Abstract
We previously demonstrated that bovine subcutaneous preadipocytes promote adipogenic gene expression in muscle satellite cells in a co-culture system. Herein we hypothesize that saturated fatty acids would promote adipogenic/lipogenic gene expression, whereas mono- and polyunsaturated fatty acids would have the opposite effect. Bovine semimembranosus satellite cells (BSC) and intramuscular preadipocytes (IPA) were isolated from crossbred steers and cultured with 10% fetal bovine serum (FBS)/Dulbecco’s Modified Eagle Medium (DMEM) and 1% antibiotics during the 3-d proliferation period. After proliferation, cells were treated for 3 d with 3% horse serum/DMEM (BSC) or 5% FBS/DMEM (IPA) with antibiotics. Media also contained 10 μg/mL insulin and 10 μg/mL pioglitazone. Subsequently, differentiating BSC and IPA were cultured in their respective media with 40 μM palmitic, stearic, oleic, or linoleic acid for 4 d. Finally, BSC and IPA were single- or co-cultured for an additional 2 h. All fatty acid treatments increased (p = 0.001) carnitine palmitoyltransferase-1 beta (CPT1β) gene expression, but the increase in CPT1β gene expression was especially pronounced in IPA incubated with palmitic and stearic acid (6- to 17- fold increases). Oleic and linoleic acid decreased (p = 0.001) stearoyl-CoA desaturase (SCD) gene expression over 80% in both BSC and IPA. Conversely, palmitic and stearic acid increased SCD gene expression three fold in co-cultured in IPA, and stearic acid increased AMPKα gene expression in single- and co-cultured BSC and IPA. Consistent with our hypothesis, saturated fatty acids, especially stearic acid, promoted adipogenic and lipogenic gene expression, whereas unsaturated fatty acids decreased expression of those genes associated with fatty acid metabolism.
Keywords: Bovine, Co-culture, Fatty Acids, Gene Expression, Preadipocytes, Satellite Cells
INTRODUCTION
The primary objective of this study was to demonstrate the effects of saturated fatty acids (palmitic and stearic acid) and unsaturated fatty acids (oleic and linoleic acid) on specific gene expression in bovine satellite cells (BSC) and intramuscular preadipocytes (IPA). The secondary objective was to document the effects of brief co-culture of BSC and IPA on adipogenic and lipogenic gene expression. We hypothesized that palmitic and stearic acid would increase expression of genes associated with lipid accumulation in BSC and IPA during differentiation. We also tested the effects of oleic acid and linoleic acid, as we predicted that they would depress adipogenic and/or lipogenic gene expression. Stearic acid is the most abundant fatty acid to pass from the rumen to the small intestine in cattle, although palmitic acid also is abundant in the small intestine of cattle (Ekeren et al., 1992; Chung et al., 2006b). Oleic acid is absorbed from the gastrointestinal tract of cattle (Ekeren et al., 1992) and is produced endogenously (St. John et al., 1991; Chang et al., 1992; Archibeque et al., 2005; Kadegowda et al., 2013), and linoleic acid is the most abundant polyunsaturated fatty acid in the diet and tissues of beef cattle (Chung et al., 2006b).
To accomplish these objectives, we documented the effects of exposure to fatty acids during culture on the expression of genes associated with adipocyte differentiation (CCAAT/enhancer-binding protein beta [C/EBPβ] and peroxisome proliferator-activated receptor gamma [PPARγ]), lipid metabolism (carnitine palmitoyltransferase-1 beta [CPT1β] and stearoyl-CoA desaturase [SCD]), and the regulation of triacylglycerol turnover (G protein-coupled protein receptor 43 [GPR43] and AMP-activated protein kinase alpha-1 [AMPKα]). In the present study, we hypothesized that co-culture of preadipocytes with myoblasts would modify the effects of saturated and unsaturated fatty acids on adipogenic and lipogenic gene expression.
MATERIALS AND METHODS
Chemicals
Fatty acids were purchased from Nu-Chek prep, Inc (Elysian, MN, USA). Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA), Invitrogen (Carlsbad, CA, USA), Calbiocem (La Jolla, CA, USA), Gibco (Grand Island, NY, USA) or Applied Biosystems (Foster City, CA, USA).
Bovine satellite cell and preadipocyte isolation
Muscle-derived BSC and IPA were isolated from semimembranosus muscle of 14-mo-old crossbred steers raised at Texas Tech University. Steers were killed with a captive bolt stunner followed by exsanguination. Using sterile techniques, approximately 500 g of the semimembranosus muscle and IPA within the muscle were dissected and transported to the laboratory. Subsequent procedures were conducted in a sterile field in a tissue culture hood. After removal of connective tissue and IPA, the muscle was passed through a sterile meat grinder. The ground muscle was incubated with 0.1% pronase (Calbiochem) in Earl’s Balanced Salt Solution (Sigma-Aldrich, USA) for 1 h at 37°C with frequent mixing. The dissected IPA was incubated with 0.1% type IV collagenase (Sigma-Aldrich, USA) in Earl’s Balanced Salt Solution for 1 h at 37°C with frequent mixing (Suryawan and Hu, 1997; Ohyama et al., 1998). After incubation, both mixtures were centrifuged at 1,500×g for 4 min, the pellets were suspended in phosphate buffered saline (Gibco) (140 mM NaCl, 1 mM KH2PO4, 3 mM KCl, 8 mM Na2HPO4), and the suspensions were centrifuged at 500×g for 10 min. The supernatants were centrifuged at 1,500×g for 10 min to pellet the mononucleated cells. The PBS wash and differential centrifugation were repeated twice. The resulting cells preparations were suspended in cold (4°C) Dulbecco’s Modified Eagle Medium (DMEM; Gibco) containing 10% fetal bovine serum (FBS) (Gibco) and 10% (vol/vol) dimethylsulfoxide (Sigma-Aldrich, USA) and frozen. Cells were stored frozen in liquid nitrogen.
