Abstract
In this study, 6-hydroxydopamine was stereotaxically injected into the right substantia nigra compact and ventral tegmental area of rats to establish Parkinson's disease models. The rats then received a transplantation of bone marrow stromal cells that were previously isolated, cultured and labeled with 5-bromo-2’-deoxyuridine in vitro. Transplantation of the bone marrow stromal cells significantly decreased apomorphine-induced rotation time and the escape latency in the Morris water maze test as compared with rats with untreated Parkinson's disease. Immunohistochemical staining showed that, 5-bromo-2’-deoxyuridine-immunoreactive cells were present in the lateral ventricular wall and the choroid plexus 1 day after transplantation. These immunoreactive cells migrated to the surrounding areas of the lateral cerebral ventricle along the corpus callosum. The results indicated that bone marrow stromal cells could migrate to tissues surround the cerebral ventricle via the cerebrospinal fluid circulation and fuse with cells in the brain, thus altering the phenotype of cells or forming neuron-like cells or astrocytes capable of expressing neuron-specific proteins. Taken together, the present findings indicate that bone marrow stromal cells transplanted intracerebroventricularly could survive, migrate and significantly improve the rotational behavior and cognitive function of rats with experimentally induced Parkinson's disease.
Keywords: bone marrow stromal cells, lateral ventricle, Parkinson's disease, behavior, cognition, neural regeneration
Abbreviations
PD, Parkinson's disease; BMSCs, bone marrow stromal cells; 6-OHDA, 6-hydroxydopamine
INTRODUCTION
Patients with Parkinson's disease (PD) show a progressive decline in cognitive function that is even apparent in early stages of PD[1,2,3], with similar findings in animal models of PD[4,5,6]. Although currently available drugs and surgical procedures can partially improve PD symptoms, their long-term efficacy is unsatisfactory. Perlow et al[7] was the first to transplant embryonic ventral mesencephalic cells into the dorsal caudate putamen in animal models of PD, and found that the engrafted cells grew well in the brain and the behavioral functional defects showed varying degrees of improvements. Accordingly, cell transplantation is now recognized as a potential treatment strategy for PD. Bone marrow stromal cells (BMSCs) are a convenient and abundant type of stem cells that can be amplified within a short time. They also allow autologous transplantation, thus avoiding allogeneic transplantation- induced immunological rejection and ethical controversy. From these properties, BMSCs are considered ideal seed cells. BMSCs transplanted into the corpus striatum of rats with PD significantly improved their behaviors[8,9,10,11]. BMSCs injected into veins or arteries may also migrate to the damaged tissue via the circulation[12], while BMSCs directly injected into the cerebral ventricle or subarachnoid space also migrate to the tissue lesions via cerebrospinal fluid circulation[13,14]. The majority of studies showed that stereotaxic transplantation of BMSCs to the corpus striatum of PD model rats improves rotational behavior[8,15].
This study aimed to observe the effects of stereotaxic transplantation of BMSCs into the lateral ventricles on the behavior and cognitive function of rats with experimentally induced PD.
RESULTS
Quantitative analysis of experimental animals
A total of 60 Sprague-Dawley rats were included in this study. Two rats with memory impairments and one rat only showing rotational behavior were excluded after the Morris water maze and apomorphine injection; the remaining 57 rats were used in this study. Eight rats were randomly selected as the normal group, while PD was induced in the other 49 rats by intracerebroventricular injection of 6-hydroxydopamine (6-OHDA). Subsequent testing confirmed PD in 34 rats (success rate 69%), which were randomly divided into three groups to receive BMSCs transplantation (n = 16), phosphate-buffered saline (PBS) injection (n = 10) or no treatment (untreated PD; n = 8). The BMSC- and PBS-treated groups received intracerebroventricular injections of BMSCs or PBS, respectively, after inducing PD. At each of 1, 3 and 7 days after transplantation, two rats in the BMSCs group were randomly selected for immunohistochemical staining to detect 5-bromo-2’-deoxyuridine (BrdU) expression. The remaining 10 rats underwent the Morris water maze test. Rats in the control, and PBS-treated and untreated PD groups only underwent the rotational behavior and cognitive function tests. At 8 weeks after transplantation, two rats in the untreated PD group underwent the Morris water maze and then killed for immunohistochemical analysis of tyrosine hydroxylase expression in the substantia nigra and to confirm the establishment of PD.
