Abstract
This study describes a detailed process for obtaining brain glioma stem cells from freshly dissected human brain glioma samples using an immunomagnetic bead technique combined with serum-free media pressure screening. Furthermore, the proliferation, differentiation and self-renewal biological features of brain glioma stem cells were identified. Results showed that a small number of CD133 positive tumor cells isolated from brain glioma samples survived as a cell suspension in serum-free media and proliferated. Subcultured CD133 positive cells maintained a potent self-renewal and proliferative ability, and expressed the stem cell-specific markers CD133 and nestin. After incubation with fetal bovine serum, the number of glial fibrillary acidic protein and microtubule associated protein 2 positive cells increased significantly, indicating that the cultured brain glioma stem cells can differentiate into astrocytes and neurons. Western blot analysis showed that tumor suppressor phosphatase and tensin homolog was highly expressed in tumor spheres compared with the differentiated tumor cells. These experimental findings indicate that the immunomagnetic beads technique is a useful method to obtain brain glioma stem cells from human brain tumors.
Keywords: brain glioma stem cells, CD133, nestin, immunomagnetic beads, glial fibrillary acidic protein, microtubule associated protein 2, neural regeneration
Abbreviations
BGSCs, brain glioma stem cells; MAP2, microtubule associated protein 2; GFAP, glial fibrillary acidic protein; PTEN, tumor suppressor phosphatase and tensin homolog
INTRODUCTION
The cancer stem cell hypothesis posits that brain glioma stem cells (BGSCs) are “seed cells” that have the ability to form any cell in the overall tumor population (multipotency) and are the cause of tumor formation, progression, and recurrence[1,2,3,4,5,6,7,8]. Currently, one of the key issues in cancer stem cell research is how to isolate and identify tumor stem cells, and to establish stable technique platform and stem cell lines for further study[9,10]. Currently, the lack of a method to isolate viable tumor stem cells is the limiting step of cancer stem cell research.
To date, several methods have been used to isolate stem cells. First, magnetism-activated cell sorting and fluorescence-activated cell sorting are two broadly applicable methods based on the tumor stem cell surface protein CD133[11,12,13,14], the only generally accepted and widely used stem cell marker[15]. CD133 is commonly expressed by stem cells and progenitor cells in various tissues especially the central nervous system[16]. CD133+ cells possess extensive self-renewal, proliferation and multipotency abilities similar to those of neural stem cells[17]. These two sorting systems have a relatively high specificity and high yield at the cost of high expenditure. Second, as cancer stem cells have higher ATP-binding cassette transporter and lower fluorescence following Hoechst 33342 staining, compared with bulk tumor cells, the side population[5,18,19] cells can be separated from bulk tumor cells based on this cell surface functional protein using fluorescence-activated cell sorting[19]. Third, as serum free media enhanced with epidermal growth factor, basic fibroblast growth factor and B27 supplement provides a stringent growth system, which negatively selects against differentiated/differentiating cells in primary central nervous system cultures, leaving stem cells free to proliferate and expand exponentially. Thus, serum free media has been used to isolate stem cells[20].
Although many studies describe how to isolate BGSCs from newly dissected specimens or glioblastoma multiforme (GBM) cell lines and the function and mechanism of BGSCs in gliomas, a complete and detailed protocol describing how to isolate, culture and identify human BGSCs is still lacking. Since isolating BGSCs from newly dissected specimens is a complicated process with numerous steps, where neglecting a particular detail often leads to very low isolation efficiency, a detailed and complete isolation protocol is important and valuable for successfully obtaining high numbers of BGSCs.
Therefore, this study describes a detailed process for obtaining BGSCs from freshly dissected human brain glioma samples using an immunomagnetic bead technique combined with serum free media pressure screening. Furthermore, this study aimed to identify whether these cultured CD133 positive cells were BGSCs, and to examine the proliferation, differentiation and self-renewal biological features of BGSCs[1,21]. In addition, the dynamic proliferation and differentiation behavior of BGSCs were observed.
RESULTS
Primary cultured CD133+ cells form tumor spheres
As CD133 is a generally accepted marker for most tumor stem cells[15], especially brain tumor stem cells, it was used in the current study to isolate BGSCs. CD133 positive cells were isolated from freshly resected brain glioma tissues using an immunomagnetic bead sorting technique followed by culturing in serum-free media containing epidermal growth factor and basic fibroblast growth factor, which preferentially induces stem cell proliferation and negatively selects against differentiated/differentiating cells[22]. An important question is whether these CD133 positive cells have the characteristics of glioma stem cells, such as extensive proliferation ability, self-renewal potential and multipotency ability. These three characteristics of glioma stem cells were tested individually in the following experiments.