Cell culture
The BSC and IPA were obtained from a single batch and experiments were replicated in three independent incubations (Choi et al., 2013a). Cells were plated at a density of 1×104 cells per well and grown at 37°C under a humidified atmosphere of 5% CO2. Upon reaching confluence after 3 d, the growth medium was replaced with 3% horse serum/DMEM plus antibiotics (BSC) or 5% FBS/DMEM plus antibiotics (IPA). Differentiation medium also contained 10 μg/mL insulin and 10 μM pioglitazone (Kang et al., 2003; Chung and Johnson, 2009), and BSC and IPA were allowed to differentiate for 3 d. Subsequently, no fatty acids (control cells) or 40 μM fatty acids (palmitic, stearic, oleic, or linoleic acid) were added to the media, and the differentiating IPA and BSC were incubated for an additional 4 d. Fatty acids were dissolved in ethanol prior to addition to the cell culture (Satory and Smith, 1999; Chung et al., 2006a). Finally, differentiated IPA and BSC were single-cultured and co-cultured for an additional 2 h in DMEM plus antibiotics in the presence of the fatty acids.
Real-time polymerase chain reaction
RNA was extracted with Tri Reagent (Sigma Chemicals, St. Louis, MO, USA) as reported previously (Smith et al., 2012). The concentration of RNA was quantified with a NanoDrop ND-100 Spectrophotometer (Thermo Scientific, Washington, DE, USA). The 260:280 ratio for all samples was greater than 1.85. Quantitative real time polymerase chain reaction (qRT-PCR) was used to analyze the expression of C/EBPβ, CPT1β, GPR43, PPARγ, AMPK-α, and SCD (primers listed in Table 1). Commercially available eukaryotic 40S ribosomal protein S9 (RPS9) RNA (Applied Biosystems; GeneBank Accession #X03205) was used as the endogenous control. Other studies in bovine adipose tissue explants (Hosseini et al., 2010) and bovine liver (Baxa et al., 2010) demonstrated that RPS9 mRNA expression was stable and suitable as a housekeeping gene under their conditions. Additionally, RPS9 was used as a housekeeping gene for the expression genes in bovine muscle (Chung and Johnson, 2009; Baxa et al., 2010).
Table 1.
Forward and reverse primers and probes for real-time PCR for specific gene mRNA
| Maker gene | Gene No. | Sequence (5′ to 3′) | |
|---|---|---|---|
| RPS9 | DT860044 | Forward | GAGCTGGGTTTGTCGCAAAA |
| Reverse | GGTCGAGGCGGGACTTCT | ||
| Taqman probe | 6FAM-ATGTGACCCCGCGGAGACCCTTC-TAMRA | ||
| AMPK-α | NM_001109802 | Forward | ACCATTCTTGGTTGCTGAAACTC |
| Reverse | CACCTTGGTGTTTGGATTTCTG | ||
| Taqman probe | 6FAM-CAGGGCGCGCCATACCCTTG-TAMRA | ||
| C/EBPβ | NM_176788 | Forward | CCAGAAGAAGGTGGAGCAACTG |
| Reverse | TCGGGCAGCGTCTTGAAC | ||
| Taqman probe | 6FAM-CGCGAGGTCAGCACCCTGC-TAMRA | ||
| CPT1β | NM_001034349 | Forward | ACACATCTACCTGTCCGTGATCA |
| Reverse | CCCCTGAGGATGCCATTCT | ||
| Taqman probe | 6FAM-TCCTGGAAGAAACGCCTGATTCGC-TAMRA | ||
| GPR43 | FJ_562212 | Forward | GGCTTTCCCCGTGCAGTA |
| Reverse | ATCAGAGCAGCGATCACTCCAT | ||
| Taqman probe | 6FAM-AAGCTGTCCCGCCGGCCC-TAMRA | ||
| PPARγ | NM_181024 | Forward | ATCTGCTGCAAGCCTTGGA |
| Reverse | TGGAGCAGCTTGGCAAAGA | ||
| Taqman probe | 6FAM-CGCGAGGTCAGCACCCTGC-TAMRA | ||
| SCD | AB075020 | Forward | TGCCCACCACAAGTTTTCAG |
| Reverse | GCCAACCCACGTGAGAGAAG | ||
| Taqman probe | 6FAM-CCGACCCCCACAATTCCCG-TAMRA |
PCR, polymerase chain reaction; RPS9, ribosomal protein S9; AMPK-α, AMP-activated protein kinase alpha; C/EBPβ, CCAAT/enhancer-binding protein beta; CPT1β, carnitine palmitoyltransferase-1 beta; GPR43, G protein-coupled protein receptor 43; PPARγ, peroxisome proliferator-activated receptor gamma; SCD, stearoyl-CoA desaturase.
Complementary DNA was produced from 1 μg RNA using Taq- Man Reverse Transcriptase Reagents (Applied Biosystems, USA) by the protocol recommended by the manufacturer. Random hexamers were used as primers in cDNA synthesis. Measurement of the relative quantity of the cDNA of interest was carried out using TAMRA PCR Master Mix (Applied Biosystems, USA), appropriate forward and reverse primers, and 1 μL of the cDNA mixture. Assays were performed in duplicate in the GeneAmp 5700 Sequence Detection System (Applied Biosystems, USA) using thermal cycling parameters recommended by the manufacturer (40 cycles of 15 s at 95°C and 1 min at 60°C). Cycle threshold (CT) values were means of duplicate measurements. The comparative CT values were employed to determine expression levels for target genes; fold change was determined as 2ΔΔCT with RPS9 as the endogenous control. Titration of the target mRNA primers against increasing amounts of cDNA gave linear responses with slopes between −2.8 and −3.0. In order to reduce the effect of assay-to-assay variation in the PCR assay, all values were calculated relative to a calibration standard run on every qRT-PCR assay. The ABI Prism 7000 detection system (Applied Biosystems, USA) was used to perform the assay utilizing the thermal cycling variables recommended by the manufacturer (50 cycles of 15 s at 95°C and 1 min at 60°C).
