Abstract
We describe here a simple method for labeling the genome of human cytomegalovirus, a large double-stranded DNA virus, with bromodeoxyuridine (BrdU). The labeled DNA was incorporated into viral particles, which were then collected in cell supernatant. To demonstrate the versatility and effectiveness of this method, labeled virions were used to study the immediate-early events of virus-host cell interaction via indirect immunofluorescence microscopy. It is our hope that this new methodology will prove useful in the study of binding, entry and viral genome deposition in diverse virus systems.
Attachment and entry of viral particles into the host cell constitute a complex process that has been studied in great depth. The advent of electron microscopy (EM) in the 1940s greatly aided the quest to capture the nature of the interaction of a virus with the host cell membrane and transport of the virion capsid (in the case of most DNA viruses) to the nuclear compartment. In the herpesvirus field, there have been several elegant studies utilizing EM to visualize the initial entry events of membrane fusion and the subsequent deposition of the capsid into the cytoplasm (13, 17, 22). Green fluorescent protein technology introduced the ability to specifically label viral capsid, tegument, and glycoproteins (and thus manufacture green virions), which could then be used to study the late life cycle events of assembly and export of herpesvirus virions in real time (6-8, 16). Although recent advances (11, 12) have enabled the visualization of very early deposition of herpesvirus DNA adjacent to intranuclear sites called ND10s (nuclear domains 10), these types of detailed studies are hampered by the tedious and often technically challenging process of dual DNA-protein detection using in situ hybridization and immunolocalization. Until now, we could not observe binding of the intact virion, transport of the DNA-containing capsid, and deposition of input DNA using just one reagent. Here we describe what we believe to be the first incorporation of the thymidine analog, bromodeoxyuridine (BrdU), to make labeled human cytomegalovirus (HCMV) viral particles, which were then used to initiate and follow a subsequent infection. It is our hope that this new technique will prove to be a valuable tool for studying all aspects of viral entry and deposition within the infected host cell, not just for HCMV but also for a broad spectrum of viruses.
A simple method for producing high-titer, labeled HCMV particles.
Low-passage human foreskin fibroblasts (FFs) (<20) were used for this study and were cultured as previously described (10). Confluent FFs were trypsinized and reseeded into T185 flasks at a density of approximately 2.5 × 106 cells/flask. After allowing for resettling, the cells were infected with the Towne strain of HCMV at a multiplicity of infection (MOI) of 0.05 and incubated overnight. Medium was removed and replaced the following morning. When the cells exhibited 80 to 90% cytopathic effect (day 5 to 6 postinfection [p.i.]), the medium was replaced with 17 ml of fresh medium containing 10 μM BrdU. Two days later, the media was spiked with additional BrdU, and the cells were incubated another 24 h. The supernatant was subsequently harvested from the cells, spun at low speed to clear debris, and frozen in aliquots for titration (21) and subsequent use. Flasks with media containing BrdU were kept in dim light during incubation to avoid photolysis of BrdU residues. Virus with titers within a normal range (∼2 × 106 to 5 × 106 PFU/ml) was recovered from these flasks, indicating that the incorporation of BrdU into the genome did not impede the life cycle of the virus.
Labeled virus can be used to study binding, transport, and entry into the nuclei of infected cells.
Confluent FFs were trypsinized, reseeded onto coverslips, and allowed to settle for approximately 2 h prior to infection at an MOI of either 1 or 3 with BrdU-labeled virus. We used two protocols for the experiments described herein. First, to study the initial events of viral entry and transport to the nucleus, we used protocol 1. In this format, cells were placed at 4°C for 30 min prior to infection. After this time, cold virus was added to the cells and allowed to adsorb to the surface for a further 30 min at 4°C prior to removal of inoculum. Coverslips were then washed in phosphate-buffered saline and either harvested immediately or refed with fresh media and transferred to 37°C for later collection. This protocol allowed for a relatively synchronous infection and was utilized for the experiments shown in Fig. 1 and 2. We found that removal of excess virions not bound after 30 min of adsorption greatly simplified our viewing of a single wave of particles entering the cell for these experiments. Protocol 2, used in the experiments shown in Fig. 3 and 4, allowed a more natural interaction of virus with host cell by adding virus directly to cells incubating at 37°C.
