Abstract
Background
Season of birth (SoB) has been linked with various health outcomes. This study aimed to examine the associations between month of birth (MoB) and adult measures of leg length (LL), body mass index (BMI), and waist circumference (WC).
Methods
We analysed survey data from 10 geographically diverse areas of China obtained through the China Kadoorie Biobank. Analysis included 487 529 adults with BMI ≥ 18.5 kg/m2. A general linear model was used to examine the associations between MoB and adult measures of LL, BMI, and WC, adjusted for survey site, sex, age, education level, smoking habit, alcohol consumption, physical activity level, sedentary leisure time, height (only for WC and LL), and hip circumference (only for LL).
Results
MoB was independently associated with both BMI and WC. Birth months in which participants had higher measures of adiposity were March–July for BMI and March–June for WC. The peak differences were 0.14 kg/m2 for BMI and 0.47 cm for WC. The association between MoB and LL depended on survey site. Participants who were born in February–August in four sites (Harbin, Henan, Gansu, and Hunan) had the shortest LL (all P < 0.01). The peak difference in mean LL was 0.21 cm. No statistically significant association between MoB and LL was noted in the other sites (Qingdao, Suzhou, Sichuan, Zhejiang, Liuzhou, and Haikou).
Conclusions
These findings suggest that MoB is associated with variations in adult adiposity measures and LL among Chinese adults. Low exposure to ultraviolet B radiation and subsequent reduced levels of vitamin D during the late second and early third trimesters may be involved in these phenomena.
Key words: month of birth, BMI, waist circumference, leg length, vitamin D deficiency
INTRODUCTION
Obesity is a serious public health issue globally,1 and China is no exception.2,3 Besides recognition of established adult lifestyle behaviours and their environmental determinants,1 much attention is being focused on the developmental origins of adult obesity.4,5 There is increasing evidence that early life conditions, beginning with the intrauterine environment and continuing through the first few years of life, have long-term impacts on later health.6,7 Leg length (LL)—both in terms of absolute size and relative to total stature—has often been used as an anthropometric marker of the quality of the environment in early life in studies testing the hypothesis that early-life exposure to certain factors may have long-term impact on adult health.8–10 The general reasoning is that the vital organs of the head, thorax, and abdomen are protected from adversity at the expense of the less vital tissues of the limbs.9 LL has been linked to maternal smoking during pregnancy,11,12 birth weight,11 breastfeeding,13 energy intake at 4 years,13 and socioeconomic adversity in childhood.11 However, there remains considerable uncertainty about the adverse exposures during the prenatal and childhood periods, which may lead to shorter LL.
Season of birth (SoB) is a well-defined variable indicating various environmental factors in early life. These factors include not only meteorological factors and sunlight exposure, but also alterations in food supply and eating habits, energy expenditure (eg, outdoor physical activity and work load), air pollution, and exposure to infectious agents.14–17 If factors responsible for programming early in life change seasonally, then it stands to reason that individuals who are born in various seasons are influenced by those factors differently. Therefore, the SoB may be related to the later-life phenotype.15 To our knowledge, no study has examined the relationship between the SoB and LL. Only a few studies have examined the relationship between the SoB and adult adiposity in the population of Western countries, and their findings and explanations of underlying mechanism were mixed.18–22 No study has reported on the association between SoB and adult adiposity in the Chinese population.
Here, we analysed the baseline survey data from the China Kadoorie Biobank (CKB) of 0.5 million adults recruited from 10 geographically diverse areas of China.23,24 The main aim of the current study was to examine the associations between the month of birth (MoB) and adult measures of LL and general (body mass index [BMI]) and central adiposity (waist circumference [WC]).
METHODS
Study design and participants
Details of the CKB study design and characteristics of the study participants have been described elsewhere.23,24 Briefly, 512 891 participants aged 30–79 years were recruited between 2004 and 2008 from 5 urban and 5 rural areas of China (see eTable 1 for geographic coordinates and climate characteristics of the 10 survey sites and eFigure 1 for locations of the 10 survey sites). Selection of the survey sites was based on local patterns of disease and exposure to certain risk factors, population stability, quality of death and disease registries, and local commitment and capacity. Within each area, permanent residents without major disabilities in each of the 100–150 administrative units (rural villages or urban residential committees) that were selected for the study were identified from local records and sent a letter and leaflet inviting them to participate. The participation rate was 33% in rural areas and 27% in urban areas, and the main reasons for non-participation (reported anecdotally by field staff) were absence from the home and reluctance to spend time visiting the screening centre.
Ethical approval for the CKB study was obtained from the Ethics Review Committee of the Chinese Center for Disease Control and Prevention (Beijing, China) and the Oxford Tropical Research Ethics Committee, University of Oxford (UK). In addition, approvals were issued by the institutional research boards at the local Center for Disease Control and Prevention at each of the 10 survey sites. Finally, written informed consent was obtained from participants.
Measures and variables
At the baseline survey, trained interviewers administered a standardised questionnaire using a laptop-based direct data-entry system, with built-in functions to prevent logical errors and missing items. The questionnaire included detailed questions on socio-demographic status, medical history and health status, smoking habit, alcohol consumption, diet, physical activity, and other lifestyle behaviours. The physical measurement procedures were standardised across the 10 survey sites, and all physical measurements were conducted by trained staff using a standard protocol and instruments. All of the utilised devices were regularly maintained and calibrated to ensure consistency in measurements.
The predictor variable of interest in this study was MoB. The outcome variables were BMI, WC, and LL. The participants did not wear shoes during their height and weight measurement. Standing height and sitting height (length of the body from buttocks to the crown of the head) were measured to the nearest 0.1 cm using a manufactured instrument. LL was calculated as the difference between standing and sitting height. Weight was measured to the nearest 0.1 kg using a TANITA TBF-300GS body composition analyser (Tanita Corp., Tokyo, Japan). BMI was calculated as weight in kilograms divided by the square of the standing height in metres. WC was measured midway between the iliac crest and the lower rib margin at the end of normal expiration, and hip circumference (HC) was measured at the widest level over the greater trochanters using a plastic flexible tape to the nearest 0.1 cm.