Statistical analysis
Data were analyzed by analysis of variance as a three-factor design with cell type (adipocytes, myoblasts), culture method (single-culture, co-culture), and treatment (fatty acids) as the main effects (SuperAnova, Abacus Concepts, Berkley, CA, USA). The model also tested all possible two- and three-way interactions among main effects. Means were separated by Fisher’s Protected LSD (SuperAnova, USA) if their respective F-test indicated significant differences (p<0.05).
RESULTS
Cell type, culture method, and fatty acid treatment main effects
There were highly significant differences (p<0.001) between IPA and BSC for AMPKα, C/EBPβ, CPT1β, PPARγ, and SCD gene expression (Table 2). The mRNA levels for AMPKα, C/EBPβ, and PPARγ were higher in IPA than in BSC after differentiation, whereas CPT1β and SCD gene expression was higher in BSC than in IPA after differentiation. The mRNA levels for GPR43 did not differ between cell types (p = 0.303).
Table 2.
Main effects for cell type, culture method, and fatty acid for gene expression in single- and co-cultured bovine satellite cells and intramuscular preadipocytes incubated in the absence and presence of 40 μM palmitic, stearic, oleic, or linoleic acid
| Genea | Main effects | SEM | Treatment p-values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Cell typeb | Culture methodc | Fatty acid treatmentd | |||||||||||
|
|
|
|
|
||||||||||
| BSC | IPA | Single | Co-culture | Control | Palmitic | Stearic | Oleic | Linoleic | CT | CM | FA | ||
| AMPKα | 0.88 | 1.41 | 0.97 | 1.32 | 1.11h | 1.13h | 1.43g | 0.99h | 1.05h | 0.05 | <0.001 | <0.001 | <0.001 |
| C/EBPβ | 0.01e | 5.02 | 2.06 | 2.95 | 0.83i | 4.39h | 5.43g | 0.60i | 1.30i | 0.51 | <0.001 | <0.001 | <0.001 |
| CPT1β | 1,823 | 17 | 953 | 886 | 198d | 1,096h | 931i | 1,450g | 923i | 141 | <0.001 | 0.197 | <0.001 |
| GPR43 | 0.92 | 1.09 | 0.53 | 1.47 | 0.89 | 0.67 | 1.37 | 1.03 | 1.05 | 0.11 | 0.303 | <0.001 | 0.136 |
| PPARγ | 1.71 | 2.48 | 1.30 | 2.89 | 1.83 | 1.70 | 2.52 | 2.04 | 2.38 | 0.15 | <0.001 | <0.001 | 0.165 |
| SCD | 1.56 | 0.98 | 1.08 | 1.48 | 1.80h | 1.45i | 2.68g | 0.29j | 0.16j | 0.15 | <0.001 | <0.001 | <0.001 |
SEM, standard error of the mean; CT, cell type; CM, culture method; FA, fatty acid treatment; BSC, bovine satellite cells; IPA, intramuscular preadipocytes; AMPK-α, AMP-activated protein kinase alpha; C/EBPβ, CCAAT/enhancer-binding protein beta; CPT1β, carnitine palmitoyltransferase-1 beta; GPR43, G protein-coupled protein receptor 43; PPARγ, peroxisome proliferator-activated receptor gamma; SCD, stearoyl-CoA desaturase.
Relative AMPKα, C/EBPβ, GPR43, PPARγ, CPT1β, and SCD mRNA levels in total RNA isolated from BSC, IPA single- or co-cultured with insulin (10 μM), and pioglitizone (10 μM). Data are for three culture preparations.
Data are means for 30 observations, pooled across culture method and fatty acid treatment.
Data are means for 30 observations, pooled across cell type and fatty acid treatment.
Data are means for 15 observations, pooled across cell type and culture method.
C/EBPβ was detectable only in control BSC and BSC incubated with 40 μM palmitic acid.
Means within a gene for fatty acid treatments with common superscripts are not different (p>0.05).
The culture method main effect (single or co-culture) was significant (p<0.001) for AMPKα, C/EBPβ, GPR43, PPARγ, and SCD gene expression (Table 2). The mRNA levels for AMPKα, C/EBPβ, GPR43, PPARγ, and SCD were greater in co-cultured cells than in single-cultured cells. Culture method had no effect on CPT1β gene expression (p = 0.197).
Fatty acid treatments affected the expression of all genes (p<0.001) except GPR43 and PPARγ (p = 0.136 and 0.165, respectively). Expression of AMPKα, C/EBPβ, and SCD was highest in cells incubated with stearic acid, whereas oleic and linoleic acid depressed SCD gene expression relative to control samples (Table 2). All fatty acids strongly increased CPT1β gene expression, and the greatest increase was caused by oleic acid.
Cell type×culture method×fatty acid three-way interactions
There were significant cell type×culture method×fatty acid interactions for AMPKα, C/EBPβ, PPARγ, and SCD gene expression (p≤0.052) (Figures 1 and 2). Relative to the control cells, palmitic acid increased AMPKαgene expression in single-cultured IPA and both palmitic and stearic acid increased AMPKα gene expression in co-cultured IPA (Figure 1A). Similarly, palmitic and stearic acid increased C/EBPβ gene expression in single cultured IPA and stearic acid increased C/EBPβ gene expression more than palmitic acid in co-cultured IPA (Figure 1B). Linoleic acid increased PPARγ gene expression in single cultured BSC and stearic, oleic, and linoleic acid increased PPARγ gene expression in single cultured IPA (Figure 2A). None of the fatty acids treatments affected PPARγ gene expression in co-cultured IPA. Relative to the control cells, palmitic acid decreased SCD gene expression in single- and co-cultured BSC but increased SCD gene expression in co-cultured IPA (Figure 2B).
