3.72 (P<0.05) | 66.714 ± 4.15 (P< 0.001) | 47.714 ± 2.37 | 13.34 ± .83 (P< 0.001) | 3.714 ± 1.63 (P< 0.05) |
| Diabetic + D. aucheri (200 mg/kg) | 61.285 ± 3.35 | 95.428 ± 7.28 (P< 0.05) | 59 ± 2.64 (P<0.01) | 19.08 ±1.45 (P<0.05) | 1.1 ± .56 (P< 0.01) |
| Diabetic + D. aucheri (400 mg/kg) | 62.625 ± 3.72 | 104 ± 9.12 | 46.625 ± 3.60 | 20.8 ± 1.82 | 5.6 ± 2.15 |
| Diabetic + GLB (0.25 mg/kg) | 62.666 ± 4.87 | 116 ± 12.54 | 49.666 ± 2.38 | 23.2 ± 2.5 | 3.983 ± 2.75 |
Results are expressed as mean ± SEM. GLB: Glibenclamide, HDL: high-density lipoprotein; VLDL: very low-density lipoprotein; LDL: low-density lipoprotein, Significant differences are versus Diabetic control group
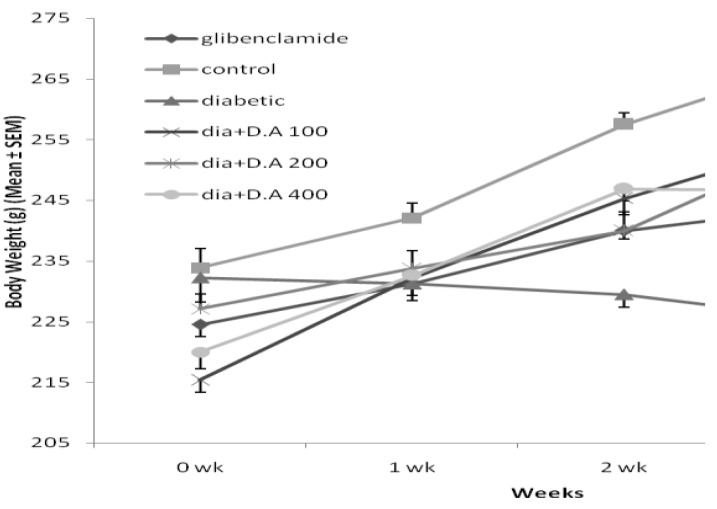
Effect D. aucheri on body weight
As demonstrated in Figure 1, induction of diabetes resulted in significant decrease in body weight of wistar rats compared with control group at the end of experiment (P< 0.01). Administration of D. aucheri extract to diabetic rats improved the body weight of animals at the end of 4 weeks which was found to be significant in dose of 200 mg/kg body weight of D. aucheri extract (P< 0.05).
Figure 1.
Effect of hydro-alcoholic extract of Dorema aucheri leave on body weight during 4 weeks in nicotinamide streptozotocin-induced diabetic rats
b (P< 0.05) and a (P< 0.01)
dia: Diabetic rat
D.A 100: Dorema aucheri leave extract (100 mg/kg)
D.A 200: Dorema aucheri leave extract (200 mg/kg)
D.A 400: Dorema aucheri leave extract (400 mg/kg)
Effect D. aucheri on serum SGPT, SGOT, alkalin phosphatase and leptin level
Table 3 shows the efficacy of D. aucheri extract on serum leptin and hepatic enzymes in diabetic rats. Serum SGOT and alkaline phosphatase levels were significantly altered in STZ-NA induced diabetic rats compared with control rats (P< 0.001). SGPT levels in diabetic rats which treated with Glibenclamide, as well as doses of 200 and 400 mg/kg body weight of D. aucheri extract, were significantly decreased compared with diabetic control group (P< 0.05).
Table 3.