To examine the proliferative ability of the acutely dissociated individual tumor cells, cells were cultured at a low density of 1 × 104 live cells/cm2 to exclude the possibility of cell aggregates forming. After plating, the dynamic proliferative behavior of CD133 positive cells was observed using inverted fluorescence microscopy each day. In serum free media, the cultured cells existed in a single, dispersed, floating state without cell division within 24 hours of being plated. Some cells showed cell fission and formed phase-bright clones comprising 2–4 cells 48 hours after being plated. After 72 hours of primary culture, greater numbers of spheres formed, containing 20 cells per sphere. Thereafter, the cell number increased rapidly and the volume of the tumor spheres reached approximately 150–200 μm in diameter at 7–10 days (Figure 1). In our system, three cell lines (two lines from GBM and one from grade III glioma tissue) of tumor spheres were obtained from eight tumor samples. The other five cases (one was GBM) failed to produce BGSCs, possibly due to the low number of cells obtained from the tumor tissues being less than the requirement for immunomagnetic bead isolation, or that the tumor contained a relatively low percentage of BGCSs. The above result indicated that CD133 positive cells could form cell spheres in vitro, highlighting extensive proliferation ability and suggesting these cells might be BGSCs.
Figure 1.
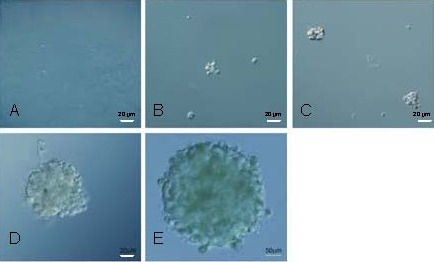
Dynamic behavior of the primary cultured tumor sphere proliferation (Olympus IX-71 inverted microscopy). Scale bars: 20 μm in A–D, 50 μm in E.
The dissociated CD133+ cells were cultured at a low density and the dynamic behavior of the primary tumor spheres was observed after being plated. The following results were observed: (A) at 24 hours, single, dispersing, floating cells and no cell fission was observed. (B) At 48 hours, floating cell aggregates consisted of 2–4 cells. (C) At 72 hours, floating spheres consisted of approximately 20 cells. (D) By 5 days, the cell number and volume of spheres had increased. (E) By 9 days, the sphere volume had increased to a diameter of 150–200 μm.
Tumor sphere cells exhibited self-renewal potential
To determine the potential self-renewal capacity of the primary cultured tumor spheres, the tumor spheres were dissociated, plated at a low density of 1×104 live cells/cm2 and secondary tumor sphere formation ability was observed. Results showed that all of the dissociated primary tumor spheres demonstrated the capacity to form secondary tumor spheres, the morphology of which was identical to that of primary spheres (data not shown). Tumor spheres derived from one GBM sample were expanded for more than 10 passages (long-term self-renewal), with an average doubling time of 4 to 5 days (data not shown). In addition, passage 3 tumor spheres were collected and identified by co-immunostaining with the stem cell surface markers CD133 and nestin. As shown in Figure 2A, the cells at the surface of the secondary tumor spheres also expressed the stem cell markers CD133 and nestin as observed by standard inverted fluorescence microscopy. Furthermore, confocal laser scanning microscopy was used to observe the expression of CD133 and nestin in the center of the spheres. Figure 2B shows that cells deep in the center of the spheres also expressed the stem cell markers CD133 and nestin. These data indicated that the cultured tumor spheres have potent self-renewal ability.
Figure 2.
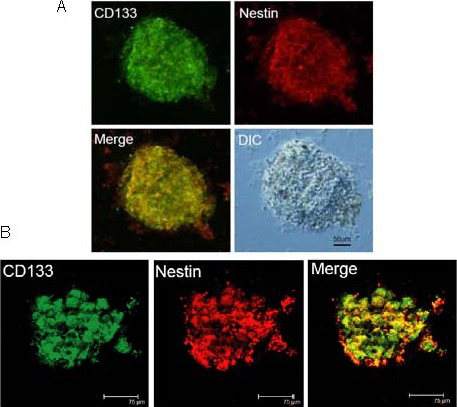
Tumor spheres expressed the tumor stem cell markers CD133 and nestin.