Figure 1.
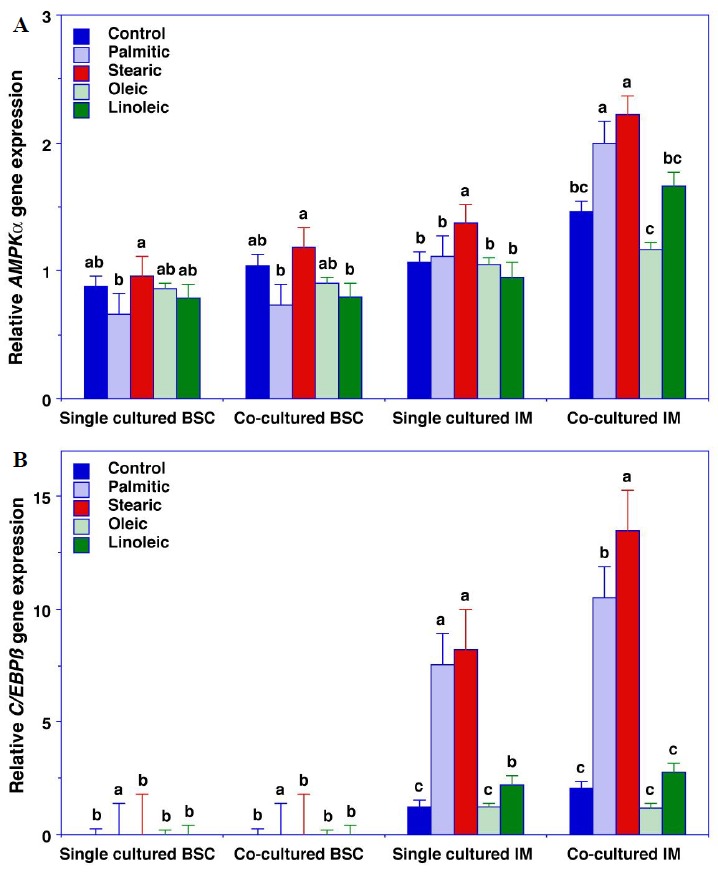
AMPKα gene expression (A) and C/EBPβ gene expression (B) in bovine satellite cells (BSC) and intramuscular preadipocytes (IPA). Cells were plated at a density of 1×104 cells per well and grown at 37°C under a humidified atmosphere of 5% CO2. Upon reaching confluence after 3 d, the growth medium was replaced with 3% horse serum/DMEM plus antibiotics (BSC) or 5% FBS/DMEM plus antibiotics (IPA). Differentiation medium also contained 10 μg/mL insulin and 10 μM pioglitazone, and BSC and IPA were allowed to differentiate for 3 d. Subsequently, no fatty acids (control cells) or 40 μM fatty acids (palmitic, stearic, oleic, or linoleic acid) were added to the media, and the differentiating IPA and BSC were incubated for anadditional 4 d. Differentiated IPA and BSC were single-cultured and co-cultured for an additional 2 h in DMEM plus antibiotics in the presence of the 40 μM fatty acids. A. AMPKα: The cell type×culture method×fatty acid three-way interaction was significant (p = 0.029). Relative to the control cells, palmitic acid increased AMPKα gene expression in single cultured IM (intramuscular) preadipocytes and both palmitic and stearic acid increased AMPKα gene expression in co-cultured IPA. B. C/EBPβ: The cell type×culture method×fatty acid three-way interaction was significant (p = 0.003). Relative to the control cells, palmitic and stearic acid increased C/EBPβ gene expression in single cultured IPA and stearic acid increased C/EBPβ gene expression more than palmitic acid in co-cultured IPA. All data are the means±SEM for 3 independent incubations. abc Means within a cell type and culture method with common superscripts are not different (p>0.05). AMPK-α, AMP-activated protein kinase alpha; CCAAT/enhancer-binding protein beta; DMEM, Dulbecco’s Modified Eagle Medium; FBS, fetal bovine serum; SEM, standard error of the mean.
Figure 2.