Effect of hydroalcoholic extract of Dorema aucheri leave on Leptin (ng/dl), SGPT, Alkalin phosphatase and SGOT levels in nicotinamide/streptozotocin-induced diabetic rats
| Parameters Groups | Leptin level | SGPT | Alkalin phosphatase | SGOT |
|---|---|---|---|---|
| Control | 1.333 ± .08 | 56.250 ± 9.24 | 117.125 ± 4.50 (P< 0.001) | 87 ± 4.34 (P< 0.001) |
| Diabetic | 1 ± 00 | 67 ± 11.40 | 139.250 ± 3.80 | 126.625 ± 10.97 |
| Diabetic + D. aucheri (100 mg/kg) | .933± .42 | 54.571 ± 7.65 | 123.875 ± 2.13 (P< 0.05) | 105.625 ± 2.39 (P< 0.05) |
| Diabetic + D. aucheri (200 mg/kg) | 1.496± .80 (P< 0.05) | 37.714 ± 2.74 (P< 0.05) | 112 ± 3.53 (P< 0.001) | 86.625 ± 2.47 (P< 0.001) |
| Diabetic + D. aucheri (400 mg/kg) | 1.166± .13 | 48.125 ± 6.01 (P< 0.05) | 130.625 ± 5.04 | 110.75 ± 5.96 |
| Diabetic + GLB (0.25 mg/kg) | 1.125 ± .87 | 39.833 ± 4.08 (P< 0.05) | 120.375 ± 3.74 (P< 0.01) | 101.5 ± 6.10 (P< 0.01) |
In diabetic rats, administration of doses of 100 and 200 mg/kg body weight of D. aucheri extract and Glibenclamide, significantly reduced SGOT and alkaline phosphatase levels compared with diabetic control rats (P< 0.05, P< 0.001 and P< 0.01 respectively) dose of 200 mg/kg body weight had better effect compared with the dose of 100 mg/kg body weight. Only administration of the doses 200 mg/kg body weight of D. aucheri extract showed significant (P< 0.05) increase in leptin levels compared with diabetic control rats.
Discussion
Type 2 diabetes, the most common metabolic syndrome, is a heterogeneous disease that is a heavy burden on individuals and families (21). In this study, we used the STZ- nicotinamide for induction of type 2 diabetes which is widely used in experimental studies by other researchers (17). Streptozotocin (STZ) is a nitrosourea compound which is generated by Streptomyces achromogenes and specifically effect on insulin producing β-cells through free radical production and DNA strand breakage in these cells which results in insulin deficiency and induction of diabetes mellitus (22).
Also, nicotinamide by its free radical scavengering property and its antioxidant activity protects pancreatic β-cells against cytotoxic activity of STZ. Thus STZ was able to exert only minor damage in these cells (23).
In this research, remarkable increase in blood glucose and reduction in insulin levels were observed in STZ/NA- induced diabetic rats compared with the control group.
Since the oxidative stress causes destruction in pancreatic β-cells and diminishes their insulin producing function, thereby insulin secretion decreases consequently (5). On the other hand blood glucose level will increase subsequently after decrement of blood insulin level. These changes are visible in diabetic patients (24). Insulin level in type 2 diabetic rats in this study and other similar experiment, imply that some insulin secretary beta cells are intact and still have the potential of insulin synthesis and secretion. Therefore, this model has found to be an advantageous tool for investigation of antioxidants and insulinotropic agents for treatment of type 2 diabetes (23). Therefore, we used D. aucheri leave extract for this purpose. Our data showed that treatment with the D. aucheri extract in diabetic rats significantly corrected hyperglycemia and insulin deficiency in them.
Several researchers have noted that flavonoids are the important ingredient in D. aucheri (14). Song and his colleagues reported that flavonoids, which have antioxidant and antidiabetic properties, are able to protect the β-cells function through free radical scavengering in pancreatic tissue (15). D. aucheri extract is able to decline oxidative stress in beta cells and improve their function and this probably is because of the flavonoides presence in this plant. So the serum level of insulin increases which leads to blood sugar reduction. Our previous study have showed that the hydro-alcoholic extracts of D. aucheri improved sperm production process and increase sperm counts in Nicotinamide-STZ- induced diabetic rats. This positive effect may originate from the presence of flavonoids as an antioxidant agent in D. aucheri plant (25).