Intracerebroventricular transplantation of BMSCs reduced rotational behavior of PD rats
The apomorphine-induced rotational times of PD rats were significantly reduced by BMSCs transplantation compared with that before transplantation (P < 0.05 or P < 0.01). By contrast, no changes in rotational times were detected in the PBS-treated and untreated PD groups (P > 0.05; Figure 1).
Figure 1.
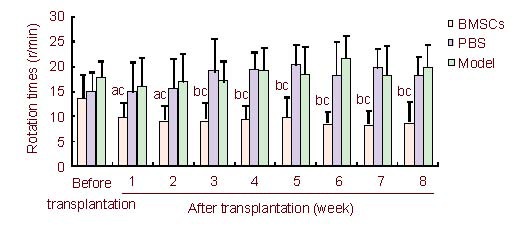
Apomorphine-induced rotation times determined before and after transplantation.
Data are expressed as mean ± SD and were compared by one-way analysis of variance with least significant difference-ttests for multiple comparisons. aP < 0.05, bP < 0.01, vs. the PBS-treated PD group, the untreated PD (model) group; cP < 0.05, vs. before transplantation.
BMSCs: Bone marrow stromal cells; PBS: phosphate-buffered saline; PD: Parkinson's disease.
Effects of inducing PD on cognitive function
Place navigation test
The escape latency of rats in each group was similar before establishing PD (P > 0.05), but was significantly longer after establishing PD compared with the control group (P < 0.05; Figure 2).
Figure 2.
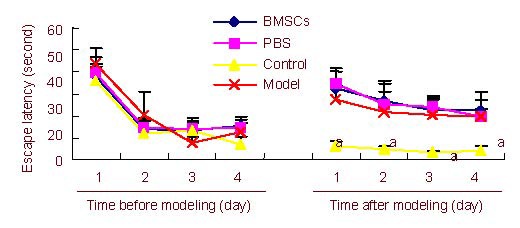
Escape latency measured in the Morris water maze before and after inducing PD.
Data are expressed as mean ± SD and were compared by one-way analysis of variance with least significant difference-ttests for multiple comparisons. aP < 0.05, vs. the BMSCs-treated PD group, the PBS-treated PD group and the untreated PD group.
BMSCs: Bone marrow stromal cells; PBS: phosphate-buffered saline; PD: Parkinson's disease.
Spatial probe test
The percentage of swimming distance and swimming time in the original platform quadrant, as well as the crossing times of rats were similar in all of the groups before and after establishing PD (P > 0.05). However, compared with the control group, these indices decreased significantly after inducing PD (P < 0.05; Figure 3).
Figure 3.
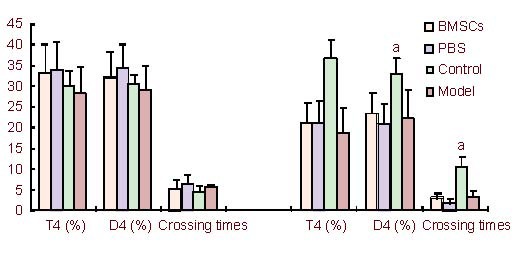
The percentage of swimming distance and swimming time in the platform quadrant and the crossing times recorded before and after inducing PD.
Data are expressed as mean ± SD and were compared by one-way analysis of variance with least significant difference-ttests for multiple comparisons. aP < 0.05, vs. the BMSCs-treated PD group, the PBS-treated PD group and the untreated PD group.
T4: The percentage of swimming time in the original platform quadrant; D4: the percentage of swimming distance in the original platform quadrant. BMSCs: Bone marrow stromal cells; PBS: phosphate-buffered saline; PD: Parkinson's disease.
Intracerebroventricular transplantation of BMSCs improved cognitive function in PD rats
Place navigation test
The escape latency of rats in the BMSCs-treated group was significantly shorter than that in the PBS-treated and untreated groups at both 4 and 8 weeks after cell transplantation (P < 0.05). Escape latency in the BMSCs-treated group was similar to that in the control group (P > 0.05; Figure 4).
Figure 4.