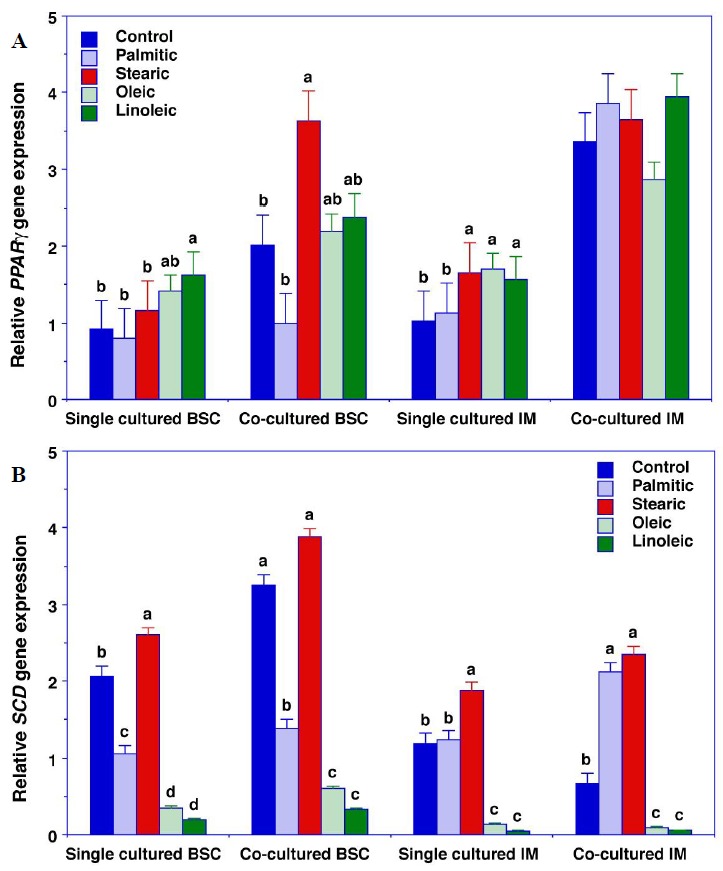
PPARγ gene expression (A) and SCD gene expression (B) in bovine satellite cells (BSC) and intramuscular preadipocytes (IPA). Cells were plated at a density of 1×104 cells per well and grown at 37°C under a humidified atmosphere of 5% CO2. Upon reaching confluence after 3 d, the growth medium was replaced with 3% horse serum/DMEM plus antibiotics (BSC) or 5% FBS/DMEM plus antibiotics (IM preadipocytes). Differentiation medium also contained 10 μg/mL insulin and 10 μM pioglitazone, and BSC and IPA were allowed to differentiate for 3 d. Subsequently, no fatty acids (control cells) or 40 μM fatty acids (palmitic, stearic, oleic, or linoleic acid) were added to the media, and the differentiating IPA and BSC were incubated for an additional 4 d. Differentiated IPA and BSC were single-cultured and co-cultured for an additional 2 h in DMEM plus antibiotics in the presence of the 40 μM fatty acids. A. PPARγ: The cell type×culture method×fatty acid three-way interaction was significant (p = 0.053). Relative to the control cells, linoleic acid increased PPARγ gene expression in single cultured BSC and stearic, oleic, and linoleic acid increased PPARγ gene expression in single cultured IPA. None of the fatty acids treatments affected PPARγ gene expression in co-cultured IPA. B. SCD: The cell type×culture method×fatty acid three-way interaction was significant (p = 0.001). Relative to the control cells, palmitic acid decreased SCD gene expression in single- and co-cultured cultured BSC but increased SCD gene expression in co-cultured IPA. All data are the means±SEM for 3 independent incubations. abc Means within a cell type and culture method with common superscripts are not different (p>0.05). PPARγ, peroxisome proliferator-activated receptor gamma; SCD, stearoyl-CoA desaturase; DMEM, Dulbecco’s Modified Eagle Medium; FBS, fetal bovine serum; IM, intramuscular adipocyte; SEM, standard error of the mean.
Culture method×fatty acid, cell type×fatty acid, and culture method×cell type two-way interactions for CPT1β and GPR43
The cell type×culture method×fatty acid interactions for CPT1β and GPR43 gene expression were not significant (p = 0.312 and 0.113, respectively) (Table 3). Nor was the culture method×fatty acid interaction significant for CPT1β and GPR43 gene expression (p = 0.341 and 0.125, respectively). The cell type×fatty acid interaction was significant for CPT1β (p<0.001) but not for GPR43 gene expression (p = 0.141). Also, the culture method x cell type was not significant for CPT1β (p = 0.141) but tended to be significant for GPR43 gene expression (p = 0.073) (Table 3).
Table 3.
Culture method×fatty acid and cell type×fatty acid interaction means for gene expression in single- and co-cultured bovine satellite cells and intramuscular preadipocytes incubated in the absence and presence of 40 μM palmitic, stearic, oleic, or linoleic acid
| Gene/culture methoda | Treatment | SEM | p-values | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Control | Palmitic | Stearic | Oleic | Linoleic | ||||
| Culture method×fatty acidb | ||||||||
| CPT1β | Single culture | 178 | 1,069 | 980 | 1,582 | 959 | 141 | 0.341 |
| Co-culture | 217 | 1,123 | 882 | 1,318 | 887 | |||
| GPR43 | Single culture | 0.58 | 0.34 | 0.50 | 0.78 | 0.46 | 0.11 | 0.125 |
| Co-culture | 1.19 | 1.01 | 2.24 | 1.29 | 1.64 | |||
| Cell type×fatty acidc | ||||||||
| CPT1β | BSC | 392g | 2,165ef | 1,835f | 2,889e | 1,832f | 141 | <0.001 |
| IPA | 3.32j | 27.91h | 26.79h | 11.93i | 14.13i | |||
| GPR43 | BSC | 0.69 | 0.46 | 1.72 | 0.93 | 0.78 | 0.11 | 0.141 |
| IPA | 1.08 | 0.89 | 1.02 | 1.14 | ||||
| Culture method×cell typed | Single culture | Co-culture | ||||||
| CPT1β | BSC | 1,883 | 1,738 | 141 | 0.141 | |||
| IPA | 12 | 22 | ||||||
| GPR43 | BSC | 0.29 | 1.54 | 0.11 | 0.073 | |||
| IPA | 0.77 | 1.41 | ||||||
SEM, standard error of the mean; CPT1β, carnitine palmitoyltransferase-1 beta; GPR43, G protein-coupled protein receptor 43; BSC, bovine satellite cells; IPA, intramuscular preadipocytes.
Relative CPT1β and GPR43mRNA levels in total RNA isolated from BSC, IPA single- or co-cultured with insulin (10 μM), and pioglitizone (10 μM).
Data are means for 6 observations, pooled over cell type.
Data are means for 6 observations, pooled over culture method.
Data are means for 30 observations, pooled over fatty acid treatment.
efghij: Means within a gene with common superscripts are not different (p>0.05).