Lee and his colleagues reported that sulfonylurea through increasing the PPARγ transcriptional activity, improves insulin sensitivity along with reduction in blood glucose (26). In fact, PPARγ is an important regulator of glucose homeostasis. Kanda and his colleagues showed that administration of PPARγ agonist in animal diabetic model, resulted in protection of beta cell mass against oxidative stress and apoptosis (27). Data of our experiment showed that glibenclamide probably reduced blood glucose in diabetic rats significantly which could happen through enhancing PPARγ transcriptional activity. However its confirmation requires more investigations.
In consistent with the previous report (28), our findings showed that the average body weight was significantly decreased in diabetic rats. In fact, STZ induced diabetes is associated with loss of body weight due to excessive breakdown of tissue proteins and wasting muscle mass because of insulin deficiency (29). Treatment of diabetic rats with the plant extract, leads to increase in body weight in comparison to diabetic control rats, probably due to the enhancing effect of extract on insulin secretion and thereby inhibition of muscle mass wasting.
In our study after induction of diabetes by STZ, a marked elevation in serum cholesterol, LDL, VLDL and triglycerides occurred in rats which are in agreement with Watcho et al. findings (30). Significant reduction in serum lipid levels in diabetic rats that received the extract, probably caused as a result of insulin levels increment, which is in agreement with the findings of Sadeghi and his colleagues. (12). It is well recognized that hyperlipidemia occurs in diabetic patients. In fact when insulin level decreased, the failure of lipoprotein lipase activation happens which increases lipolysis cycle (23), and leads to hypertriglyceridemia and increase in plasma free fatty acid concentration (31).
Reduction in SGPT, SGOT and alkaline phosphatase levels in treatments groups implied that D. aucheri extract had protective effect on liver function. Previous studies indicated that flavonoides are potent suppressor of nitric oxide synthase (NOS) (32). It has been proved previously that those components which are able to decrease the generation of NO in the liver, have hepatoprotective effects (33). Thus it was assumed that the hepatoprotective effect of D. aucheri extract is mainly associated to the presence of flavonoides in this plant. As liver is an important organ involved in carbohydrate metabolism, the positive effect of the D. aucheri on liver function may be associated with its antihyperglycemic characteristic.
In our study, D. aucheri extract and glibenclamide increased the leptin level which has decreased in diabetic rats. Reduction of leptin level in diabetic rats had been previously reported (34) and it may be due to the reduction of uptake and metabolism of glucose in adipose tissue. Since insulin is able to induce the glucose uptake and oxidation in adipocytes, it could cause elevation of leptin level and the extract effect on maintaining leptin normal level in diabetic rats may attribute to its beneficial effect on bate cells for insulin secretion (35).
Conclusion
The data of our study suggest that D. aucheri leave extract has antihyperglycemic effects and also has optimal effects on improvement of lipid profiles levels and hepatic enzymes activities of diabetic rats and could be useful in treatment of diabetes mellitus as mentioned in traditional medicine. However, comprehensive pharmacological and chemical experiments are required to discover the active component(s) responsible for these effects.
Acknowledgment
This study is financially supported by Vice Chancellor of Research Affairs of Ahvaz Jundishapur University of Medical Sciences (D-9103). The authors also thank the Health Research institute, Diabetes Research Center of AJUMS experienced personnel.