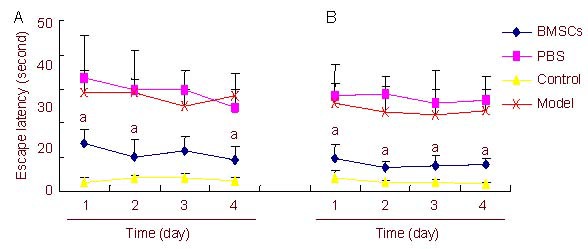
Escape latency measured in the Morris water maze at 4 (A) and 8 (B) weeks after BMSCs transplantation.
Data are expressed as mean ± SD and were compared with one-way analysis of variance with least significant difference-ttests for multiple comparisons. aP < 0.05, vs. PBS-treated PD group and untreated PD group.
BMSCs: Bone marrow stromal cells; PBS: phosphate- buffered saline; PD: Parkinson's disease.
Spatial probe test
The percentage of swimming distance and swimming time in the original platform quadrant, as well as the crossing times of rats within 2 minutes were significantly greater in the BMSCs-treated group than in the PBS-treated and untreated groups at both 4 and 8 weeks after transplantation (P < 0.05). The values in the BMSCs-treated group were similar to those in the control group (P > 0.05; Figure 5).
Figure 5.
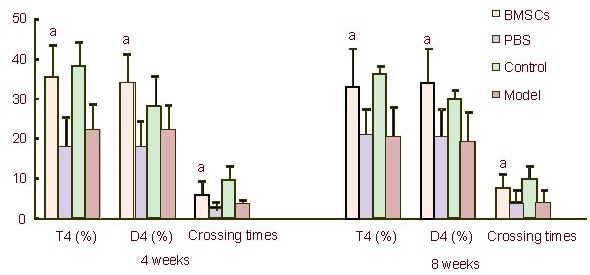
The percentage of swimming distance and swimming time in the platform quadrant and the crossing times within 2 minutes recorded at 4 and 8 weeks after (BMSCs) transplantation.
Data are expressed as mean ± SD and were compared by one-way analysis of variance with least significant difference-ttests for multiple comparisons.
aP < 0.05, vs. PBS-treated PD group and untreated PD group. T4: The percentage of swimming time in the original platform quadrant; D4: the percentage of swimming distance in the original platform quadrant.
BMSCs: Bone marrow stromal cells; PBS: phosphate-buffered saline; PD: Parkinson's disease.
6-OHDA successfully induced PD
At 8 weeks after inducing PD, immunohistochemical staining revealed the absence of tyrosine hydroxylase-positive neurons and nerve fiber expression in the substantia nigra compacta, the surrounding the site of 6-OHDA (Figure 6). These findings confirm that 6-OHDA successfully induced PD.
Figure 6.
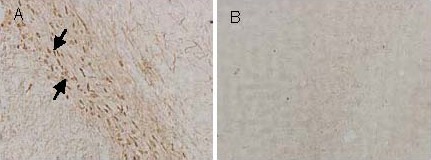
Tyrosine hydroxylase-positive cells (arrows) in the substantia nigra of rats with experimentally induced Parkinson's disease (immunohistochemical staining, × 100).
(A) The substantia nigra not injected with 6-hydroxydopamine.
(B) The substantia nigra injected with 6-hydroxydopamine.
Effects of intracerebroventricular transplantation of BMSCs on BrdU-positive neurons in PD rats
Numerous BrdU-positive cells were visible in the wall of the lateral ventricle and choroid plexus at 1 day after transplantation. By day 3, a small number of cells had migrated along the corpus callosum. BrdU-positive cells were still visible in the choroid plexus and brain parenchyma surrounding the corpus callosum and lateral ventricle at 7 days after transplantation. No BrdU-positive cells were found 8 weeks after transplantation (Figure 7).
Figure 7.
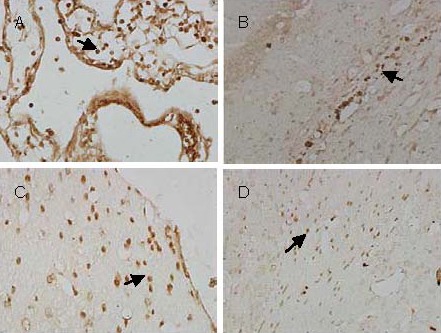
Immunohistochemical localization of 5-bromo-2’-deoxyuridine (BrdU)-positive cells (arrows) following transplantation of bone marrow stromal cells into the lateral cerebral ventricle of rats with experimentally induced Parkinson's disease.
(A) BrdU-positive cells are present in the choroid plexus 1 day after transplantation (× 400).
