Abstract
Detection of the mouse hepatitis virus receptor within the central nervous system (CNS) has been elusive. Receptor expression on microglia was reduced during acute infection and restored following immune-mediated virus control. Receptor down regulation was independent of neutrophils, NK cells, gamma interferon, or perforin. Infection of mice devoid of distinct inflammatory cells revealed CD4+ T cells as the major cell type influencing receptor expression by microglia. In addition to demonstrating receptor expression on CNS resident cells, these data suggest that transient receptor down regulation on microglia aids in establishing persistence in the CNS by assisting virus infection of other glial cell types.
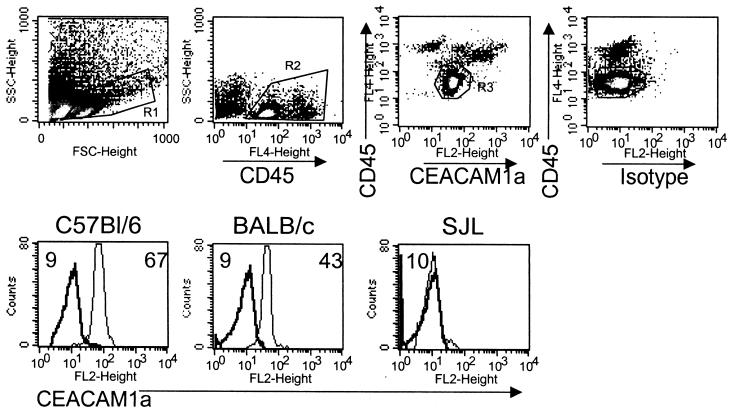
Interactions between the mouse hepatitis virus (MHV) spike (S) protein and the virus receptor influences tropism, spread, and pathogenesis (8, 17). The primary MHV receptor is the four-immunoglobulin isoform of CEACAM1a (1, 4). Low-affinity binding to related molecules and receptor-independent infection in vitro have also been described (5, 7, 9, 25-27). Despite limited in vitro receptor usage by the neurotropic JHM strain of MHV (JHMV) (5, 25, 27), central nervous system (CNS) ependymal cells, microglia, oligodendroglia, astrocytes, and neurons are infected in vivo (22). Low levels of MHV receptor mRNA are expressed by the CNS (5, 24). However, no receptor was identified by a direct binding assay (23), and only CNS endothelial cells, but not glial cells, expressed the receptor by immunohistochemistry (10). The absence of CNS expression led to proposals of alternate lower-affinity receptors (5) or a receptor-independent mechanism of virus infection (15). To resolve this issue, CEACAM1a expression on CD45low microglia isolated from the CNS of adult mice was examined. CNS mononuclear cells were isolated from homogenates by using a Percoll step gradient as described previously (2, 3). CNS-derived cells were labeled with phycoerythrin, fluorescein isothiocyanate, or allophycocyanin-labeled monoclonal antibodies (MAb) specific for CD45 (30-F11), CD4 (RM4-5), CD8 (53-6.7), MHC class II I-A/I-E (2G9), Ly-6G (1A8), CD11b (M1/70) (BD PharMingen, San Diego, Calif.), and F4/80 (Serotec, Raleigh, N.C.). Receptor expression was detected by using biotinylated MAb CC-1 (23) and phycoerythrin-conjugated avidin. Biotinylated immunoglobulin G1 (BD PharMingen) was used as an isotype control. Cells were analyzed on a FACS Calibur flow cytometer (Becton Dickinson, San Jose, Calif.), using Cellquest software. CEACAM1a was expressed by microglia from naïve susceptible C57BL/6 and BALB/c mice but not by microglia from resistant SJL mice (National Cancer Institute, Fredrick, Md.) (Fig. 1).
FIG. 1.