Other covariates included survey site, sex, age, the highest education completed (no formal school, primary school, middle school, high school, or college/university), smoking habit (never, occasional, former, or current regular), alcohol consumption (never, occasional, former, or current regular),25 total physical activity in metabolic equivalent hours per day (MET-hours/day),26 and sedentary leisure time (hours/week).26
Statistical analyses
The current analysis included participants who had a weight between 30 kg and 160 kg, a height between 145 cm and 200 cm for males or between 140 cm and 200 cm for females, a BMI between 18.5 kg/m2 and 45.0 kg/m2, and a WC between 50 cm and 150 cm. A total of 487 529 participants remained in this analysis. A general linear model was used to test for differences in mean BMI, WC, and LL between 12 MoB groups, adjusting for survey site, sex, age, education, smoking habit, alcohol consumption, amount of physical activity, and sedentary leisure time. Analyses using WC or LL as outcome measures were additionally adjusted for height. Further, considering that a thicker gluteo-femoral fat thickness will increase sitting height and artificially decrease LL,27 we additionally adjusted for HC in the analysis of LL. Bonferroni’s method was used to adjust the P-values for multiple comparisons. The presence of interactions of MoB with sex and survey site was also tested. All of the statistical analyses were performed using Stata version 13.1 (StataCorp LP., College Station, TX, USA).28
RESULTS
Overall, of the 487 529 participants included in the analysis, 200 529 (41.1%) were men, and 268 962 (55.2%) resided in rural areas. At the time of the survey, the mean age was 51.3 ± 10.6 years. More men (58.7%) than women (44.1%) finished middle school or above. Both the prevalence of current smoking (60.6% compared with 2.2%) and of drinking (33.7% compared with 2.0%) were higher among men than women. Men were also less physically active (25.3 compared with 26.8 MET-hours/day) and spent more time on leisure-time sedentary behaviors (21.8 compared with 20.8 hours/week) than women. Women had slightly higher BMI (24.1 compared with 23.7 kg/m2) and larger HC (91.6 compared with 91.1 cm) but lower WC (79.8 compared with 82.7 cm) and shorter LL (71.0 compared with 76.9 cm) than men (Table 1).
Table 1. Main characteristics of study participants by sex.
| Men (n = 200 529) |
Women (n = 287 000) |
P for gender difference |
|
| Age, years (mean ± SD) | 52.1 ± 10.8 | 50.7 ± 10.3 | <0.001 |
| Education (%) | <0.001 | ||
| Illiterate | 8.5 | 24.7 | |
| Elementary | 32.8 | 31.2 | |
| Middle school | 32.8 | 25.8 | |
| High school | 17.8 | 13.8 | |
| College and above | 8.1 | 4.5 | |
| Tobacco use (%) | <0.001 | ||
| Nonsmoker | 14.5 | 95.2 | |
| Occasional smoker | 11.4 | 1.8 | |
| Former smoker | 13.4 | 0.8 | |
| Regular smoker | 60.6 | 2.2 | |
| Alcohol use (%) | <0.001 | ||
| Nondrinker | 19.7 | 63.0 | |
| Occasional drinker | 38.0 | 34.1 | |
| Former drinker | 8.7 | 0.9 | |
| Regular drinker | 33.7 | 2.0 | |
| Physical activity, MET-hours/daya (mean ± SD) | 25.3 ± 12.0 | 26.8 ± 10.3 | <0.001 |
| Sedentary leisure time, hours/weeka (mean ± SD) | 21.8 ± 10.7 | 20.8 ± 10.9 | <0.001 |
| Height, cma (mean ± SD) | 165.3 ± 6.4 | 154.4 ± 5.7 | <0.001 |
| LL, cma (mean ± SD) | 76.9 ± 4.0 | 71.0 ± 3.6 | <0.001 |
| Weight, kga (mean ± SD) | 65.0 ± 10.4 | 57.5 ± 8.9 | <0.001 |
| BMI, kg/m2 a (mean ± SD) | 23.7 ± 3.1 | 24.1 ± 3.2 | <0.001 |
| WC, cma (mean ± SD) | 82.7 ± 9.4 | 79.8 ± 9.1 | <0.001 |
| HC, cma (mean ± SD) | 91.1 ± 6.6 | 91.6 ± 6.6 | <0.001 |
MET-hours/day, metabolic equivalent hours per day; LL, leg length; BMI, body mass index; WC, waist circumference; HC, hip circumference.
aP-values for gender difference were adjusted for age and survey site.
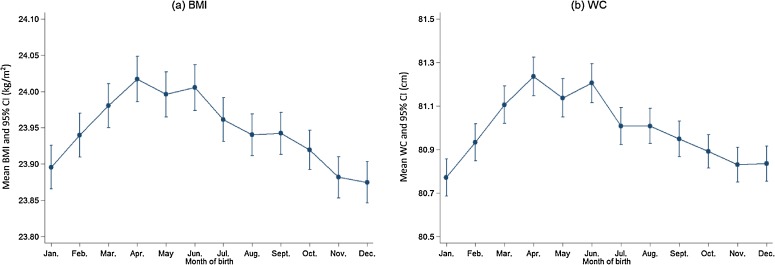
MoB was independently associated with both BMI (F = 9.70, P < 0.001) and WC (F = 12.05, P < 0.001) after accounting for survey site, sex, age, education, smoking, alcohol use, physical activity, sedentary leisure time, and height (adjusting only for WC). Associations of MoB with BMI and WC were independent of gender (P for interaction = 0.725 for BMI and 0.459 for WC) and survey site (P for interaction = 0.080 for BMI and 0.115 for WC). Adjusted mean BMI and WC by MoB are presented in Table 2 and Figure 1. After correcting for multiple comparisons, participants who were born in March–July had higher BMI than those who were born in other months. The maximum difference in mean BMI across twelve months was 0.14 kg/m2 (maximum: April, 24.02 kg/m2; minimum: December, 23.87 kg/m2). A similar trend was also noted for WC. Participants who were born in March–June had larger WC than those who were born in other months. The maximum difference in mean WC across 12 months was 0.47 cm (maximum: April, 81.24 cm; minimum: January, 80.77 cm). Adjusted prevalence of general and central adiposity by MoB presented similar trends (Table 2).