(B) BrdU-positive cells are present in the corpus callosum 3 days after transplantation (× 200).
(C) BrdU-positive cells are attached to the wall of the lateral cerebral ventricle 7 days after transplantation (× 400).
(D) BrdU-positive cells surround the lateral cerebral ventricle 7 days after transplantation (× 200).
DISCUSSION
6-OHDA is a neurotoxin with a similar structure to dopamine. Therefore, it is often mistaken as a neurotransmitter and is taken up by dopaminergic neurons. By forming hydroxyl radicals and inhibiting mitochondrial oxidative respiratory chain complexes I and IV, 6-OHDA disrupts ATP synthesis and selectively causes dopaminergic neuronal death[16,17,18]. Additionally, 6-OHDA may be mistaken for an endogenous catecholamine (dopamine, norepinephrine) in some amine uptake systems[19], leading to selective damage of catecholaminergic neurons and exhausting dopamine, epinephrine and norepinephrine levels. This is important because the norepinephrine and dopamine pathways are closely associated with learning and memory[20,21]. In addition, the prefrontal cortex can process working memory information through the cortex-basal ganglia loop modulated by dopaminergic projection fibers elicited from substantia nigra pars compacta[22,23]. The substantia corpus striatum is also involved in spatial memory structure through the connections of the thalamus with the cerebral cortex and hippocampus[24]. In this study, 6-OHDA was injected into the substantia nigra pars compacta and ventral tegmental area to destroy the nigrostriatal pathway. This intervention not only affects prefrontal cortical function, but also disturbs the connections from the thalamus to the cerebral cortex and the hippocampus, ultimately leading to significant spatial memory impairment.
The results of this study indicate that apomorphine-induced rotation times were significantly decreased at 1 week after BMSCs transplantation into the lateral cerebral ventricle of the 6-OHDA-induced PD rats. The learning and memory ability of PD rats were tested using the Morris water maze test at 4 and 8 weeks after transplantation. The results show that, the escape latencies of rats in the place navigation test and spatial probe test were significantly reduced after BMSCs transplantation compared with the untreated PD group, while the percentage of swimming distance and swimming time in the original platform quadrant, as well as the crossing times, were significantly increased. Our results are consistent with those of previous studies[25,26,27,28] in that intracerebroventricular transplantation of BMSCs significantly improves spatial learning and memory of rats with 6-OHDA-induced PD.
In this study, immunohistochemical staining analysis showed that the transplanted BrdU-positive BMSCs were visible in the lateral cerebral ventricle at 1, 3 and 7 days after transplantation, and these cells migrated to tissues surrounding the lateral ventricle. This provides evidence that BMSCs transplanted into the lateral cerebral ventricle can migrate into other regions of the brain. The cognitive behavior of PD rats was also improved by the migration of BMSCs to periventricular brain tissue via cerebrospinal fluid and fuse with cells in brain, thus altering the cell phenotype or forming neuron-like cells or astrocytes that could express nerve-specific proteins[29]. The implanted BMSCs can not only differentiate into neurons and astrocytes, but also enhance cellular proliferation in the ependyma and subventricular zone, thus increasing neuronal plasticity[30]. Interestingly, no BrdU-positive cells were found 8 weeks after transplantation, which may be due to the short half-life of BrdU or scattered distribution of the implanted cells.
In summary, stereotaxic transplantation of BMSCs into the lateral cerebral ventricle significantly improved apomorphine-induced rotational behavior and cognitive function of rats with 6-OHDA-induced PD. These results provide experimental evidence supporting the clinical use of autologous BMSCs transplantation for the treatment of PD. Further studies are required to confirm the mechanisms involved in these effects of BMSCs.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment.
Time and setting
Experiments were performed at the Brain Aging and Cognitive Neuroscience Laboratory, the First Hospital of Hebei Medical University, China, between October 2007 and July 2008.
Materials
The BMSCs were collected from 20 healthy female Sprague-Dawley rats, aged 1 month, weighing approximately 120 g. Sixty healthy female Sprague-Dawley rats, aged 3 months, weighing 200–220 g were also obtained from the Laboratory Animal Center, Hebei Medical University, China (license No. SCXK (Ji) 2003-1-003). Rats were housed at 22–25°C, under a 12-hour light/dark cycle, with free access to food and water. All experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[31].