CEACAM1a expression on microglia from adult mice. Single-cell brain suspensions from naïve mice were stained with anti-CD45, anti-CEACAM1a, or isotype control MAb. In the top row are representative density plots showing forward-side scatter and staining patterns. R1 indicates the live cell gate used for analysis. CD45 expression differentiates CD45low microglia and CD45high infiltrating cells (combined in R2). R3 and R4 highlight CD45low microglia stained with CC-1 or isotype control MAb. Shown in the bottom row is CEACAM1a expression (light lines) on microglia from C57Bl/6 and BALB/c mice but not SJL mice (R3). Dark lines represent isotype control MAb (R4); numbers are MFI values for CEACAM1a (right) and the isotype control (left).
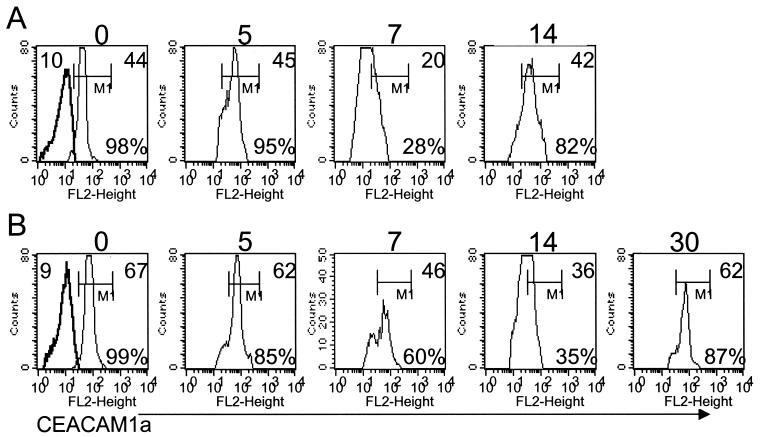
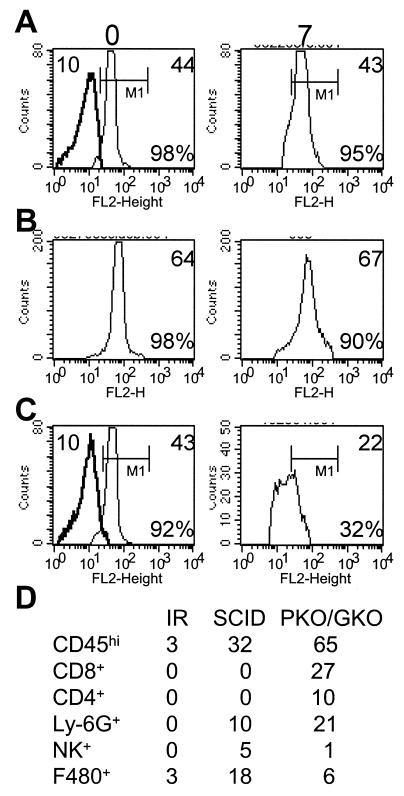
Microglia from BALB/c mice infected intracerebrally with 500 PFU of the J.2.2v-1 MAb-derived variant of JHMV (8) were examined at various times postinfection (p.i.). Whereas receptor expression was unaltered at 5 days p.i, expression decreased at 7 days p.i. based on mean fluorescence intensity (MFI) (Fig. 2A). The majority of microglia regained CEACAM1a expression by day 14 p.i. (Fig. 2A), and this expression returned to uninfected levels by day 30 p.i. (data not shown) concomitantly with virus clearance and partial resolution of inflammation. Similarly, microglia from C57BL/6 mice infected with 250 PFU of JHMV also exhibited reduced receptor expression by 7 days p.i. (Fig. 2B). Although down regulation was protracted in infected C57BL/6 mice compared to BALB/c mice (Fig. 2A and B), receptor was reexpressed at naïve levels by day 30 p.i. (Fig. 2B). These results suggest that either virus or inflammation regulates microglia receptor expression. Intracellular S protein-receptor interaction (18) or extracellular progeny could inhibit receptor detection. Sequestration appears unlikely, as only a small fraction of microglia are infected (22). To distinguish between a viral effect and inflammation, receptor expression was investigated during uncontrolled virus replication in mice immunosuppressed by irradiation (850 rad) 24 h prior to infection (16, 28) and in immunodeficient SCID mice (3). At day 7 p.i., receptor expression on microglia from infected irradiated BALB/c mice (Fig. 3A) and C57BL/6 mice (data not shown) was equivalent to that on microglia from naïve mice. No loss in receptor expression was noted in a limited number of survivors at day 9 p.i. (data not shown). CD45high inflammatory cells (Fig. 3D) were below the detection level (16, 28). Unaltered receptor expression despite uncontrolled virus replication (16, 28) indicates that neither intracellular trapping, extracellular binding of viral particles, nor soluble S protein masks receptor detection.