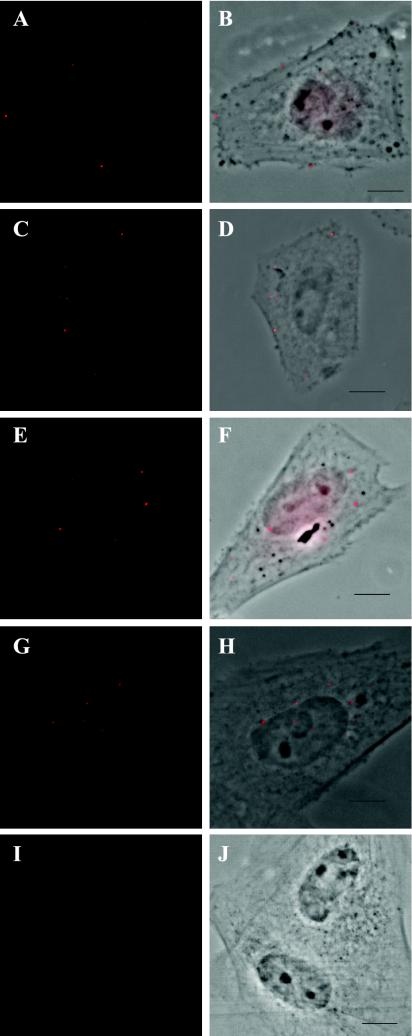
FIG. 1.
A time course of the progression of labeled HCMV into the nucleus. Human FFs were seeded onto glass coverslips, infected at an MOI of 1 by using protocol 1, harvested and stained for BrdU as indicated in the text (rat anti-BrdU Ab OBT030CX from Accurate Chemicals Scientific Corp., anti-rat tetramethyl rhodamine isothiocyanate-labeled secondary Ab from Jackson Immunoresearch Laborato-ries). Cells were analyzed on a Nikon Eclipse E800 epifluorescence microscope equipped with a digital camera and Metamorph imaging software. BrdU staining (red in Panels A, C, E, G, and I) was overlaid onto phase-contrast images in panels B, D, F, H, and J. (A and B) Cells incubated with virus on ice for 30 min prior to harvesting. (C and D) Cells harvested at 15 min post-cold release. (E and F) Cells harvested at 30 min post-cold release. (G and H) Cells harvested at 1 h post-cold release. (I and J) Cells incubated with supernatant gathered from mock-infected cells after treatment for 3 days with BrdU. Cells were harvested at 30 min p.i. Bar = 5 μm for all figures.
FIG. 2.
BrdU-labeled cells are pp65 positive. Cells were seeded, infected at an MOI of 1 using protocol 1, harvested at 3 h p.i. and stained for pp65 (green label) using Ab 1205S (The Rumbaugh-Goodwin Institute) followed by anti-mouse immunoglobulin G1 (IgG1) Alexa Fluor-coupled secondary Ab (Molecular Probes). After a second fixation, cells were stained for BrdU (red label) as described in the text. (A) pp65 staining; (B) BrdU staining; (C) phase contrast with BrdU overlay.
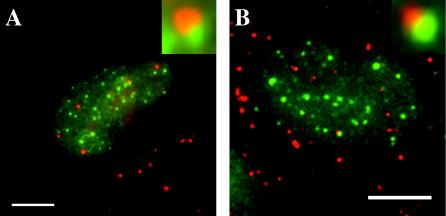
FIG. 3.