Table 2. Means of BMI and WC and prevalence of general and central adiposity by MoB among study participants.
| MoB | n | BMI (kg/m2) | WC (cm) | |||||||||||||||
| 24.0 to <27.9 (%)a |
≥28.0 (%)a |
Meana | (SE) | Bonferroni-corrected multiple comparisonb |
Men ≥85 Women ≥80 (%)a |
Meana | (SE) | Bonferroni-corrected multiple comparisonb |
||||||||||
| Jan. | 38 987 | 34.0 | 10.9 | 23.90 | (0.02) | A | B | 43.1 | 80.77 | (0.04) | A | |||||||
| Feb. | 38 850 | 34.8 | 11.0 | 23.94 | (0.02) | A | B | C | D | 43.7 | 80.93 | (0.04) | A | B | C | |||
| Mar. | 38 743 | 35.3 | 11.2 | 23.98 | (0.02) | C | D | E | 44.5 | 81.11 | (0.04) | C | D | E | ||||
| Apr. | 36 424 | 35.1 | 11.7 | 24.02 | (0.02) | E | 45.2 | 81.24 | (0.05) | E | ||||||||
| May | 36 677 | 34.8 | 11.6 | 24.00 | (0.02) | D | E | 44.5 | 81.14 | (0.05) | C | D | E | |||||
| Jun. | 35 638 | 35.2 | 11.5 | 24.01 | (0.02) | D | E | 45.0 | 81.21 | (0.05) | D | E | ||||||
| Jul. | 39 504 | 34.8 | 11.3 | 23.96 | (0.02) | B | C | D | E | 44.1 | 81.01 | (0.04) | B | C | D | |||
| Aug. | 43 111 | 34.7 | 11.1 | 23.94 | (0.01) | A | B | C | D | 43.9 | 81.01 | (0.04) | B | C | D | |||
| Sept. | 42 780 | 34.6 | 11.0 | 23.94 | (0.01) | A | B | C | D | 43.6 | 80.95 | (0.04) | A | B | C | |||
| Oct. | 48 669 | 34.7 | 10.9 | 23.92 | (0.01) | A | B | C | 43.6 | 80.89 | (0.04) | A | B | |||||
| Nov. | 44 086 | 34.6 | 10.5 | 23.88 | (0.01) | A | 43.3 | 80.83 | (0.04) | A | B | |||||||
| Dec. | 44 060 | 34.4 | 10.6 | 23.87 | (0.01) | A | 43.5 | 80.83 | (0.04) | A | B | |||||||
BMI, body mass index; WC, waist circumference; MoB, month of birth; SE, standard error.
aPrevalences and means adjusted for survey sites, sex, age, education, smoking, alcohol use, physical activity, sedentary leisure time, and height (for WC only).
bMeans sharing the same letter were not significantly different at Bonferroni-corrected level of significance.
Figure 1. Adjusted means of BMI and WC with 95% CI by month of birth among study participants.