FIG. 2.
CEACAM1a expression on microglia is down regulated following JHMV infection. CD45low microglia from naïve mice compared with those from infected BALB/c (A) or C57Bl/6 (B) mice at days 5, 7, 14 and 30 p.i. were analyzed for CEACAM1a expression. The number above each histogram is the number of days p.i. Regions and gates are described in Fig. 1. Numbers in the upper parts of the histograms are MFI values for MAb CC1 (right) and the isotype control (left). Percentages of microglia positive for CEACAM1a expression are given in the bottom parts of the histograms. The data presented are representative of three to five experiments.
FIG. 3.
CEACAM1a expression on microglia from immunodeficient mice. (A to C) CD45low microglia isolated from naïve (left) or infected irradiated BALB/c (A), SCID (B), or PKO/GKO (C) mice at day 7 p.i. (right) stained for CEACAM1a (light line) or the isotype control (dark line). Regions and gates are described in Fig. 1. Numbers in the upper parts of the histograms are MFI values for CEACAM1a (right) or the isotype control (left). Percentages of microglia expressing CEACAM1a are given in the bottom right parts of the histograms. (D) Percentages of CD45high infiltrating cells and distinct phenotypic populations in irradiated BALB/c (IR), SCID, and PKO/GKO mice at 7 days p.i. The data presented are representative of three to five experiments.
CNS infection induces the recruitment of innate and adaptive immune components (2, 3, 29). SCID mice were infected with 500 PFU of JHMV to determine whether neutrophils, NK cells, or macrophages regulate receptor expression (Fig. 3D). Microglia from uninfected and infected SCID mice exhibited no difference in receptor expression at 7 days p.i. (Fig. 3B) or at 10 days p.i. (data not shown). These data confirm that decreased receptor expression is not associated with virus replication and suggest that neutrophils, matrix metalloprotease-9 secretion (29), NK cells, and macrophages are not sufficient to influence receptor expression. To test gamma interferon (IFN-γ) secretion and perforin-mediated cytolysis as potential mediators, mice deficient in both functions (PKO/GKO mice) (2) were infected with 500 PFU of JHMV. Receptor expression on microglia from infected PKO/GKO mice was decreased at day 7 p.i. (Fig. 3C), indicating that neither of these anti-viral effector mechanisms are sufficient to mediate decreased receptor expression.
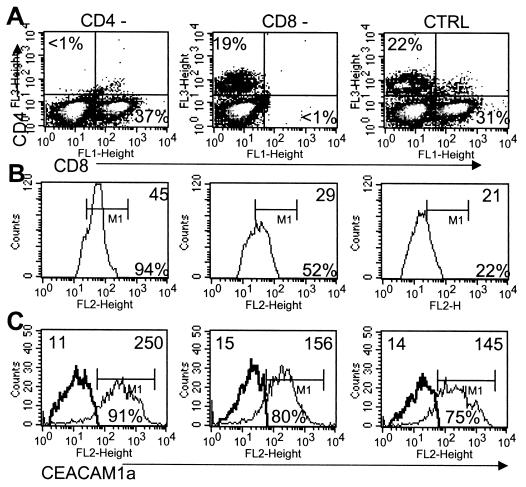
The fact that receptor expression in infected SCID mice was distinct from that in infected PKO/GKO mice suggested a link between T-cell infiltration and reduced receptor levels. Mice were therefore depleted of CD4+ T cells (MAb GK1.5) or CD8+ T cells (MAb 2.43) by MAb treatment on days −1, +1, and + 3 relative to infection (Fig. 4A). Controls received anti-β-galactosidase MAb GL113. The majority of microglia isolated from infected CD4+-T-cell-deficient mice at day 7 p.i. retained receptor expression compared to controls (Fig. 4B). Receptor loss was less severe on microglia from CD8+-T-cell-depleted mice (Fig. 4B), despite CD4+-T-cell accumulation in the CNS similar to that for infected controls (data not shown). CD45high CD11b+/class II+ macrophages from the CNS of CD4+-T-cell-deficient mice also retained receptor expression at 7 days p.i., while a minority of macrophages within the CNS of CD8-deficient mice exhibited decreased expression (Fig. 4C). These data indicate that CD4+ T cells and, to a lesser extent, CD8+ T cells influence the loss of receptor expression on both microglia and infiltrating macrophages.