(A) Photos taken by standard inverted fluorescence microscopy (Olympus IX-71 inverted microscopy; scale bar: 50 μm).
(B) Photos taken by confocal laser scanning microscopy (Leica TCS SP2 microscopy; scale bars: 75 μm). The cultured tumor spheres were costained with CD133 (green) and nestin (red) at passage 3. The photos showed that all cells in the tumor spheres expressed tumor stem cell markers CD133 and nestin, indicating that the CD133 positive cells had self-renewal ability.
Tumor sphere cells were multipotent
The third question was whether the tumor spheres have multipotency ability, in other words, multilineage differentiation capacity. To address this, tumor spheres were cultured in media containing 10% fetal bovine serum and the dynamic differentiation behavior of tumor spheres was observed using Olympus IX71 invert fluorescence microscopy each day at the same field. After incubation with 10% fetal bovine serum for 6 hours, all spheres attached to the coverslips without any observed pseudopodia. Six hours later, some pseudopodia were observed although the cell did not migrate. After 24 hours, a few cells had migrated out of the sphere. Thereafter, the number of cells trafficking from the sphere increased gradually. By 48 hours, increased numbers of cells migrated out of the sphere, and the round spheres became flat and oval. After differentiation for 6 days, all cells migrated out of the sphere, and morphology of the emigrated cells was identical to that of the attached cells and the sphere disappeared (Figure 3).
Figure 3.
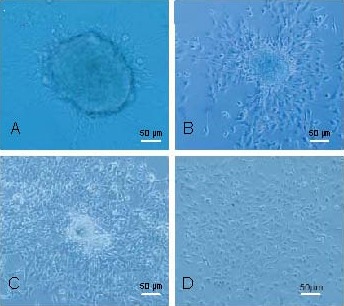
Dynamic differentiation behavior of tumor spheres (Olympus IX-71 inverted microscopy). Scale bars: 50 μm.
The tumor spheres were plated onto coverslips coated with 0.01% poly-D-lysine in serum free media containing 10% fetal bovine serum and the dynamic differentiation behavior was observed after being plated. (A) At 12 hours, a few cell pseudopodia were apparent but no cells migrated out of the sphere. (B) After 24 hours, many cells had migrated out of the sphere. (C) At 48 hours, the number of migrating cells increased and the sphere became flat and oval. (D) By 6 days, the morphology of the migrating cells was identical to the attached cells and the sphere had disappeared.
This suggested that CD133 positive cells could proliferate after serum induction.
As BGSCs can differentiate to glial fibrillary acidic protein (GFAP)-positive astrocytes and microtubule associated protein (MAP2)-positive neurons under certain conditions. This characteristic of the differentiated cells was examined here by co-immunostaining with GFAP and MAP2. Results showed that the majority of differentiated cells expressed GFAP (green) and a small minority of differentiated cells expressed MAP2, while some cells expressed both GFAP and MAP2 (Figure 4). All of the differentiated cells were negative for CD133 and nestin, two cancer stem cell markers (Figure 5). These data indicated that the cultured tumor spheres have multipotency abilities.
Figure 4.
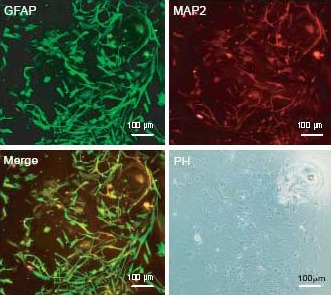
The differentiated cells expressed glial fibrillary acidic protein (GFAP) and/or microtubule associated protein (MAP2) (Olympus IX-71 inverted microscopy). Scale bar: 100 μm.
The differentiated cells were costained with GFAP and MAP2 after 7 days of differentiation. The majority of differentiated cells expressed GFAP (green) and a small minority of differentiated cells expressed MAP2 (red), while some cells expressed both GFAP and MAP2
Figure 5.
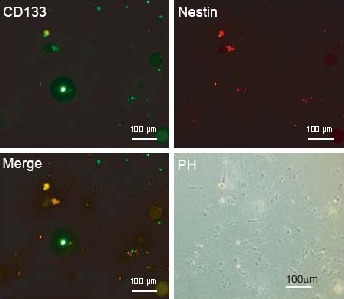
Differentiated cells did not express CD133 or nestin (Olympus IX-71 inverted microscopy). Scale bar: 100 μm.