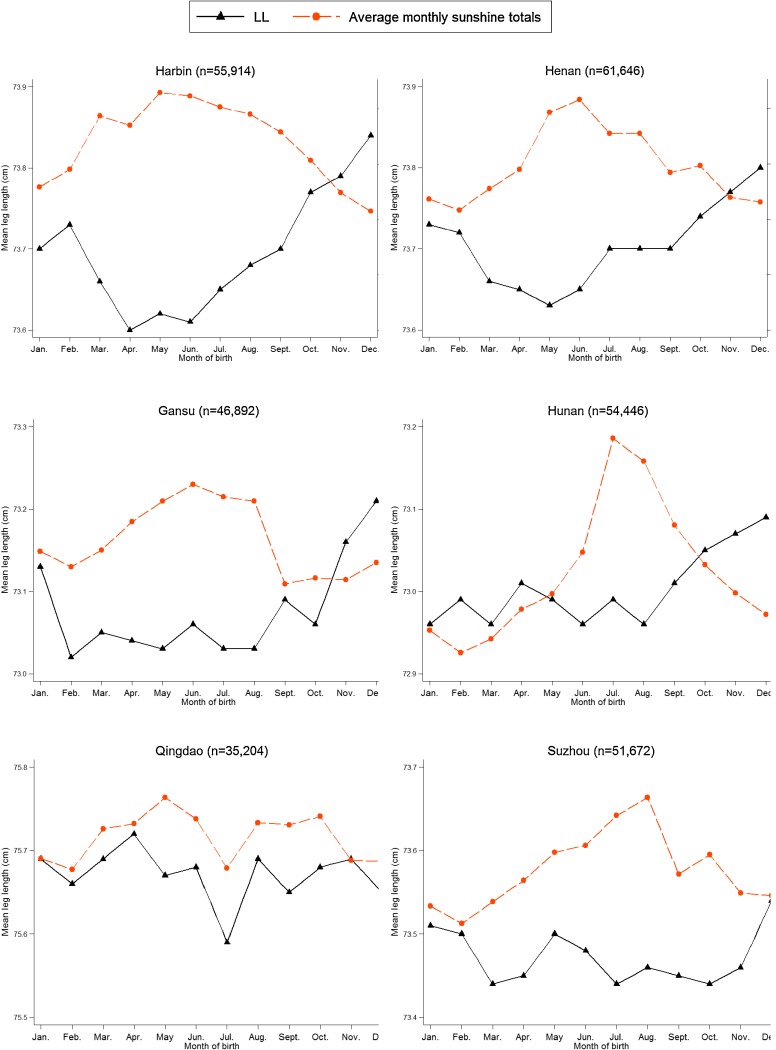
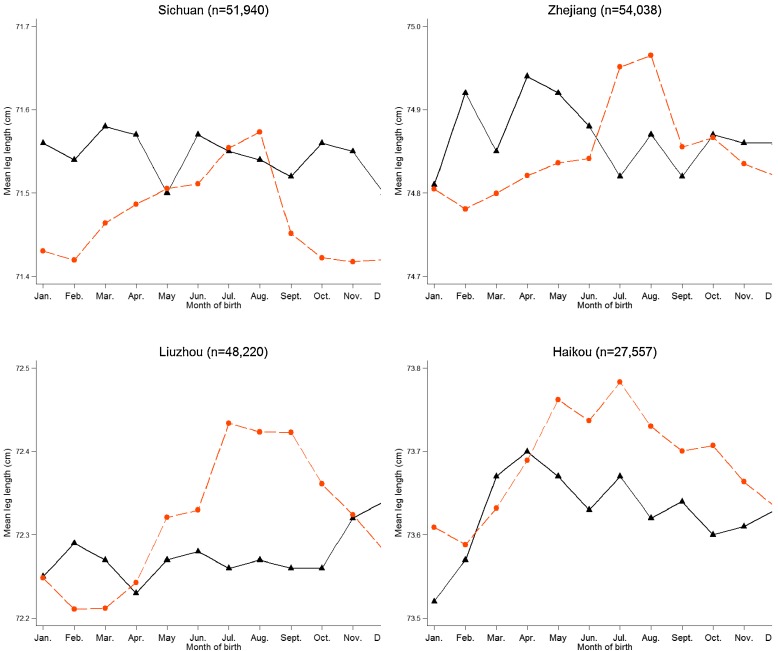
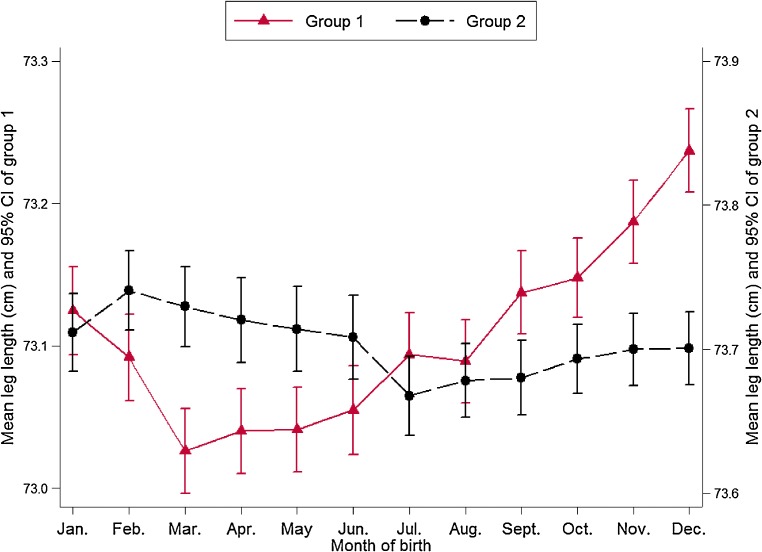
Association of MoB with LL was independent of gender (P for interaction = 0.854) but dependent on survey site (P for interaction < 0.001), after adjusting for survey site, sex, age, education level, smoking habit, alcohol consumption, physical activity amount, sedentary leisure time, height, and HC. MoB was independently associated with LL among participants in Harbin (P < 0.001), Henan (P < 0.001), Gansu (P < 0.001), and Hunan (P = 0.006) (Figure 2). These four survey sites showed similar seasonal variations in LL; participants who were born at the end of the year had longer LL. No statistically significant association between MoB and LL was detected in the other six survey sites. The means of LL by MoB, stratified by whether or not the survey sites showed seasonal variation in LL, are presented in Table 3 and Figure 3. Group 1, including participants from Harbin, Henan, Gansu, and Hunan, showed statistically significant variation in LL associated with MoB. Participants who were born in February–August had shorter LL, and those who were born in November–December had longer LL. The maximum difference in mean LL across twelve months was 0.21 cm (maximum: December, 73.24 cm; minimum: March, 73.03 cm). No statistically significant association between MoB and LL was found in group 2, which included participants from Qingdao, Suzhou, Sichuan, Zhejiang, Liuzhou, and Haikou.
Figure 2. Monthly variation in mean leg length (cm) by survey site, with average monthly sunshine totals as reference. The sunshine data were collected by the weather station nearest to each survey site from 1951 to 1980, which were made available through the Database for Climate Resources (http://www.data.ac.cn). The average monthly sunshine total was used to measure the duration of sunshine in a month and was expressed as an average of 30 years from 1951 to 1980.