FIG. 4.
CEACAM1a expression on microglia and macrophages is regulated by CD4+ T cells. Mice were treated with anti-CD4, anti-CD8, or control MAb. CNS cells from infected CD4-depleted (CD4−; left), CD8-depleted (CD8−; middle), or control (CTRL; right) mice confirm the absence of specific cell types (A). Percentages of CD4+ or CD8+ T cells within the CNS infiltrates are indicated. CD45low microglia (B) or CD45high infiltrating CD11b+/class II+ macrophages (C) at day 7 p.i. were analyzed for CEACAM1a expression. In panels B and C, numbers in the upper parts of the histograms are MFI values and percentages in the bottom right parts of the histograms are those of cells expressing CEACAM1a. The data presented represent one of three similar experiments.
Regulation of receptor expression by T cells appears to be a novel method by which immune responses influence the course of viral infection in an immune-privileged site via inhibition of attachment and entry. While protective, cytotoxic mechanisms in the CNS also induce immune-mediated pathology (3), decreased microglial susceptibility may thus be a mechanism to avoid overt immune pathology. The mechanism(s) by which CD4+ T cells regulate receptor expression on microglia is unclear but is likely to involve soluble factors, although the possibility of a reversible conformational change cannot be ruled out. JHMV infection results in the release of proinflammatory cytokines, chemokines, and matrix metalloproteinases (13, 16, 28). Analysis of IFN-γ-deficient mice excluded the possibility of a role for IFN-γ in receptor regulation in vivo, despite contradicting in vitro studies (21). IFN-α/β mRNA is also increased in the CNS of immunodeficient mice (unpublished observation), suggesting no correlation with receptor down regulation. However, unlike many potential proinflammatory cytokines that are decreased prior to diminished receptor expression (16), RANTES is maintained until JHMV infection and inflammation resolve (13), correlating with the transient down regulation of CEACAM1a on microglia. Reduced receptor expression occurs as infectious virus declines and thus may contribute to the limiting of virus spread, cytopathology, and potential glial cell-mediated damage. Viral persistence in vitro is associated with both reduced cytopathology (19, 20) and reduced receptor expression (6, 19), while increased expression enhances virus-induced cytopathology (18). Receptor down regulation in vivo appears to be widespread, although only a few microglia or macrophages are infected (22). Down regulation may be specific for only microglia and macrophages, thereby facilitating the infection of other glial cell types not responsive to receptor down regulation. Alternatively, reduced expression may modulate lymphocyte function. CEACAM1a signaling increases T-cell chemotaxis and cytokine secretion (12, 14) as well as B-cell activation (11). Thus, reduced receptor on CNS resident antigen-presenting cells may reduce T-cell recruitment, cytokine secretion, and possibly cytolysis in an attempt to limit immune-mediated pathology.
Acknowledgments
This work was supported by National Institutes of Health grants NS18146, NS40667, AI47248, and AI25231.