DISCUSSION
Most studies of the regulation of adipogenesis have been conducted in cultured primary preadipocyte cultures or 3T3-L1 secondary preadipocyte cultures. A few studies have investigated the differentiation of bovine adipocytes and myoblasts in single cultures (Chung et al., 2006a; Ouellette et al., 2009), and we are aware of three studies describing the interaction between adipocytes and myoblastic cells in a co-culture system (Hausman et al., 2005; Choi et al., 2013a; Park et al., 2013), two of which originated from our laboratory (Choi et al., 2013a; Park et al., 2013). We have used this set of genetic markers of adipose tissue differentiation (C/EBPβ, PPARγ) and lipid metabolism (AMPKα, CPT1β, GPR43, SCD) in studies of the effects of fatty acids on bovine preadipocytes (Chung et al., 2006a) and mature adipose tissues (Smith et al., 2012; Choi et al., 2013a). The expression of PPARγ is promoted strongly by C/EBPβ, which initiates preadipocyte differentiation (Wu et al., 1996; Saladin et al., 1999). Recent studies demonstrated that inhibition of SCD catalytic activity reduced the activities of genes associated with de novo fatty acid synthesis, such as fatty acid synthase while increasing expression of genes associated with fatty acid oxidation, such as CPT1β (Kim et al., 2011; Kadegowda et al., 2013). Acetate and propionate activate the GPR43 receptor (Brown et al., 2003); this leads to a reduction in lipolysis (Ge et al., 2008), which in turn increases lipid accumulation in adipocytes and promotes metabolism of fatty acids and glucose in other tissues (Kimura et al., 2013). These effects may be attenuated by AMPKα (Yin et al., 2003). Expression of AMPKα promotes fatty acid oxidation by upregulating PPAR gamma coactivator-1alpha (PGC-1α) gene expression (Wan et al., 2013). In our previous study (Choi et al., 2013a), co-culture of subcutaneous (SC) preadipocytes with BSC increased GPR43 gene expression nearly fivefold, which would antagonize the actions of AMPKα if activated by appropriate ligands (i.e., volatile fatty acids). In this study, co-culture increased GPR43 gene expression two- to five fold. Although culture method had no effect on CPT1β gene expression, the elevated GPR43 gene expression induced by co-culture suggests that the presence of IPA may increase lipid accumulation in BSC.
Co-culture generally increased PPARγ gene expression in BSC and IPA, which we did not observe previously in SC preadipocytes co-cultured with BSC (Choi et al., 2013a). However, PPARγ gene expression was profoundly higher is SC preadipocytes in our previous study than in the IPA of the current study. Conversely, C/EBPβ gene expression was not detectable in SC preadipocytes (Choi et al., 2013a) but readily measurable in IPA in the current study. The expression of C/EBPβ occurs soon after preadipocytes are induced to differentiation (Darlington et al., 1998). These data suggest that IPA still were in early stages of differentiation even after 7 d of differentiation.
In an early study, we demonstrated high levels of SCD gene expression in bovine longissimus dorsi and SC adipose tissue (Cameron et al., 1994). In a recent study (Choi et al., 2014), intramuscular (IM) adipose tissue explants were removed from cattle at 12, 14, or 16 mo of age and cultured for 48 h in the absence and presence of 40 μM α-linolenic acid, trans-10, cis-12 conjugated linoleic acid (CLA), oleic acid, stearic acid, and trans-vaccenic acid. The mRNA levels for SCD increased over threefold between 12 and 16 mo of age in IM adipose tissue, similar to the pattern of SCD gene expression in bovine SC adipose tissue (Martin et al., 1999). However, whereas unsaturated fatty acids strongly depressed SCD gene expression in both BSC and IPA in this study, they had no effect on SCD gene expression in IM adipose tissue explants (Choi et al., 2014). We previously demonstrated that trans-10, cis-12 CLA strongly depressed SCD expression in SC preadipocytes (Chung et al., 2006b; Choi et al., 2013a), but CLA only slightly depressed SCD gene expression in intact IM adipose tissue in our recent study (Choi et al., 2014). Conversely, whereas stearic acid strongly stimulated C/EBPβ gene expression in BSC and IPA, stearic acid had no effect on C/EBPβ gene expression in IM adipose tissue explants (Choi et al., 2014). Based on this and previous studies (Chung et al., 2006b; Choi et al., 2013a), we conclude that fatty acids can exert effects on adipogenic or lipogenic gene expression only during the early phases of preadipocyte differentiation.
The saturated fatty acids, palmitic and stearic acid are noteworthy for their strong effects on gene expression. Palmitic acid increased C/EBPβ gene expression in both BSC and IPA (although C/EBPβ gene expression remained barely detectable in BSC). Palmitic acid also increased AMPKα and SCD gene expression in co-cultured IPA. Dietary palmitate, fed in the form of palm oil, increased de novo fatty acid synthesis, enzymes associated with fatty acid synthesis, and adipocyte volume in bovine SC adipose tissue (Choi et al., 2013b). Elevated palmitic acid in plasma or muscle of young cattle may promote trans-differentiation of satellite cells, leading to muscle steatosis. This observation certainly warrants further investigation into the effects of palmitic acid on myogenic gene expression.
Of the fatty acids tested in this study, stearic acid elicited the most consistent increases in adipogenic and lipogenic gene expression. The pronounced effects of stearic acid on PPARγ, C/EBPβ, and SCD gene expression in IPA suggests that stearic acid may promote adipogenesis and fatty acid desaturation during growth in cattle. Stearic acid is the most abundant fatty acid in the digesta of cattle, and is the most abundant saturated fatty acid in bovine plasma (Ekeren et al., 1992; Chung et al., 2006b; Brooks et al., 2010), and thus is readily available to promote adipogenesis. Circulating oleic and linoleic acid may antagonize the effects of saturated fatty acids in promoting SCD gene expression in cattle, consistent with an early study with 3T3-L1 preadipocytes (Sessler et al., 1996) but not PPARγ, C/EBPβ, or AMPKα gene expression, as these unsaturated fatty acids had no effect on the expression of these genes in IPA.