A subset of BrdU foci is juxtaposed to PML sites in infected nuclei. Cells were infected at an MOI of 3 using protocol 2, harvested at 3.5 h p.i., and stained for PML (green label) using Santa Cruz Ab SC966 detected with goat anti-mouse IgG1 Alexa Fluor-coupled secondary Ab (Molecular Probes). After a second round of fixation, cells were stained for BrdU as described in the text (red label). The small insets are further magnification of the overlapping regions within the nuclei pictured. (A) Cells were washed after 30 min p.i. (B) Cells were unwashed for the duration of the experiment.
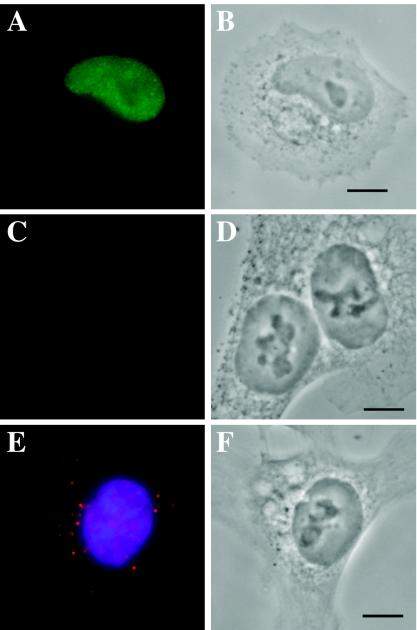
FIG. 4.
HCT116 cells are blocked for nuclear import. HCT116 cells were seeded onto polylysine-coated coverslips, infected at an MOI of 1 using protocol 2, and harvested at 24 h p.i. as described. All images were taken by using Z series. (A) FS2 cells stained for immediate-early protein IE1 using Ab p63-27 (a gift from William Britt, University of Alabama) followed by IgG2A anti-mouse Alexa Fluor-conjugated secondary Ab (Molecular Probes). (B) Phase-contrast image of cells in panel A. (C) HCT116 cells stained for IE1. (D) Phase-contrast image of Panel C. (E) Overlaid image of HCT116 nuclei stained for BrdU (red label) and DNA (blue label). (F) Phase-contrast image of panel E.
Harvested coverslips were washed in phosphate-buffered saline (PBS) prior to fixation in 3% formaldehyde for 10 min at room temperature (RT), followed by permeabilization with 1% Triton X-100 for 5 min. After several rounds of PBS washing, cells were treated for 10 min at RT with a 4 N HCl solution (diluted in water) to expose the BrdU residues for staining. The cells were then washed several times with RT PBS. Coverslips were blocked with 30% fetal bovine serum in a blocking solution (PBS with 1% bovine serum albumin and 0.01% Tween 20) for 15 min. Cells were then stained with a rat anti-BrdU antibody (Ab), followed by detection using a cross-adsorbed anti-rat rhodamine-labeled secondary Ab. Cells were counterstained with Hoechst dye to visualize total nuclear DNA.
In all of the figures, the viral particles are seen as small red foci with an average diameter of approximately 250 nm. This is directly parallel to what has been described earlier as single genome “point sized particles,” using in situ hybridization techniques (11, 12). Given that the size of an HCMV capsid is approximately 130 nm (3) and that 50 to 100 nm must be added to this figure to account for primary and secondary Ab binding (1), it is not unreasonable to assume that each 250-nm focus is a single genome-containing capsid. However, we cannot formally rule out the possibility that these spots may represent two adjacent capsids.