REFERENCES
- 1.Beauchemin, N., P. Draber, G. Dveksler, P. Gold, S. Gray-Owen, F. Grunert, S. Hammarstrom, K. V. Holmes, A. Karlsson, M. Kuroki, S. H. Lin, L. Lucka, S. M. Najjar, M. Neumaier, B. Obrink, J. E. Shively, K. M. Skubitz, C. P. Stanners, P. Thomas, J. A. Thompson, M. Virji, S. von Kleist, C. Wagener, S. Watt, and W. Zimmermann. 1991. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 252:243. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann, C. C., B. Parra, R. Hinton, R. Chandran, M. Morrison, and S. A. Stohlman. 2003. Perforin-mediated effector function within the central nervous system requires IFN-gamma-mediated MHC up-regulation. J. Immunol. 170:3204-3213. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann, C. C., B. Parra, D. R. Hinton, C. Ramakrishna, K. C. Dowdell, and S. A. Stohlman. 2004. Perforin and interferon gamma mediated control of coronavirus central nervous system infection by CD8 T cells in the absence of CD4 T cells. J. Virol. 78:1739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blau, D. M., C. Turbide, M. Tremblay, M. Olson, S. Letourneau, E. Michaliszyn, S. Jothy, K. V. Holmes, and N. Beauchemin. 2001. Targeted disruption of the Ceacam1 (MHVR) gene leads to reduced susceptibility of mice to mouse hepatitis virus infection. J. Virol. 75:8173-8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, D. S., M. Asanaka, K. Yokomori, F. Wang, S. B. Hwang, H. P. Li, and M. M. Lai. 1995. A pregnancy-specific glycoprotein is expressed in the brain and serves as a receptor for mouse hepatitis virus. Proc. Natl. Acad. Sci. USA 92:12095-12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, W., and R. S. Baric. 1996. Molecular anatomy of mouse hepatitis virus persistence: coevolution of increased host cell resistance and virus virulence. J. Virol. 70:3947-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dveksler, G. S., C. W. Dieffenbach, C. B. Cardellichio, K. McCuaig, M. N. Pensiero, G. S. Jiang, N. Beauchemin, and K. V. Holmes. 1993. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus-A59. J. Virol. 67:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming, J. O., M. D. Trousdale, F. El-Zaatari, S. A. Stohlman, and L. P. Weiner. 1986. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J. Virol. 58:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher, T. M., M. J. Buchmeier, and S. Perlman. 1992. Cell receptor-independent infection by a neurotropic murine coronavirus. Virology 191:517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfraind, C., N. Havaux, K. V. Holmes, and J. P. Coutelier. 1997. Role of virus receptor-bearing endothelial cells of the blood-brain barrier in preventing the spread of mouse hepatitis virus-A59 into the central nervous system. J. Neurovirol. 3:428-434. [DOI] [PubMed] [Google Scholar]
- 11.Greicius, G., E. Severinson, N. Beauchemin, B. Obrink, and B. B. Singer. 2003. CEACAM1 is a potent regulator of B cell receptor complex-induced activation. J. Leukoc. Biol. 74:126-134. [DOI] [PubMed] [Google Scholar]
- 12.Kammerer, R., D. Stober, B. B. Singer., B. Obrink, and J. Reimann. 2001. Carcinoembryonic antigen-related cell adhesion molecule 1 on murine dendritic cells is a potent regulator of T cell stimulation. J. Immunol. 166:6537-6544. [DOI] [PubMed] [Google Scholar]
- 13.Lane, T. E., M. T. Liu, B. P. Chen, V. C. Asensio, R. M. Samawi, A. D. Paoletti, I. L. Campbell, S. L. Kunkel, H. S. Fox, and M. J. Buchmeier. 2000. A central role for CD4+ T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J. Virol. 74:1415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakajima, A., H. Iijima, M. F. Neurath, T. Nagaishi, E. E. Nieuwenhuis, R. Raychowdhury, J. Glickman, D. M. Blau, S. Russell, K. V. Holmes, and R. S. Blumberg. 2002. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J. Immunol. 168:1028-1035. [DOI] [PubMed] [Google Scholar]