Table 3. Mean leg length of two groups of survey sitesa by MoB among study participants.
| MoB | Group 1 (n = 218 898) | Group 2 (n = 268 631) | ||||||||
| Mean LL, cmb |
(SE) | Bonferroni-corrected multiple comparisonc |
Mean LL, cmb |
(SE) | Bonferroni-corrected multiple comparisonc |
|||||
| Jan. | 73.12 | (0.02) | B | C | D | 73.71 | (0.01) | F | ||
| Feb. | 73.09 | (0.02) | A | B | C | 73.74 | (0.01) | F | ||
| Mar. | 73.03 | (0.02) | A | 73.73 | (0.01) | F | ||||
| Apr. | 73.04 | (0.02) | A | 73.72 | (0.02) | F | ||||
| May | 73.04 | (0.02) | A | 73.71 | (0.01) | F | ||||
| Jun. | 73.05 | (0.02) | A | B | 73.71 | (0.01) | F | |||
| Jul. | 73.09 | (0.02) | A | B | C | 73.67 | (0.01) | F | ||
| Aug. | 73.09 | (0.01) | A | B | C | 73.68 | (0.01) | F | ||
| Sept. | 73.14 | (0.01) | C | D | 73.68 | (0.01) | F | |||
| Oct. | 73.15 | (0.01) | C | D | 73.69 | (0.01) | F | |||
| Nov. | 73.19 | (0.01) | D | E | 73.70 | (0.01) | F | |||
| Dec. | 73.24 | (0.01) | E | 73.70 | (0.01) | F | ||||
LL, leg length; MoB, month of birth; SE, standard error.
aGroup 1 including Harbin, Henan, Gansu, and Hunan; group 2 including Qingdao, Suzhou, Sichuan, Zhejiang, Liuzhou, and Haikou.
bMeans LL adjusted for survey sites, sex, age, education, smoking, alcohol use, physical activity, sedentary leisure time, height, and HC.
cMeans sharing the same letter were not significantly different at Bonferroni-corrected level of significance.
Figure 3. Mean leg length (cm) with 95% confidence intervals by month of birth among 218 898 participants of group 1 and 268 631 participants of group 2.
DISCUSSION
The present study is the largest population-based study ever to report on the associations of MoB with adult adiposity and LL measures. The study’s findings suggest that spring- and early summer-born adults had higher BMI and WC and shorter LL than autumn- and winter-born adults in China. Participants at all 10 survey sites showed seasonal variations in BMI and WC, but only 4 sites showed seasonal variation in LL. The associations between MoB and adult measures of adiposity and LL were independent of gender.
Few studies have examined the association between SoB and adult adiposity. Inconsistent findings and mixed explanations of underlying mechanism have been reported, including speculation that early temperature exposure is associated with physiological regulation of birth weight or that extremely low ambient temperature influences the conceived embryo or the selection of the sperm that fuses with the ovum.19–21 However, the findings from the current study appeared to more strongly suggest the possible etiological role of prenatal maternal exposure to ultraviolet B radiation (UVB) and vitamin D status in adult chronic diseases.29–31
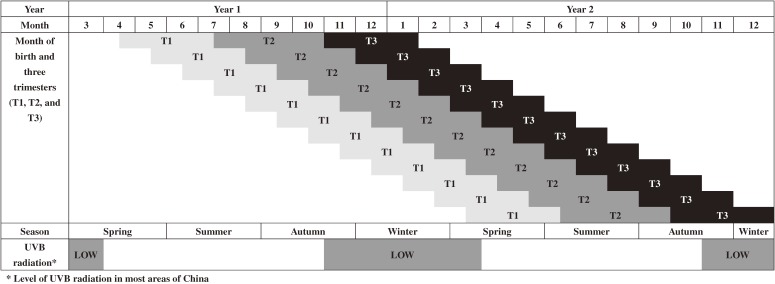
Sun exposure provides one of the major ways to meet vitamin D requirements during pregnancy, especially for those of older generations. For latitudes with distinct seasonal changes in availability of UVB, there is a well-documented seasonal cycle in vitamin D status, with a maximum in late summer and a minimum in late winter.32 Low vitamin D during gestation may strongly influence susceptibility to obesity later in life.33
However, relatively little is known about the critical period of fetal growth at which low vitamin D is of particular importance. Low vitamin D during the second or third trimester of pregnancy has been suggested to be associated with adult obesity.31,33 In the current study, spring and early summer peaks and winter troughs for BMI and WC were evident. Mothers with babies born in spring and early summer had low sun-related UVB exposure in winter, and hence, lower vitamin D exposure for their offspring during the late second and early third trimesters (Figure 4). Babies who were born in winter had high UVB exposure during the late second and early third trimesters, which occurred in summer and early autumn. Up to now, a few studies have examined the association between lower maternal vitamin D levels in later pregnancy and offspring adiposity at age 6 years34; at birth, age 9 months and age 9 years35; at ages 5 and 9.5 years36; and at age 35 years,37 and shown inconsistent results. Further studies are warranted to test the association between prenatal vitamin D status and adult adiposity in an adult population older than 35 years.
Figure 4. Month of birth and three trimesters in relation to the calendar month of year, season, and the level of UVB radiation.
To our knowledge, no previous study has examined the relationship between prenatal vitamin D status and LL. LL in the present study followed an opposite seasonal trend to that of adult adiposity measures, for which maternal UVB exposure (and hence vitamin D status) is still a reasonable explanation. For spring- and summer-born babies, shorter LL, together with higher adiposity measures, could be the adverse outcome of lower UVB exposure in winter during their second and early third trimesters.
However, this association between MoB and LL was only observed in four survey sites. Three of them, including Harbin, Henan, and Gansu, have higher latitudes. Lucas et al found that seasonal variation in vitamin D status in Australian adults (living in an area spanning 27°–43° South) had greater amplitude compared to Australian adults living in a different region, and vitamin D deficiency increased with increasing latitude, reflecting stronger seasonal variation in ambient UVB and less skin exposure due to colder temperatures.38 Hunan, being another site presenting seasonal variation in LL, has lower latitude than seven other study sites but the greatest yearly sunshine variation of all the study sites (see eTable 1). Besides significant seasonal variation in sunshine, peak and trough windows are also supposed to last long enough to influence vitamin D status. These might explain why null results were found for the other six survey sites. Results in the present study imply that prenatal vitamin D status might be a potential pathway linking LL with adult risks of obesity39,40 and a range of chronic diseases.8–10 However, seasonal variation in LL depending on region suggests that LL can only be used as anthropometric biomarker of prenatal vitamin D status in some geographic regions.