Finally, the results of this study confirm our earlier report (Choi et al., 2013a) that co-culture of BSC and IPA alters the expression of genes associated with adipogenesis and lipogenesis. These data suggest that the coexistence of BSC and IPA within intact muscle would promote IPA differentiation and may even cause trans-differentiation of BSC to IM adipocytes.
ACKNOWLEDGMENTS
Supported by the Beef Checkoff, a Korea Research Foundation Grant funded by the Korean Government (KRF-2008-357-F00030) and the Rural Development Administration (PJ00931701), Republic of Korea.
REFERENCES
- Archibeque SL, Lunt DK, Gilbert CD, Tume RK, Smith SB. Fatty acid indices of stearoyl-CoA desaturase do not reflect actual stearoyl-CoA desaturase enzyme activities in adipose tissues of beef steers finished with corn-, flaxseed-, or sorghum-based diets. J Anim Sci. 2005;83:1153–1166. doi: 10.2527/2005.8351153x. [DOI] [PubMed] [Google Scholar]
- Baxa TJ, Hutcheson JP, Miller MF, Brooks JC, Nichols WT, Streeter MN, Yates DA, Johnson BJ. Additive effects of a steroidal implant and zilpaterol hydrochloride on feedlot performance, carcass characteristics, and skeletal muscle messenger ribonucleic acid abundance in finishing steers. J Anim Sci. 2010;88:330–337. doi: 10.2527/jas.2009-1797. [DOI] [PubMed] [Google Scholar]
- Brooks MA, Choi CW, Lunt DK, Kawachi H, Smith SB. Subcutaneous and intramuscular adipose tissue stearoyl-coenzyme A desaturase gene expression and fatty acid composition in calf- and yearling-fed Angus steers. J Anim Sci. 2011;89:2556–2570. doi: 10.2527/jas.2010-3369. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Cameron PJ, Rogers M, Oman J, May SG, Lunt DK, Smith SB. Stearoyl coenzyme A desaturase enzyme activity and mRNA levels are not different in subcutaneous adipose tissue from Angus and American Wagyu steers. J Anim Sci. 1994;72:2624–2628. doi: 10.2527/1994.72102624x. [DOI] [PubMed] [Google Scholar]
- Chang JHP, Lunt DK, Smith SB. Fatty acid composition and fatty acid elongase and stearoyl-CoA desaturase activities in tissues of steers fed high oleate sunflower seed. J Nutr. 1992;122:2074–2080. doi: 10.1093/jn/122.11.2074. [DOI] [PubMed] [Google Scholar]
- Choi SH, Chung KY, Johnson BJ, Go GW, Kim KH, Choi CW, Smith SB. Co-culture of bovine muscle satellite cells with preadipocytes increases PPARγ and C/EBPβ gene expression in differentiated myoblasts and increases GPR43 gene expression in adipocytes. J Nutr Biochem. 2013a;24:539–543. doi: 10.1016/j.jnutbio.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Choi SH, Gang GO, Sawyer JE, Johnson BJ, Kim KH, Choi CW, Smith SB. Fatty acid biosynthesis and lipogenic enzyme activities in subcutaneous adipose tissue of feedlot steers fed supplementary palm oil or soybean oil. J Anim Sci. 2013b;91:2091–2098. doi: 10.2527/jas.2012-5801. [DOI] [PubMed] [Google Scholar]
- Choi SH, Silvey DT, Johnson BJ, Doumit ME, Chung KY, Sawyer JE, Go GW, Smith SB. Conjugated linoleic acid (t-10, c-12) reduces fatty acid synthesis de novo, but not expression of genes for lipid metabolism in bovine adipose tissue ex vivo. Lipids. 2014;49:15–24. doi: 10.1007/s11745-013-3869-0. [DOI] [PubMed] [Google Scholar]
- Chung KY, Choi CB, Kawachi H, Yano H, Smith SB. Trans-10, cis-12 conjugated linoleic acid antagonizes arginine-promoted differentiation of bovine preadipocytes. Adipocytes. 2006a;2:93–100. [Google Scholar]
- Chung KY, Johnson BJ. Melengestrol acetate enhances adipogenic gene expression in cultured muscle-derived cells. J Anim Sci. 2009;87:3897–3904. doi: 10.2527/jas.2008-1645. [DOI] [PubMed] [Google Scholar]
- Chung KY, Lunt DK, Choi CB, Chae SH, Rhoades RD, Adams TH, Booren B, Smith SB. Lipid characteristics of subcutaneous adipose tissue and M. longissimus thoracis of Angus and Wagyu steers fed to us and Japanese endpoints. Meat Sci. 2006b;73:432–441. doi: 10.1016/j.meatsci.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes inadipocyte differentiation. J Biol Chem. 1998;273:30057–30060. doi: 10.1074/jbc.273.46.30057. [DOI] [PubMed] [Google Scholar]
- Ekeren PA, Smith DR, Lunt DK, Smith SB. Ruminal biohydrogenation of fatty acids from high-oleate sunflower seeds. J Anim Sci. 1992;70:2574–2580. doi: 10.2527/1992.7082574x. [DOI] [PubMed] [Google Scholar]
- Ge H, Li X, Weiszmann J, Wang P, Baibault H, Chen JL, Tian H, Li Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149:4519–4526. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- Hausman GJ, Poulos SP. A method to establish co-cultures of myotubes and preadipocytes from collagenase digested neonatal pig semitendinosus muscles. J Anim Sci. 2005;83:1010–1016. doi: 10.2527/2005.8351010x. [DOI] [PubMed] [Google Scholar]