The differentiated cells were costained with CD133 and nestin after 7 days of differentiation. The cells did not express CD133 or nestin.
Tumor sphere cells expressed high levels of tumor suppressor phosphatase and tensin homolog (PTEN)
The PTEN protein level was determined using western blot analysis. The tumor suppressor PTEN was highly expressed in tumor spheres (Figure 6), compared with that of attached differentiated cells, consistent with a report by Qiang et al[14].
Figure 6.
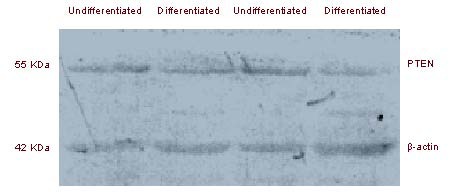
Tumor spheres expressed a high level of tumor suppressor phosphatase and tensin homolog (PTEN).
Protein extracted from tumor spheres and attached differentiated cells, was analyzed by western blot using mouse anti-PTEN antibody or mouse anti-β-actin antibody.
DISCUSSION
With the cancer stem cell hypothesis being supported by increasing numbers of studies, cancer stem cell research has became a “hot spot” in the cancer research field[5,6,7]. One key issue is tumor stem cell isolation and identification to establish a stable technique platform and stem cell lines. In this study, BGSCs were isolated from freshly dissected human brain tumors using an immunomagnetic bead technique combined with serum free media pressure screening. The results were as follows: (1) The isolated CD133+ glioma cells formed floating spheres and identical spheres after being passaged in serum free media, indicating self-renewal ability[12,23,24].(2) Cells in tumor spheres expressed CD133 and nestin, both stem cell markers. (3) The spheres differentiated to GFAP positive astrocytes and/or MAP2 positive neurons in media containing 10% fetal bovine serum and the differentiated cells did not express CD133 or nestin. The above findings suggested that the CD133 positive cells obtained from glioma samples were glioma stem cells and retained the characteristic biological features of the primary tumor, consistent with previous reports[25,26]. Interestingly, it was found that the tumor suppressor PTEN was highly expressed in tumor spheres compared with the differentiated tumor cells, indicating PTEN may play a special role in the maintenance and proliferation of cancer stem cells.
Isolation of cancer stem cells
To date, several methods have been used to isolate stem cells including magnetism-activated cell sorting and fluorescence-activated cell sorting, which are based on the tumor stem cell surface protein CD133. These sorting systems have a relatively high specificity and high yield at the cost of high expenditure. Other methods, such as obtaining stem cells based on cell surface functional protein ATP-binding cassette transporter using fluorescence-activated cell sorting[19,27] or serum free media pressure have less specificity than fluorescence-activated cell sorting. In terms of isolation samples, cancer stem cells could be obtained from tumor cell lines or from freshly dissected tumor tissues[28,29]. Although obtaining cancer stem cells from tumor cell lines is relatively easy, the stem cells may undergo biological changes and may not reflect the original characteristics of the primary tumor due to different growth environments between in vitro and in vivo conditions. Isolating cancer stem cells from freshly dissected tumor tissues can obtain a high yield of stem cells but can also maintain the in vivo features of stem cells.
In this study, BGSCs were isolated from freshly dissected human brain tumors using magnetism-activated cell sorting combined with serum free media pressure screening. This system not only maintained the high efficiency of cell sorting but also overcame the problem of low specificity of serum free media pressure screening, and maintained the in vivo features of stem cells. The cells were cultured at a low density of 1×104 live cells/cm2 to exclude the possibility of cell aggregates forming. Finally, three lines of BGSCs were obtained from eight freshly dissected tumor tissues (two lines from GBM and one from grade III glioma tissues) and tumor spheres were derived from one GBM sample and were expanded for more than 10 passages (long-term self-renewal). The percentage of cancer stem cells has been estimated to be approximately 1% among many solid tumors but higher percentages of stem cells have been observed in more malignant tumors[30]. Thus, the cause of failure to obtain BGSCs from the other five cases (one was GBM) might be due to the low number of cells obtained from the tumor tissues, which was either less than the requirement for immunomagnetic bead isolation, or that the tumor contained a relatively low percentage of BGCSs. These results suggested that to obtain a high yield of BGSCs, highly malignant tumors and tumor tissues should be used if possible.