The large sample size of the current study ensured sufficient statistical power to identify a relatively small difference. The study participants, who resided across a range of latitudes, provided a sound basis for observing that individuals living in low-latitude and lower- to middle-latitude regions of China also had seasonal low UVB exposure, and hence, higher measures of adult adiposity. Although the current study is unable to precisely determine the reason for MoB variation in adult measures of LL, general adiposity, and central adiposity, the study improves our current understanding of a potential association of prenatal low exposure to vitamin D during late second and early third trimesters with short LL and adiposity in later life. Vitamin D insufficiency during pregnancy is highly prevalent in a diverse range of populations living at various latitudes and in both developed and developing societies.29,33 The adverse effects of vitamin D insufficiency on offspring during development and later in life have also been extended greatly.29–31 From a public health perspective, the potential to prevent common chronic diseases via low-cost, simple, and safe food fortification is an attractive option.33
Some potential limitations of this study warrant consideration. First, we used MoB in the analysis and then speculated the period of trimester during which the mothers may have had low UVB exposure without considering preterm birth. Second, participants were geographically grouped based on their residence at the time of the survey rather than their place of birth. Given that these survey sites were not located in old, well-developed, large cities, it is unlikely that many local residents, especially those of older residents, were migrants from distant locations across the provinces. Third, we adjusted the analyses for socioeconomic and lifestyle variables at the time of the survey, but we failed to include additional potential confounders in early life, such as mother’s socio-demographic characteristics, health conditions, and lifestyle habits during pregnancy; adverse birth outcomes; postnatal feeding; and child growth and development, potentially biasing the findings. A lack of documented prenatal, birth, and child health records for a vast majority of Chinese citizens is the main reason for this missing information. Fourth, Bogin et al suggested that there is a significant bias when LL are estimated from stature and sitting height in populations with a high percentage of overweight and obesity.27 Lower extremity length should be measured directly. Although China’s obesity problem is increasing rapidly, the overall obesity rate is still relatively low. In addition, HC was included in the model to adjust gluteo-femoral fat thickness, mitigating any bias.
In summary, the present study provides the largest population evidence to date that MoB is associated with variations in adult measures of both general and central adiposity among Chinese adults. The association between MoB and LL was only seen in areas with possibly greater variation in seasonal UVB exposure. On average, individuals who were born in spring and early summer had higher BMI and WC and shorter LL than those who were born in autumn and winter. This is also the first-ever population-based evidence linking low prenatal sun-related UVB exposure (and lower vitamin D by proxy) to LL and the susceptibility to obesity later in life and also suggesting that late second and early third trimesters might be a critical period for the above-mentioned effects Although such evidence is non-specific, it warrants further studies in this area. Long-term follow-up data from this CKB study may provide prospective evidence for possible associations between MoB and many later health outcomes.
ONLINE ONLY MATERIALS
ACKNOWLEDGEMENTS
We thank Judith Mackay in Hong Kong; Yu Wang, Gonghuan Yang, Zhengfu Qiang, Lin Feng, Maigeng Zhou, Wenhua Zhao and Yan Zhang at China CDC; Lingzhi Kong, Xiucheng Yu and Kun Li at the Chinese Ministry of Health; and Yiping Chen, Sarah Clark, Martin Radley, Mike Hill, Hongchao Pan, and Jill Boreham at the CTSU, Oxford, for assisting with the design, planning, organization, and conduct of the study. The most important acknowledgement is to the participants in the study and the members of the survey teams in each of the 10 regional centres, as well as to the project development and management teams based at Beijing, Oxford, and the 10 regional centres.
Conflicts of interest: None declared.
Funding
The CKB baseline survey and the first re-survey were supported by the Kadoorie Charitable Foundation in Hong Kong. The long-term follow-up of the project during 2009–14 is supported by the Wellcome Trust in the UK [088158/Z/09/Z] and the National Key Technologies Research and Development Program in the 12th Five-year Plan, Chinese Ministry of Science and Technology [2011BAI09B01, 2012–14]. Support for this analysis is provided by the National Natural Science Foundation of China [No. 81373082]. The sponsors had no role in the design, analysis, interpretation or drafting of this manuscript.