Methods
BMSCs separation, culture and labeling
BMSCs were isolated from the femurs and tibias of Sprague-Dawley rats under aseptic conditions. The bone marrow cavity was rinsed 2–3 times with phosphate buffered saline, and the cell suspension was transferred to a centrifuge tube and centrifuged at 800 r/min for 5 minutes. After discarding the supernatant, the BMSCs were precipitated and incubated with DMEM containing 20% fetal bovine serum (Gibco, New York, NY, USA) at 37°C in a 5% CO2 atmosphere. BMSCs were subcultured after culture for 7–8 days, and they had a long spindle-like shape at this time. BMSCs in passage 3 were labeled with BrdU (1 mL/L; Sigma, St. Louis, MO, USA), cultured for 24 hours, digested with 0.25% trypsin and centrifuged at 800 r/min for 5 minutes. The supernatant was discarded and cells were rinsed twice with 0.01 M PBS, and resuspended in 0.01 M PBS. Finally, the cells were prepared into single cell suspension at 1 × 1011/L for transplantation.
Animal screening
Before inducing PD, the rats underwent behavioral screening tests. One rat with rotational behavior was excluded after intraperitoneal injection of apomorphine (0.5 mg/kg; Sigma), and two rats were eliminated because they showed memory impairments in the Morris water maze test. The remaining rats were randomly allocated to the control group (n = 8), or for the establishment of PD (n = 49).
Establishment of PD
Rats were anesthetized by an intraperitoneal injection of 10% chloral hydrate (4 mL/kg). Using stereotaxic coordinates of the rat brain[32], two injection points were selected, the substantia nigra compacta (4.4 mm posterior to the bregma, 1.2 mm right of the sagittal suture, subdural 7.8 mm) and the ventral tegmental area (4.8 mm posterior to the bregma, 1.0 mm right of the sagittal suture, subdural 7.8 mm). Each point was injected with 6-OHDA 2 μL (4 μg/L; Sigma). The needle was slowly inserted, held in place for 10 minutes after injection, and then slowly withdrawn. Penicillin (30 000 U/d) was injected intraperitoneally for 1 week after surgery to prevent infection. At 21 days after surgery, the rats were intraperitoneally injected with apomorphine (0.5 mg/kg) to induce rotation. Establishment of PD was considered successful if the mean speed of rotation was > 7 r/min.
Cell transplantation
Cell transplantation was performed at 22 days after injecting 6-OHDA. Rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate (4 mL/kg) and placed on cerebral stereotactic instrument. A suspension of BMSCs (10 μL; approximately 1 × 106 cells) or an identical volume of PBS was injected into the right lateral ventricle (1.5 mm posterior to the bregma, 1.0 mm right of the sagittal suture, subdural 3.8 mm). Penicillin (30 000 U/d) was injected intraperitoneally for 1 week after surgery to prevent infection.
Behavioral tests
At 1–8 weeks after BMSCs transplantation (or equivalent times in the other groups), rats were intraperitoneally injected with apomorphine (0.5 mg/kg) on the 1st day of each week to induce rotation. The number of rotations per minute for each rat was recorded.
Assessment of spatial learning and memory using the Morris water maze test
Before and after inducing PD, and at 4 and 8 weeks after transplantation, rats underwent the Morris water maze test, 24 hours after the apomorphine-induced rotation test. The Morris water maze test lasted 5 days. The Morris water maze (Huaibei Zhenghua Biological Equipment Co., Ltd., Huaibei, Anhui province, China) consists of a circular pool and an automatic camera system. The bottom wall of the pool was gray, 120 cm in diameter, 50 cm in height, and the water temperature was maintained at 22 ± 1°C. The pool was divided into four quadrants with four equidistant points on the pool wall and numerous spatial references surrounding the maze. A transparent glass platform (14 cm in diameter and 20 cm in height) was randomly placed in one of the quadrants within the pool, submerged to a depth of 1.5–2.0 cm. The camera was placed 3 m above the pool.
Place navigation test
Rats were tested four times each day for 4 days. Rats were placed into the pool in different quadrants each time. The time taken for the rat to find the platform was recorded as the escape latency. If a rat did not find the platform within 120 seconds, the time was recorded as 120 seconds, and the rat was allowed to take a rest on the platform for 10 seconds before the next trial. The mean of four trials was calculated for each rat each day.