Identification of glioma stem cells
Cancer stem cell identification consists of surface marker identification and functional identification. CD133 and nestin are commonly used as tumor stem cell markers while GFAP and MAP2 are used as differentiation markers. The two main generally accepted features of cancer stem cells are self-renewal and multipotency. The self-renewal capacity of stem spheres was assayed by dissociation of primary tumor spheres, plating of cells at a low density and observing the ability of secondary tumor sphere formation. BGSCs could differentiate into astroglia or neurons and expressed cell surface proteins GFAP or MAP2 under certain conditions, respectively[13,31].
In this study, the dynamic proliferative behavior of the dissociated CD133 positive cells was observed and it was found that the cell spheres formed from a single cell. The redissociated CD133 positive cells from the primary tumor spheres could form secondary spheres morphology identical to that of the primary sphere, indicating self-renewal capacity. In addition, each cell in the cultured cell spheres expressed the stem cell marker CD133 and nestin before serum induction. During serum induction, the cells gradually migrated from the spheres and formed an even monolayer. Furthermore, after serum induction, the tumor spheres differentiated to GFAP positive astroglia and/or MAP2 positive neurons, respectively. The differentiated cells did not express stem cell markers. The above results indicated that the isolated cells were BGSCs.
Highly expressed PTEN in the BGSCs
It has been reported that tumor suppressor PTEN is highly mutated in many types of human tumors. In glioma, PTEN was one of the two most commonly mutated tumor suppressor genes (http://tcga-data.nci.nih.gov/tcga/ findArchives.htm)[18]. Apart from the important function of PTEN in bulk glioma cells, PTEN can control glioma stem/progenitor cell renewal and differentiation and loss of PTEN increases the number of side population cells[5,18]. In the present study, the PTEN protein level was examined using western blot analysis. Interestingly, contrary to the reports that PTEN is mutated or lost in gliomas, this study demonstrated that PTEN was highly expressed in tumor spheres compared with attached differentiated cells, consistent with the report by Qiang et al[14]. The high level of PTEN in BGSCs suggested that PTEN might have an unknown role in maintaining BGSC features.
Taken together, the results of this study suggested that obtaining BGSCs from human brain tumors using an immunomagnetic bead technique combined with serum free media pressure screening is a useful method for further cancer stem cell research and novel cancer therapeutic drug development in vitro. This study described the detailed procedures for isolation, cultivation and identification of BGCSs but also recorded the dynamic behavior of isolated CD133 positive cells, which might help elucidate the differentiation and proliferation characteristics of BGSCs. This study will provide readers with helpful guidance about how to isolate, culture and identify BGCSs. Due to the contrary expression level of PTEN in glioma bulk cells and BGSCs, it will be interesting to further study the role and the regulatory mechanism of PTEN in BGSCs.
MATERIALS AND METHODS
Design
Repetitive measurements for in vitro experiments.
Time and setting
These experiments were conducted at the Laboratory of Neurosurgery, Xuzhou Medical College, China from September 2009 to March 2010.
Materials
Human glioma tissues (two grade II, three grade III and three grade VI glioma tissues) were obtained from patients who underwent surgical resection at the Affiliated Hospital of Xuzhou Medical College (Xuzhou, China). All samples used in this study were primary glioma tissues without any treatment and were confirmed by a histological pathology report according to the World Health Organization guidelines. Written informed consent was obtained from each patient according to the Administrative Regulations on Medical Institution[32].
Methods
BGSCs isolation
Non-electric coagulated and non-cystic changed tumor samples were chopped into 0.5 mm × 0.5 mm × 0.5 mm pieces and washed repeatedly in D-Hanks solution. Then the tumors were subjected to enzymatic dissociation with 0.25% trypsin-ethylone tetraacetic acid for 15–20 minutes at 37°C, followed by trituration with pipettes and the single-cell suspension was filtered and centrifuged at 1 000 r/min for 5 minutes. The BGSCs were magnetically labeled using the Miltenyi Biotec CD133 MicroBead Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and magnetic separation was carried out on the miniMACS Separator (Miltenyi Biotec) according to the manufacturer's recommendations with minor modifications. Briefly, the vital isolated cells were incubated in CD133 MicroBeads for 30 minutes at 2–8°C. After incubation, the cells were washed, centrifuged and resuspended. The cell suspension was added into a pre-rinsed MS column, which was placed in the magnetic field of a MiniMACS Separator. After washing twice with 2 mL of buffer, the unlabeled cells were removed. The column was removed from the separator and placed on a suitable collection tube. The magnetically labeled cells were flushed out by firmly pushing the plunger into the column after 1 mL of buffer was pipetted into the column. The whole isolation process was performed within 2 hours.