Members of China Kadoorie Biobank collaborative group
(a) International steering committee
Liming Li (PI), Junshi Chen, Rory Collins, Richard Peto, Zhengming Chen (PI)
(b) Study coordinating Centres
International Co-ordinating Centre, Oxford: Zhengming Chen, Garry Lancaster, Xiaoming Yang, Alex Williams, Margaret Smith, Ling Yang, Yumei Chang, Iona Millwood, Yiping Chen, Sarah Lewington
National Co-ordinating Centre, Beijing: Yu Guo, Zheng Bian, Can Hou, Yunlong Tan, Huiyan Zhou
Regional Co-ordinating Centres, 10 areas in China:
Qingdao
Qingdao CDC: Zengchang Pang, Shaojie Wang, Yun Zhang, Kui Zhang
Licang CDC: Silu Liu, Wei Hou
Heilongjiang
Provincial CDC: Zhonghou Zhao, Shumei Liu, Zhigang Pang
Nangang CDC: Weijia Feng, Shuling Wu, Liqiu Yang, Huili Han, Hui He, Bo Yu
Hainan
Provincial CDC: Xianhai Pan, Shanqing Wang, Hongmei Wang
Meilan CDC: Xinhua Hao, Chunxing Chen, Shuxiong Lin, Xiangyang Zheng
Jiangsu
Provincial CDC: Xiaoshu Hu, Minghao Zhou, Ming Wu, Ran Tao
Suzhou CDC: Yeyuan Wang, Yihe Hu, Liangcai Ma, Renxian Zhou, Guanqun Xu, Yan Lu
Guangxi
Provincial CDC: Baiqing Dong, Naying Chen, Ying Huang
Liuzhou CDC: Mingqiang Li, Jinhuai Meng, Zhigao Gan, Jiujiu Xu, Yun Liu, Jingxin Qing
Sichuan
Provincial CDC: Xianping Wu, Yali Gao, Ningmei Zhang
Pengzhou CDC: Guojin Luo, Xiangsan Que, Xiaofang Chen
Gansu
Provincial CDC: Pengfei Ge, Jian He, Xiaolan Ren
Maiji CDC: Hui Zhang, Enke Mao, Guanzhong Li, Zhongxiao Li, Jun He, Yulong Lei, Xiaoping Wang
Henan
Provincial CDC: Guohua Liu, Baoyu Zhu, Gang Zhou, Shixian Feng
Huixian CDC: Yulian Gao, Tianyou He, Li Jiang, Jianhua Qin, Huarong Sun
Zhejiang
Provincial CDC: Liqun Liu, Min Yu, Ruying Hu, Yaping Chen
Tongxiang CDC: Zhixiang Hu, Jianjin Hu, Yijian Qian, Zhiying Wu, Chunmei Wang, Lingli Chen
Hunan
Provincial CDC: Wen Liu, Guangchun Li, Huilin Liu
Liuyang CDC: Xiangquan Long, Xin Xu, Youping Xiong, Zhongwen Tan, Xuqiu Xie, Yunfang Peng, Weifang Jia
REFERENCES
- 1.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378:804–14. 10.1016/S0140-6736(11)60813-1 [DOI] [PubMed] [Google Scholar]
- 2.Wildman RP, Gu D, Muntner P, Wu X, Reynolds K, Duan X, et al. Trends in overweight and obesity in Chinese adults: between 1991 and 1999–2000. Obesity (Silver Spring). 2008;16:1448–53. 10.1038/oby.2008.208 [DOI] [PubMed] [Google Scholar]
- 3.Xi B, Liang Y, He T, Reilly KH, Hu Y, Wang Q, et al. Secular trends in the prevalence of general and abdominal obesity among Chinese adults, 1993–2009. Obes Rev. 2012;13:287–96. 10.1111/j.1467-789X.2011.00944.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med Princ Pract. 2010;19:87–98. 10.1159/000273066 [DOI] [PubMed] [Google Scholar]
- 5.Remacle C, Bieswal F, Bol V, Reusens B. Developmental programming of adult obesity and cardiovascular disease in rodents by maternal nutrition imbalance. Am J Clin Nutr. 2011;94(6Suppl):1846S–52S. 10.3945/ajcn.110.001651 [DOI] [PubMed] [Google Scholar]
- 6.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. 10.1056/NEJMra0708473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker DJ. Mothers, babies, and health in later life. 2nd ed. Edinburgh; New York: Churchill Livingstone; 1998. ix, 217 p. [Google Scholar]
- 8.Batty GD, Shipley MJ, Gunnell D, Huxley R, Kivimaki M, Woodward M, et al. Height, wealth, and health: an overview with new data from three longitudinal studies. Econ Hum Biol. 2009;7:137–52. 10.1016/j.ehb.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 9.Bogin B, Varela-Silva MI. Leg length, body proportion, and health: A review with a note on beauty. Int J Environ Res Public Health. 2010;7:1047–75. 10.3390/ijerph7031047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang N, Zhang X, Xiang YB, Yang G, Li HL, Gao J, et al. Associations of adult height and its components with mortality: a report from cohort studies of 135,000 Chinese women and men. Int J Epidemiol. 2011;40:1715–26. 10.1093/ije/dyr173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, Dangour AD, Power C. Early life influences on adult leg and trunk length in the 1958 British birth cohort. Am J Hum Biol. 2007;19:836–43. 10.1002/ajhb.20649 [DOI] [PubMed] [Google Scholar]
- 12.Leary S, Davey Smith G, Ness A. Smoking during pregnancy and components of stature in offspring. Am J Hum Biol. 2006;18:502–12. 10.1002/ajhb.20518 [DOI] [PubMed] [Google Scholar]
- 13.Wadsworth ME, Hardy RJ, Paul AA, Marshall SF, Cole TJ. Leg and trunk length at 43 years in relation to childhood health, diet and family circumstances; evidence from the 1946 national birth cohort. Int J Epidemiol. 2002;31:383–90. 10.1093/ije/31.2.383 [DOI] [PubMed] [Google Scholar]
- 14.Reffelmann T, Ittermann T, Empen K, Dörr M, Felix SB. Is cardiovascular mortality related to the season of birth? Evidence from more than 6 million cardiovascular deaths between 1992 and 2007. J Am Coll Cardiol. 2011;57:887–8. 10.1016/j.jacc.2010.10.021 [DOI] [PubMed] [Google Scholar]
- 15.Krenz-Niedbała M, Puch EA, Kościński K. Season of birth and subsequent body size: the potential role of prenatal vitamin D. Am J Hum Biol. 2011;23:190–200. 10.1002/ajhb.21101 [DOI] [PubMed] [Google Scholar]
- 16.McGrath JJ, Keeping D, Saha S, Chant DC, Lieberman DE, O’Callaghan MJ. Seasonal fluctuations in birth weight and neonatal limb length; does prenatal vitamin D influence neonatal size and shape? Early Hum Dev. 2005;81:609–18. 10.1016/j.earlhumdev.2005.03.013 [DOI] [PubMed] [Google Scholar]