- 15.Ontiveros, E., T. S. Kim, T. M. Gallagher, and S. Perlman. 2003. Enhanced virulence mediated by the murine coronavirus, mouse hepatitis virus strain JHM, is associated with a glycine at residue 310 of the spike glycoprotein. J. Virol. 77:10260-10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parra, B., D. R. Hinton, M. T. Lin, D. J. Cua, and S. A. Stohlman. 1997. Kinetics of cytokine mRNA expression in the central nervous system following lethal and nonlethal coronavirus-induced acute encephalomyelitis. Virology 233:260-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips, J. J., M. M. Chua, E. Lavi, and S. R. Weiss. 1999. Pathogenesis of MHV-4/MHV-A59 recombinant viruses: the murine coronavirus spike protein is a major determinant of neurovirulence. J. Virol. 73:7752-7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao, P. V., and T. M. Gallagher. 1998. Intracellular complexes of viral spike and cellular receptor accumulate during cytopathic murine coronavirus infections. J. Virol. 72:3278-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawicki, S. G., J. H. Lu, and K. V. Holmes. 1995. Persistent infection of cultured cells with mouse hepatitis virus (MHV) results from the epigenetic expression of the MHV receptor. J. Virol. 69:5535-5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stohlman, S. A., A. Y. Sakaguchi, and L. P. Weiner. 1979. Characterization of the cold-sensitive murine hepatitis virus mutants rescued from latently infected cells by cell fusion. Virology 98:448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassao, R. C., M. T. de Franco, D. Hartz, M. Modolell, A. E. Sippel, and C. A. Pereira. 2000. Down-regulation of Bgp1(a) viral receptor by interferon-gamma is related to the antiviral state and resistance to mouse hepatitis virus 3 infection. Virology 274:278-283. [DOI] [PubMed] [Google Scholar]
- 22.Wang, F. I., D. R. Hinton, W. Gilmore, M. D. Trousdale, and J. O. Fleming. 1992. Sequential infection of glial cells by the murine hepatitis virus JHM strain (MHV-4) leads to a characteristic distribution of demyelination. Lab. Investig. 66:744-754. [PubMed] [Google Scholar]
- 23.Williams, R. K., G.-S. Jiang, S. W. Snyder, M. F. Frana, and K. V. Holmes. 1990. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (MHV)-A59 from mouse liver and identification of a nonfunctional, homologous protein in MHV-resistant SJL/J mice. J. Virol. 64:3817-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yokomori, K., and M. M. Lai. 1992. Mouse hepatitis virus utilizes two carcinoembryonic antigens as alternative receptors. J. Virol. 66:6194-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokomori, K., and M. M. Lai. 1992. The receptor for mouse hepatitis virus in the resistant mouse strain SJL is functional: implications for the requirement of a second factor for viral infection. J. Virol. 66:6931-6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokomori, K., M. Asanaka, S. A. Stohlman, and M. M. Lai. 1993. A spike protein-dependent cellular factor other than the viral receptor is required for mouse hepatitis virus entry. Virology 196:45-56. [DOI] [PubMed] [Google Scholar]
- 27.Zelus, B. D., D. R. Wessner, R. K. Williams, M. N. Pensiero, F. T. Phibbs, M. deSouza, G. S. Dveksler, and K. V. Holmes. 1998. Purified, soluble recombinant mouse hepatitis virus receptor, Bgp1(b), and Bgp2 murine coronavirus receptors differ in mouse hepatitis virus binding and neutralizing activities. J. Virol. 72:7237-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou, J., S. A. Stohlman, R. Atkinson, D. R. Hinton, and N. W. Marten. 2002. Matrix metalloproteinase expression correlates with virulence following neurotropic mouse hepatitis virus infection. J. Virol. 76:7374-7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou, J., S. A. Stohlman, D. R. Hinton, and N. W. Marten. 2003. Neutrophils promote mononuclear cell infiltration during viral-induced encephalitis. J. Immunol. 170:3331-3336. [DOI] [PubMed] [Google Scholar]