Figure 1 illustrates the ability of our reagent to detect virion binding and entry, capsid traverse of the cytoplasm, and movement of the viral DNA into the interior of the nucleus. Figure 1A and B depict the binding of the virions to the outside of the plasma membrane using protocol 1 (MOI = 1). It should be noted that the foci are in close proximity to but not inside the membrane, as would be expected under these conditions, which promote binding in the absence of penetration (4, 22). After the initial 30 min of preadsorption, the cells were washed, released to 37°C, and monitored for the next 3 h. We observed punctate staining of BrdU within the cytoplasm of the infected cells as early as 15 min p.i. (Fig. 1C and D). This is in clear agreement with earlier EM studies showing capsids within the cytoplasm at approximately 20 min p.i. (13, 17). We also visualized foci “rimming” the nucleus by 30 min p.i. (Fig. 1E and F), which we believe is indicative of viral particles preparing to deposit their genomes into the nucleoplasm. Forty-five to sixty minutes p.i., we saw punctate staining within the nucleus itself, with multiple foci per nucleus (Fig. 1G and H). The number of foci within each nucleus continued to increase over the 3-h time course (Fig. 2).
If the same time course was observed under conditions allowing small incremental stepping (250 nm) of the microscope stage in the Z axis (Z series), we observed the same pattern as that shown in Fig. 1 and 2 (data not shown). That is, if an object appeared as a sharp, bright spot, it was within the focal plane as viewed in the phase image. Therefore objects that are sharply in focus within the nucleus in Fig. 1G and H and Fig. 2B are within the nuclear interior. The punctate staining we observed during our time course was not apparent in cells infected with either mock or unlabeled Towne virus and otherwise treated identically to those in this experiment (data not shown). This staining pattern was also not observed in cells treated with supernatant gathered from mock-infected cells after treatment for 3 days with 10 μM BrdU (Fig. 1I and J). The number of foci per cell and the number of cells displaying foci were MOI dependent (Fig. 3 [MOI = 3] and data not shown).
To support our assertion that the BrdU foci were in fact viral particles, we looked for uninfected cells in our infection analyzed in Fig. 1. Using the well-accepted assertion that an infected cell not blocked for viral entry accumulates the tegument protein pp65 within its nucleus (9), we assayed for pp65 positivity after incubation with our viral supernatant. As seen in Fig. 2, only cells that were pp65+ contained BrdU foci. If a cell was pp65−, it did not show any BrdU staining above our background level.
We presumed that some of the foci we were observing represented defective genomes, since we infected at an MOI of 1 for Fig. 1 and 2 and yet were seeing up to 5 times that many foci per cell. Maul and colleagues (11, 12) have seen this phenomenon of numerous “extra” genomes during their in situ hybridization experiments. They have shown that only the small proportion of viral genomes that localize to ND10 sites are capable of beginning immediate-early transcription of viral templates and are therefore functional. To increase the number of potential genomes within the nucleus, we performed an infection at an MOI of 3 using protocol 2. We did these experiments either including (Fig. 3A) or excluding (Fig. 3B) the wash step at 30 min p.i. To confirm that both active and nonfunctional genomes were present, we double labeled our coverslips for both BrdU residues and promyelocytic leukemia (PML) protein, to mark the sites of ND10 localization. This double labeling was accomplished by slightly modifying the detection protocol described above. After initial fixation, the cells were stained for PML, followed by an anti-mouse Alexa Fluor-coupled secondary Ab for detection. They were then fixed again in formaldehyde and subsequently carried through the acid treatment and BrdU staining. Just as observed by Maul and colleagues, we found a small subset of the BrdU foci juxtaposed to PML sites within the nucleus (Fig. 3A and B). Although the number of BrdU foci within the nucleus increases with the exclusion of the washing step (Fig. 3B), the subset localized next to the PML remains small. As described above, these images were taken by using a Z series, allowing for sectioning of the cell at increments of approximately 250 nm. Therefore, the objects in clear focus are indeed in very close proximity.
In both Fig. 3A and B, numerous foci remain in the cytoplasm at this 3.5-h p.i. time point. This too was observed by Maul and colleagues (11), who hypothesized that it might be due to capsids that remained uncoated or at least released their virions very slowly. Perhaps this extended localization in the cytoplasm is also due in part to a phenomenon described by Ogawa-Goto and colleagues (14). While studying transport of HCMV capsids along microtubules, they found that if microtubules were depolymerized, a slow, very inefficient infection still occurred, possibly resulting from random diffusion of the capsids toward the nucleus.