- Hosseini A, Sauerwein H, Mielenz M. Putative reference genes for gene expression studies in propionate and β-hydroxybutyrate treated bovine adipose tissue explants. J Anim Physiol Anim Nutr (Berlin) 2010;94:e178–184. doi: 10.1111/j.1439-0396.2010.01002.x.Epub. [DOI] [PubMed] [Google Scholar]
- Janovick-Guretzky NA, Dann HM, Carlson DB, Murphy MR, Loor JJ, Drackley JK. Housekeeping gene expression in bovine liver is affected by physiological state, feed intake, and dietary treatment. J Dairy Sci. 2007;90:2246–2252. doi: 10.3168/jds.2006-640. [DOI] [PubMed] [Google Scholar]
- Kadegowda AKG, Burns TA, Pratt SL, Duckett SK. Inhibition of stearoyl-CoA desaturase 1 reduces lipogenesis in primary bovine adipocytes. Lipids. 2013;48:967–976. doi: 10.1007/s11745-013-3823-1. [DOI] [PubMed] [Google Scholar]
- Kang K, Liu W, Albright KJ, Park Y, Pariza MW. trans-10,cis-12 CLA inhibits differentiation of 3T3-L1 adipocytes and decreases PPARγ expression. Biochem Biophys Res Commun. 2003;303:795–799. doi: 10.1016/s0006-291x(03)00413-3. [DOI] [PubMed] [Google Scholar]
- Kim E, Lee JH, Ntambi JM, Hyun CK. Inhibition of stearoyl-CoA desaturase1 activates AMPK and exhibits beneficial lipid metabolic effects in vitro. Eur J Pharmacol. 2011;672:38–44. doi: 10.1016/j.ejphar.2011.09.172. [DOI] [PubMed] [Google Scholar]
- Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediate fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GS, Lunt DK, Britain KG, Smith SB. Postnatal development of stearoyl coenzyme A desaturase gene expression and adiposity in bovine subcutaneous adipose tissue. J Anim Sci. 1999;77:630–636. doi: 10.2527/1999.773630x. [DOI] [PubMed] [Google Scholar]
- Ohyama M, Matsuda K, Torii S, Matsui T, Yano H, Kawada T, Ishihara T. The interaction between vitamin A and thiazolidinedione on bovine adipocyte differentiation in primary culture. J Anim Sci. 1998;76:61–65. doi: 10.2527/1998.76161x. [DOI] [PubMed] [Google Scholar]
- Ouellette SE, Li J, Sun W, Tsuda W, Walker DK, Hersom MJ, Johnson SE. Leucine/glutamic acid/lysine protein 1 is localized to subsets of myonuclei in bovine muscle fibers and satellite cells. J Anim Sci. 2009;87:3134–3141. doi: 10.2527/jas.2009-1998. [DOI] [PubMed] [Google Scholar]
- Park SK, Baek KH, Choi CB. Suppression of adipogenic differentiation by muscle cell-induced decrease in genes related to lipogenesis in muscle and fat co-culture system. Cell Biol Int. 2013;37:1003–1009. doi: 10.1002/cbin.10150. [DOI] [PubMed] [Google Scholar]
- Saladin R, Fajas L, Dana S, Halvorsen YD, Auwerx J, Briggs M. Differential regulation of peroxisome proliferator activated receptor γ1 (PPARγ1) and PPARγ2 messenger RNA expression in the early stages of adipogenesis. Cell Growth Differ. 1999;10:43–48. [PubMed] [Google Scholar]
- Satory DL, Smith SB. Conjugated linoleic acid inhibits proliferation but stimulates lipid filling of murine 3T3-L1 preadipocytes. J Nutr. 1999;129:92–97. doi: 10.1093/jn/129.1.92. [DOI] [PubMed] [Google Scholar]
- Sessler AM, Kaur N, Palta JP, Ntambi JM. Regulation of stearoyl-CoA desaturase 1 mRNA stability by polyunsaturated fatty acids in 3T3-L1 adipocytes. J Biol Chem. 1996;271:29854–29858. doi: 10.1074/jbc.271.47.29854. [DOI] [PubMed] [Google Scholar]
- Smith SB, Go GW, Johnson BJ, Chung KY, Choi SH, Sawyer JE, Silvey DT, Gilmore LA, Ghahramany G, Kim KH. Adipogenic gene expression and fatty acid composition in subcutaneous adipose tissue depots of Angus steers between 9 and 16 months of age. J Anim Sci. 2012;90:2505–2514. doi: 10.2527/jas.2011-4602. [DOI] [PubMed] [Google Scholar]
- St. John LC, Lunt DK, Smith SB. Fatty acid elongation and desaturation enzyme activities of bovine liver and subcutaneous adipose tissue microsomes. J Anim Sci. 1991;69:1064–1073. doi: 10.2527/1991.6931064x. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Hu CY. Effect of retinoic acid on differentiation of cultured pig preadipocytes. J Anim Sci. 1997;75:112–117. doi: 10.2527/1997.751112x. [DOI] [PubMed] [Google Scholar]
- Wan Z, Root-McCaig J, Castellani L, Kemp BE, Steinberg GR, Wright DC. Evidence for the role of AMPK in regulating PGC-1 alpha expression and mitochondrial proteins in mouse epididymal adipose tissue. Obesity (Silver Spring) 2014;22:730–738. doi: 10.1002/oby.20605. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bucher NLR, Farmer SF. Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol Cell Biol. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Mu J, Birnbaum MJ. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J Biol Chem. 2003;278:43074–43080. doi: 10.1074/jbc.M308484200. [DOI] [PubMed] [Google Scholar]