Spatial probe test
The platform was removed on day 5. The rats were placed into the first quadrant of the pool facing the wall, and their swimming path was recorded for 2 minutes. The percentage of swimming distance and swimming time in the original platform quadrant, as well as the crossing times were calculated from the recordings.
Assessment of BMSCs survival and migration, and confirmation of PD
After anesthetizing the rats with 10% chloral hydrate (4 mL/kg), the left ventricle was rapidly perfused with 200 mL saline and fixed with 4% paraformaldehyde. The midbrain tissue (for BMSCs transplantation) and substantia nigra (for tyrosine hydroxylase detection) were removed, dehydrated, embedded in paraffin and cut into 5 μm thick slices. The midbrain tissue slices were incubated with mouse anti-rat BrdU monoclonal antibody (1:400; Sigma) overnight at 4°C followed by goat anti-mouse IgG for 30 minutes at 37°C. Substantia nigra tissue slices were incubated with rabbit anti-rat tyrosine hydroxylase monoclonal antibody (1:5 000; Chemicon, Temecula, CA, USA) overnight at 4°C followed by goat anti-rabbit IgG (1:100; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for 30 minutes at 37°C. Finally, all tissues were counterstained with diaminobenzidine (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.). The slices were then observed under a fluorescence microscope (Nikon, Tokyo, Japan) to determine the survival and migration of the transplanted BrdU-positive cells, and to confirm successful establishment of PD (loss/damage of tyrosine hydroxylase-positive cells).
Statistical analysis
Data were analyzed using SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA) and were expressed as mean ± SD. Rotation time and outcomes of the Morris water maze test were compared using one-way analysis of variance, with least significant difference-t tests for multiple comparisons. Statistically significant differences were accepted at P < 0.05.
Acknowledgments:
We would like to thank the staff at the Brain Aging and Cognitive Neuroscience Laboratory, Hebei Province, China for providing experimental equipment and workspace.
Footnotes
Funding: This study was supported by the Natural Science Foundation of Hebei Province, No. C2008000993.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, the First Hospital of Hebei Medical University, China.
(Edited by Luo W, Shang HF/Yang Y/Song LP)
REFERENCES
- 1.Weintraub D, Doshi J, Koka D, et al. Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch Neurol. 2011;68(12):1562–1568. doi: 10.1001/archneurol.2011.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bugalho P, Vale J. Brief cognitive assessment in the early stages of Parkinson disease. Cogn Behav Neurol. 2011;24(4):169–173. doi: 10.1097/WNN.0b013e3182350a1f. [DOI] [PubMed] [Google Scholar]
- 3.Cheng ZH, Wang L. Memory impairment in the patients with Alzheimer's disease, Parkinson's disease and cerebral infarction. Zhongguo Xingwei Yixue Kexue. 2005;14(7):610–612. [Google Scholar]
- 4.Linert W, Bridge MH, Huber M, et al. In vitro and in vivo studies investigating possible antioxidant actions of nicotine: relevance to Parkinson's and Alzheimer's diseases. Biochim Biophys Acta. 1999;1454(2):143–152. doi: 10.1016/s0925-4439(99)00029-0. [DOI] [PubMed] [Google Scholar]
- 5.Tadaiesky MT, Dombrowski PA, Figueiredo CP, et al. Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson's disease. Neuroscience. 2008;156(4):830–840. doi: 10.1016/j.neuroscience.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Li XH, Wang JY, Gao G, et al. High-frequency stimulation of the subthalamic nucleus restores neural and behavioral functions during reaction time task in a rat model of Parkinson's disease. J Neurosci Res. 2010;88(7):1510–1521. doi: 10.1002/jnr.22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perlow MJ, Karoum F, Braun D, et al. Adrenergic and dopaminergic response to chronic chair restraint in the rhesus monkey. Psychosom Med. 1979;41(2):139–145. doi: 10.1097/00006842-197903000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Gu P, Wang K, Wang YY, et al. Comparison of neural stem cell and bone marrow stromal cell transplantation in treating Parkinson disease rats. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2008;12(12):2221–2226. [Google Scholar]
- 9.Hellmann MA, Panet H, Barhum Y, et al. Increased survival and migration of engrafted mesenchymal bone marrow stem cells in 6-hydroxydopamine-lesioned rodents. Neurosci Lett. 2006;395(2):124–128. doi: 10.1016/j.neulet.2005.10.097. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Chen J, Wang L, et al. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine mouse model of Parkinson's disease. Neurosci Lett. 2001;316(2):67–70. doi: 10.1016/s0304-3940(01)02384-9. [DOI] [PubMed] [Google Scholar]