Labeled viral particles can be used in studies of permissiveness.
Viral tropism and permissiveness are issues that often must be addressed in the field of herpesvirus virology. It is often very time consuming and difficult to determine at which stage a particular cell type may be blocked for viral entry and/or gene expression. Our labeled virus can be used to very quickly and efficiently perform preliminary analyses to determine where this block might occur. Infecting at an MOI of 1 using protocol 2, we asynchronously infected FFs (which are highly permissive for the virus) and a colon carcinoma cell line, HCT116 (a gift from Bert Vogelstein) (2), that appeared to be blocked at a very early stage of infection, as witnessed by its lack of immediate-early protein staining even after 24 h p.i. (compare Fig. 4C and D to Fig. 4A and B). Even after an extended infection time of 24 h (without a wash to remove the inoculum), we were unable to see BrdU foci progress further than the rim of the nuclear membrane in the HCT116 cells (Fig. 4E, red label, and 4F, phase image). These findings are similar to results obtained by Slobbe van-Drunun and colleagues while studying the permissiveness of human umbilical vein endothelial cells for the HCMV laboratory passaged strain AD 169 (20). Using in situ hybridization techniques, they showed a distinct accumulation of viral DNA at the periphery of the nucleus, with only a very small percentage of cells (<5%) eventually allowing import of the genome and subsequent viral gene expression. Although others have argued that the viral particles do not actually make it to the nuclear periphery of human umbilical vein endothelial cells but instead are defective in transport through the cytoplasm (19), the end conclusion is the same; at our limit of detection (60 nm/pixel, or half the size of an HCMV capsid), we see no import of the viral genomes into the nuclei of these cells.
Conclusions.
We are well aware that this is not the first time that BrdU has been used to label viral DNA. Indeed, BrdU has often been incorporated into newly replicated viral DNA as a means of localizing replication compartments in many viral systems (for herpesvirus-specific references, see 5, 18, and 23). BrdU has even been used to follow newly synthesized HCMV DNA from replication centers within the nucleus out into the cytoplasm in chase experiments (15). This is, however, the first description of using labeled virions to study all three processes of binding, transport, and genome deposition with one reagent. We have demonstrated the utility of our technique and can envision that it will greatly simplify and lead to innovative studies of the immediate-early events within the virus life cycle. We also imagine that this technique will work equally well for labeling RNA viral genomes using bromouridine residues. In addition, with the incorporation of fluorescently labeled nucleotides, the technique could be adapted to real-time studies of the localization of viral particles. This last modification would be especially useful for tracking viral vectors used for gene delivery to ascertain which cells the vectors have infected in vivo. Studies in our lab are under way, using our reagent to look at early localization of cellular proteins to the sites of viral input within the nucleus.
Acknowledgments
This work was supported in part by NIH grant RO1-AI51463 to E.A.F.
We thank Veronica Sanchez, Jill Johnson, and Gary Daughdrill for helpful discussions in the preparation of the manuscript.
REFERENCES
- 1.Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts, and J. D. Watson. 1994. The immune system, p. 1207. In Molecular biology of the cell, 3rd ed. Garland Publishing, New York, N.Y.