Declaration of interest
The authors had no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Zare K, Fatemi Tabatabaei SR, Shahriari A, Jafari RA. The effect of butter oil on avoidance memory in normal and diabetic rats. Iran J Basic Med Sci . 2012; 15:983–989. [PMC free article] [PubMed] [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract . 2011; 94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Perez FJ, Aguilar-Salinas CA, Almeda-Valdes P, Cuevas-Ramos D, Lerman Garber I, Rull JA. HbA1c for the diagnosis of diabetes mellitus in a developing country. Arch Med Res . 2010; 4:302–308. doi: 10.1016/j.arcmed.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Ghorbani A. Phytotherapy for diabetic dyslipi-demia: evidence from clinical trials. Clin Lipidol . 2013; 8:311–319. [Google Scholar]
- 5.Yilmaz O, Ersan Y, Dilek Ozsahin A, Ozturk AI, Ozkan Y. Consequences of the combined α-tocopherol, ascorbic acid and α-lipoic acid on the glutathione, cholesterol and fatty acid composition in muscle and liver of diabetic rats. Iran J Basic Med Sci. 2013; 16:165–172. [PMC free article] [PubMed] [Google Scholar]
- 6.Chander AP, Reddy RRA, Puchchakayala G. Hypoglycemic and antidiabetic activity of glochidion velutinum on streptozotocin-nicotinamide induced Type 2 diabetic rats. Eur J Biol Sci. 2011; 3:126–130. [Google Scholar]
- 7.Kumar S, Rashmi N, Kumar D. Evaluation of antidiabetic activity of Euphorbia hirta Linn in streptozotocin induced induced diabetic mice. Indian J Nat Prod Resour. 2010; 1:200–203. [Google Scholar]
- 8.Ghorbani A. Best herbs for managing diabetes: A review of clinical studies. Braz J Pharm Sci . 2013; 49:413–422. [Google Scholar]
- 9.Zargari A. Pharmaceutical plants (persian) 7th ed. Tehran: Tehran University Press; 1997. pp. 212–214. [Google Scholar]
- 10.Dashti GR, Teimourinejad A, Salehi M, Sajadi SE, Torabinia N. The effect of hydroalcoholic extract of Dorema aucheri on CD40 protein expression in thoracic aorta of male white rabbits fed with hypercholesterolemic diet. J Isfahan Med Sci. 2012; 29:1–11. [Google Scholar]
- 11.Mokhtari M, Sharifi S, Parang A. Effect of alcoholic extract of Dorema auchrei on homogram in adult male rats. ZUMS J . 2008; 16:37–44. [Google Scholar]
- 12.Sadeghi H, Mirzaee A, Delaviz H. Antihyper-lipidaemic and anti-hypercholesterolaemic effects of Dorema aucheri. Iran J Pharm Res. 2004; 3:52–59. [Google Scholar]
- 13.Roghani M, Baluchnejadmojarad T, Vaez- Mahdavi MR, Roghani-Dehkordi F. Mechanisms underlying quercetin-induced vasorelaxation in aorta of subchronic diabetic rats: an in vitro study. Vascul Pharmacol . 2004; 42:31–35. doi: 10.1016/j.vph.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Brahmachari G. 6. Bio-flavonoids with promising antidiabetic potentials: A critical survey. Opportunity, Challenge and Scope of Natural Products in Medicinal Chemistry. Res Signpost . 2011; 2: 2187–212. [Google Scholar]
- 15.Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and sstemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr . 2005; 24:376–384. doi: 10.1080/07315724.2005.10719488. [DOI] [PubMed] [Google Scholar]
- 16.Ahangarpour A, Mohammadian M, Mahin D. Antidiabetic effect of hydroalcoholic urtica dioica leaf extract in male rats with fructose-induced insulin resistance. Iran J Med Sci . 2012; 37:181–186. [PMC free article] [PubMed] [Google Scholar]
- 17.Shirwaikar A, Rajendran K, Punitha ISR. Antidiabetic activity of alcoholic stem extract of Coscinium fenestratum in streptozotocin-nicotinamide induced type 2 diabetic rats. J Ethnopharmacol . 2005; 97:369–374. doi: 10.1016/j.jep.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 18.Zamami Y, Takatori S, Goda M, Koyama T, Iwatani Y, Jin X, et al. Royal jelly ameliorates insulin resistance in fructose-drinking rats. Biol Pharm Bull . 2008; 31:2103–2107. doi: 10.1248/bpb.31.2103. [DOI] [PubMed] [Google Scholar]
- 19.Shahgholiabasi R, Iranshahi F, Seyedhoseini MA, Farbod M. Increased IL-6 and is associated with decreased glucose concentration during cycling exercise. J Bus Econ Stat. 2012; 2:40–46. [Google Scholar]