- 11.Pavón-Fuentes N, Blanco-Lezcano L, Martínez-Martín L, et al. Stromal cell transplant in the 6-OHDA lesion model. Rev Neurol. 2004;39(4):326–334. [PubMed] [Google Scholar]
- 12.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 13.Ohta M, Suzuki Y, Noda T, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 2004;187(2):266–278. doi: 10.1016/j.expneurol.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Ide C, Nakai Y, Nakano N, et al. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 2010;1332:32–47. doi: 10.1016/j.brainres.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 15.Wang MW, Gu P, Li YM, et al. Subarachnoid transplantation of autologous bone marrow stromal cells in treatment of Parkinson's plus syndrome. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2009;13(10):1819–1822. [Google Scholar]
- 16.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 17.Blum D, Torch S, Lambeng N, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol. 2001;65(2):135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 18.Kim TW, Moon Y, Kim K, et al. Dissociation of progressive dopaminergic neuronal death and behavioral impairments by Bax deletion in a mouse model of Parkinson's diseases. PLoS One. 2011;6(10):e25346. doi: 10.1371/journal.pone.0025346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sachs C, Jonsson G. Effects of 6-hydroxydopamine on central noradrenaline neurons during ontogeny. Brain Res. 1975;99(2):277–291. doi: 10.1016/0006-8993(75)90029-3. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi H, Kato M, Takano H, et al. Differential contributions of prefrontal and hippocampal dopamine D(1) and D(2) receptors in human cognitive functions. J Neurosci. 2008;28(46):12032–12038. doi: 10.1523/JNEUROSCI.3446-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mather M, Schoeke A. Positive outcomes enhance incidental learning for both younger and older adults. Front Neurosci. 2011;5:129. doi: 10.3389/fnins.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faw B. Pre-frontal executive committee for perception, working memory, attention, long-term memory, motor control, and thinking: a tutorial review. Conscious Cogn. 2003;12(1):83–139. doi: 10.1016/s1053-8100(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 23.den Braber A, van’t Ent D, Cath DC, et al. Brain activation during cognitive planning in twins discordant or concordant for obsessive-compulsive symptoms. Brain. 2010;133(10):3123–3140. doi: 10.1093/brain/awq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang XC, Zhang JX, Yang B, et al. The impaired learning and memory in rats of Parkinson's disease regulated by the serum and glucocorticoid-inducible kinase. Zhongguo Xingwei Yixue Kexue. 2007;16(4):292–295. [Google Scholar]
- 25.Zhao Z, Hu H, Feng G. Learning and memory amelioration of transplantation of the neural stem cells modified with human brain-derived neurotrophic factor gene on Alzheimer disease model rat. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19(5):331–334. [PubMed] [Google Scholar]
- 26.Gao C, Wang JZ, Chen ME, et al. Effects of intravenous administration of marrow stromal stem cells on cognitive impairment of vascular dementia rat models. Zhonghua Laonian Yixue Zazhi. 2004;11(23):808–812. [Google Scholar]
- 27.Gong ZL, Zheng J, Zhao SF. The effection of the transplantation of bone marrow stromal cells on the spatial learning and remembering of global forebrain ischemia rat. Zhongguo Yaoye. 2006;15(16):12–14. [Google Scholar]
- 28.Sakurai K, Iizuka S, Shen JS, et al. Brain transplantation of genetically modified bone marrow stromal cells corrects CNS pathology and cognitive function in MPS VII mice. Gene Ther. 2004;11(19):1475–1481. doi: 10.1038/sj.gt.3302338. [DOI] [PubMed] [Google Scholar]
- 29.Zhang ZX. Shijiazhuang: Hebei Medical University; 2009. Effects of BMSCs intracerebroventrcular transplantation on the behavior and cognition of parkinson disease rats. [Google Scholar]
- 30.Qu T, Brannen CL, Kim HM, et al. Human neural stem cells improve cognitive function of aged brain. Neuroreport. 2001;12(6):1127–1132. doi: 10.1097/00001756-200105080-00016. [DOI] [PubMed] [Google Scholar]
- 31.The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- 32.Bao XM, Shu SY. Beijing: People's Medical Press; 1991. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]