- 2.Bunz, F., A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497-1501. [DOI] [PubMed] [Google Scholar]
- 3.Butcher, S. J., J. Aitken, J. Mitchell, B. Gowen, and D. J. Dargan. 1998. Structure of the human cytomegalovirus B capsid by electron cryomicroscopy and image reconstruction. J. Struct. Biol. 124:70-76. [DOI] [PubMed] [Google Scholar]
- 4.Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191:387-395. [DOI] [PubMed] [Google Scholar]
- 5.de Bruyn Kops, A., and D. M. Knipe. 1988. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell 55:857-868. [DOI] [PubMed] [Google Scholar]
- 6.Desai, P., and S. Person. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 72:7563-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott, G., and P. O'Hare. 1999. Live-cell analysis of a green fluorescent protein-tagged herpes simplex virus infection. J. Virol. 73:4110-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enquist, L. W., M. J. Tomishima, S. Gross, and G. A. Smith. 2002. Directional spread of an alpha-herpesvirus in the nervous system. Vet. Microbiol. 86:5-16. [DOI] [PubMed] [Google Scholar]
- 9.Fortunato, E. A., M. L. Dell'Aquila, and D. H. Spector. 2000. Specific chromosome 1 breaks induced by human cytomegalovirus. Proc. Natl. Acad. Sci. USA 97:853-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortunato, E. A., and D. H. Spector. 1998. p53 and RPA are sequestered in viral replication centers in the nuclei of cells infected with human cytomegalovirus. J. Virol. 72:2033-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishov, A. M., R. M. Stenberg, and G. G. Maul. 1997. Human cytomegalovirus immediate early interaction with host nuclear structures: definition of an immediate transcript environment. J. Cell Biol. 138:5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maul, G. G., A. M. Ishov, and R. D. Everett. 1996. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology 217:67-75. [DOI] [PubMed] [Google Scholar]
- 13.Morgan, C., H. M. Rose, and B. Mednis. 1968. Electron microscopy of herpes simplex virus. I. Entry. J. Virol. 2:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa-Goto, K., K. Tanaka, W. Gibson, E. Moriishi, Y. Miura, T. Kurata, S. Irie, and T. Sata. 2003. Microtubule network facilitates nuclear targeting of human cytomegalovirus capsid. J. Virol. 77:8541-8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penfold, M. E., and E. S. Mocarski. 1997. Formation of cytomegalovirus DNA replication compartments defined by localization of viral proteins and DNA synthesis. Virology 239:46-61. [DOI] [PubMed] [Google Scholar]
- 16.Potel, C., K. Kaelin, I. Gautier, P. Lebon, J. Coppey, and F. Rozenberg. 2002. Incorporation of green fluorescent protein into the essential envelope glycoprotein B of herpes simplex virus type 1. J. Virol. Methods 105:13-23. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal, K. S., J. Killius, C. M. Hodnichak, T. M. Venetta, L. Gyurgyik, and K. Janiga. 1989. Mild acidic pH inhibition of the major pathway of herpes simplex virus entry into HEp-2 cells. J. Gen. Virol. 70:857-867. [DOI] [PubMed] [Google Scholar]
- 18.Sarisky, R. T., and G. S. Hayward. 1996. Evidence that the UL84 gene product of human cytomegalovirus is essential for promoting oriLyt-dependent DNA replication and formation of replication compartments in cotransfection assays. J. Virol. 70:7398-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinzger, C., M. Kahl, K. Laib, K. Klingel, P. Rieger, B. Plachter, and G. Jahn. 2000. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J. Gen. Virol. 81:3021-3035. [DOI] [PubMed] [Google Scholar]
- 20.Slobbe-van Drunen, M. E., A. T. Hendrickx, R. C. Vossen, E. J. Speel, M. C. van Dam-Mieras, and C. A. Bruggeman. 1998. Nuclear import as a barrier to infection of human umbilical vein endothelial cells by human cytomegalovirus strain AD169. Virus Res. 56:149-156. [DOI] [PubMed] [Google Scholar]
- 21.Tamashiro, J. C., L. J. Hock, and D. H. Spector. 1982. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J. Virol. 42:547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Topilko, A., and S. Michelson. 1994. Hyperimmediate entry of human cytomegalovirus virions and dense bodies into human fibroblasts. Res. Virol. 145:75-82. [DOI] [PubMed] [Google Scholar]
- 23.Zhong, L., and G. S. Hayward. 1997. Assembly of complete, functionally active herpes simplex virus DNA replication compartments and recruitment of associated viral and cellular proteins in transient cotransfection assays. J. Virol. 71:3146-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]