- 17.Watson PE, McDonald BW. Seasonal variation of nutrient intake in pregnancy: effects on infant measures and possible influence on diseases related to season of birth. Eur J Clin Nutr. 2007;61:1271–80. 10.1038/sj.ejcn.1602644 [DOI] [PubMed] [Google Scholar]
- 18.Hillman RW, Conway HC. Season of birth and relative body weight. Am J Clin Nutr. 1972;25:279–81. [DOI] [PubMed] [Google Scholar]
- 19.Phillips DI, Young JB. Birth weight, climate at birth and the risk of obesity in adult life. Int J Obes Relat Metab Disord. 2000;24:281–7. 10.1038/sj.ijo.0801125 [DOI] [PubMed] [Google Scholar]
- 20.Wattie N, Ardern CI, Baker J. Season of birth and prevalence of overweight and obesity in Canada. Early Hum Dev. 2008;84:539–47. 10.1016/j.earlhumdev.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 21.Schreier N, Moltchanova E, Forsén T, Kajantie E, Eriksson JG. Seasonality and ambient temperature at time of conception in term-born individuals—influences on cardiovascular disease and obesity in adult life. Int J Circumpolar Health. 2013;72:21466. 10.3402/ijch.v72i0.21466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soreca I, Cheng Y, Frank E, Fagiolini A, Kupfer DJ. Season of birth is associated with adult body mass index in patients with bipolar disorder. Chronobiol Int. 2013;30:577–82. 10.3109/07420528.2012.754452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z, Chen J, Collins R, Guo Y, Peto R, Wu F, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–66. 10.1093/ije/dyr120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Lee L, Chen J, Collins R, Wu F, Guo Y, et al. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC). Int J Epidemiol. 2005;34:1243–9. 10.1093/ije/dyi174 [DOI] [PubMed] [Google Scholar]
- 25.Millwood IY, Li L, Smith M, Guo Y, Yang L, Bian Z, et al. Alcohol consumption in 0.5 million people from 10 diverse regions of China: prevalence, patterns and socio-demographic and health-related correlates. Int J Epidemiol. 2013;42:816–27. 10.1093/ije/dyt078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du H, Bennett D, Li L, Whitlock G, Guo Y, Collins R, et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: the China Kadoorie Biobank study. Am J Clin Nutr. 2013;97:487–96. 10.3945/ajcn.112.046854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogin B, Varela-Silva MI. Fatness biases the use of estimated leg length as an epidemiological marker for adults in the NHANES III sample. Int J Epidemiol. 2008;37:201–9. 10.1093/ije/dym254 [DOI] [PubMed] [Google Scholar]
- 28.StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 29.Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: biology, outcomes, and interventions. Nutr Rev. 2010;68:465–77. 10.1111/j.1753-4887.2010.00306.x [DOI] [PubMed] [Google Scholar]
- 30.Lucas RM, Ponsonby AL, Pasco JA, Morley R. Future health implications of prenatal and early-life vitamin D status. Nutr Rev. 2008;66:710–20. 10.1111/j.1753-4887.2008.00126.x [DOI] [PubMed] [Google Scholar]
- 31.Pasco JA, Wark JD, Carlin JB, Ponsonby AL, Vuillermin PJ, Morley R. Maternal vitamin D in pregnancy may influence not only offspring bone mass but other aspects of musculoskeletal health and adiposity. Med Hypotheses. 2008;71:266–9. 10.1016/j.mehy.2008.01.033 [DOI] [PubMed] [Google Scholar]
- 32.Webb AR. Who, what, where and when—influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol. 2006;92:17–25. 10.1016/j.pbiomolbio.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen R, Abrahamsen B, Bauerek M, Holst C, Jensen CB, Knop J, et al. The influence of early exposure to vitamin D for development of diseases later in life. BMC Public Health. 2013;13:515. 10.1186/1471-2458-13-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crozier SR, Harvey NC, Inskip HM, Godfrey KM, Cooper C, Robinson SM; SWS Study Group . Maternal vitamin D status in pregnancy is associated with adiposity in the offspring: findings from the Southampton Women’s Survey. Am J Clin Nutr. 2012;96:57–63. 10.3945/ajcn.112.037473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. 10.1038/sj.ejcn.1602680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnaveni GV, Veena SR, Winder NR, Hill JC, Noonan K, Boucher BJ, et al. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: the Mysore Parthenon Study. Am J Clin Nutr. 2011;93:628–35. 10.3945/ajcn.110.003921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tornhammar P, Ueda P, Hult M, Simila H, Eyles D, Norman M. Season of birth, neonatal vitamin D status, and cardiovascular disease risk at 35 y of age: a cohort study from Sweden. Am J Clin Nutr. 2014;99:472–8. 10.3945/ajcn.113.072520 [DOI] [PubMed] [Google Scholar]
- 38.Lucas RM, Valery P, van der Mei I, Dwyer T, Pender MP, Taylor B, et al. Sun exposure over a lifetime in Australian adults from latitudinally diverse regions. Photochem Photobiol. 2013;89:737–44. 10.1111/php.12044 [DOI] [PubMed] [Google Scholar]
- 39.Velásquez-Meléndez G, Silveira EA, Allencastro-Souza P, Kac G. Relationship between sitting-height-to-stature ratio and adiposity in Brazilian women. Am J Hum Biol. 2005;17:646–53. 10.1002/ajhb.20423 [DOI] [PubMed] [Google Scholar]
- 40.Asao K, Kao WH, Baptiste-Roberts K, Bandeen-Roche K, Erlinger TP, Brancati FL. Short stature and the risk of adiposity, insulin resistance, and type 2 diabetes in middle age: the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Diabetes Care. 2006;29:1632–7. 10.2337/dc05-1997 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.