- 20.Mousavi SE, Shahriari A, Ahangarpour A, Vatanpour H, Jolodar A. Effects of teucrium polium ethyl acetate extract on serum, liver and muscle triglyceride content of sucrose-induced insulin resistance in rat. Iran J Pharm Res . 2012; 11:347–355. [PMC free article] [PubMed] [Google Scholar]
- 21.Afkhami M, Rashidi M. Type 2 diabetes risk factors. J Rafsanjan Med Sci Univ . 2005; 4:348–365. [Persian] [Google Scholar]
- 22.Kumar S, Kumar V, Prakash O. Antidiabetic and anti-lipemic effects of Cassia siamea leaves extract in streptozotocin induced diabetic rats. Asian Pac J Trop Med . 2010; 3:871–873. [Google Scholar]
- 23.Kumar R, Patel D K, Prasad SK, Laloo D, Krishnamurthy S, Hemalatha S. Type 2 antidiabetic activity of bergenin from the roots of Caesalpinia digyna Rottler. Fitoterapia . 2012; 83:395–401. doi: 10.1016/j.fitote.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Bruce MK, Bruce AS. Berne & Levy Physiology. Updated sixth Edition. Philadelphia: Mosby Elsevier; 2010. pp. 710–711. [Google Scholar]
- 25.Ahangarpour A, Oroojan AA, Heydari H. Effect of hydro-alcoholic extract of Dorema aucheri on serum levels of testosterone, FSH and sperm count in nicotinamide-STZ- induced diabetic rat models. ZUMS J . 2013; 21:22–31. [Persian] [Google Scholar]
- 26.Lee KW, Ku YH, Kim M, Ahn BY, Chung SS, Park KS. Effects of sulfonylureas on peroxisome proliferator-activated receptor γ activity and on glucose uptake by thiazolidinediones. Diabetes Metab J . 2011; 35:340–347. doi: 10.4093/dmj.2011.35.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanda Y, Shimoda M, Hamamoto S, Tawaramoto K, Kawasaki F, Hashiramoto M, et al. Molecular mechanism by which pioglitazone preserves pancreatic beta-cells in obese diabetic mice: evidence foracute and chronic actions as a PPARgamma agonist. Am J Physiol Endocrinol Metab . 2010; 298:278–286. doi: 10.1152/ajpendo.00388.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghamarian A, Abdollahi M, Xiaogang S, Amiri A, Ahadi A, Nowrouzi A. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. Daru . 2012; 20:2–9. doi: 10.1186/2008-2231-20-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rangachari B, Savarimuthu I. Antidiabetic and hypolipidemic effect of methanol extract of Lippia nodiflora L. in streptozoto induced diabetic rats. Asian Pac J Trop Biomed. 2012; 1:1–7. [Google Scholar]
- 30.Watcho P, Gildas AJ, Ulrich MC, Modeste WN, Benoît NT, Kamanyi A. Hypoglycemic and hypolipidemic effects of Bersama engleriana leaves in nicotinamide/streptozotocin-induced type 2 diabetic rats. BMC Complement Altern Med . 2012; 26:1–6. doi: 10.1186/1472-6882-12-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirwaikar A, Rajendran K, Dinesh Kumar C, Bodla R. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin nicotinamide type 2 diabetic rats. J Ethnopharmacol . 2004; 91:171–175. doi: 10.1016/j.jep.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Toker G, Kupeli E, Memisoglu M, Yesilada E. Flavonoids with antinoceceptive and anti-inflammatory activities from the leaves of Tilia argentea (silver linden) J Ethnopharmacol. 2004; 95:393–397. doi: 10.1016/j.jep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Majano PL, Medina J, Zubia I, Sunyer L, Lara-Pezzi E, Maldonado-Rodriguez A, et al. N-Acetyl-cysteine modulates inducible nitric oxide synthase gene expression in human hepatocytes. J Hepatol . 2004; 40:632–637. doi: 10.1016/j.jhep.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Andaloussi AB, Louis M, Vuong T, Meddah B, Madiraju P, Settaf A, et al. The in vivo antidiabetic activity of nigella sativa is mediated through activation of the AMPK pathway and increasedMuscle Glut4 content. Evid Based Complement Alternat Med . 2011; 538671:1–9. doi: 10.1155/2011/538671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X-F, Benny K-H. Antidiabetic property of ethanolic extract of Andrographis paniculata in streptozotocin-diabetic rat. Acta Pharmacol Sin. 2000; 21:1157–1164. [PubMed] [Google Scholar]