Primary tumor sphere culture and passage
Primary cells were plated in 25 cm2 tissue culture flasks at a density of 1 × 104 live cells/cm2 in Dulbecco's modified Eagle's media/F-12 media (serum free media; Invitrogen, Carlsbad, CA, USA) containing 20 ng/mL of both recombinant human epidermal growth factor (20 ng/mL; Peprotech, Invitrogen, Carlsbad, CA, USA) and recombinant human basic fibroblast growth factor (20 ng/mL; Peprotech, Invitrogen). The culture media was half changed every 2–3 days; epidermal growth factor and basic fibroblast growth factor were added per day to maintain the concentration. The tumor spheres were observed under Olympus IX-71 inverted microscopy (Olympus, Tokyo, Japan) every day.
After 7–10 days of primary culture, the tumor spheres were transferred to a 15 mL tube and centrifuged. The tumor sphere pellet was resuspended and digested with 0.25% trypsin-ethylone tetraacetic for 3–5 minutes at 37°C. After stopping the digestion with fetal bovine serum (Invitrogen), the suspension was centrifuged at 300 × g for 10 minutes. The cell pellet was resuspended in serum free media and triturated to a single-cell suspension with pipettes. The live cell number was determined by 0.4% trypan blue staining and the cells were plated at a density of 1 × 104 live cells/cm2 in serum free media containing epidermal growth factor and basic fibroblast growth factor. The tumor spheres were passaged every 5–7 days according to above methods.
Immunocytochemical staining
For immunostaining of undifferentiated tumor spheres, passage 3 healthy tumor spheres were plated onto 0.01% poly-D-lysine coated coverslips in DMEM/F12 containing 10% fetal bovine serum for 4 hours. Cells were washed with phosphate buffered saline and fixed with 4% paraformaldehyde for 30 minutes. Coverslips were washed twice with phosphate buffered saline, blocked with 5% normal goat serum for 15 minutes, and then incubated overnight with primary mouse anti-CD133 (1:10; Miltenyi Biotec) or rabbit anti-nestin antibody (1:200; Abcam, Cambridge, MA, UK) at 4°C in a humidified chamber. After washing with phosphate buffered saline three times, cells were incubated with fluorescence conjugated secondary antibodies (Alexa 488 goat anti-mouse and Alexa 555 goat anti-rabbit; 1:500, Invitrogen Molecular Probes) for 1 hour at room temperature.
To evaluate the differentiation ability of the tumor spheres, passage 4 spheres were plated onto 0.01% poly-D-lysine coated coverslips in DMEM/F12 containing 10% fetal bovine serum and observed every day by differential interference contrast microscopy. For immunostaining of differentiated tumor cells, immunocytochemistry was performed as described above at 7 days after serum induction. The cells were costained with anti-CD133, nestin or mouse anti-GFAP monoclonal antibody (1:500; Millipore, Billerica, MA, USA) and rabbit anti-MAP2 polyclonal antibody (1:500, Millipore). After washing with phosphate buffered saline three times, coverslips were mounted onto the slides. Images were taken by Olympus IX-71 inverted microscopy or Leica TCS SP2 confocal laser scanning microscopy (Leica, Solms, Germany).
Western blot analysis
The cell spheres and attached differentiated cells were washed with phosphate buffered saline and lysed with lysis buffer (150 mM NaCl, 5 mM ethylone tetraacetic acid, 1% Triton X-100, 1 mM sodium orthovanadate, 50 mM NaF, 1 mM phenylmethanesulfonyl fluoride, 1 mM aprotinin, 1 mM leupeptin, 5 mM dithiothreitol, and 10 mM Tris-HCl; pH 7.4). Protein lysates were concentrated using bicinchoninic acid protein assay kit (Thermo Scientific) and equal amounts of protein lysates were separated with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Millipore). Immune complexes were formed by incubation of the proteins with mouse anti-PTEN monoclonal antibody (Millipore) or mouse anti-β-actin monoclonal antibody (Millipore) overnight at 4°C. Blots were washed and incubated for 1 hour with horseradish peroxidase conjugated anti-mouse secondary antibody. Immunoreactive protein bands were detected with the ECL system (Pierce, Rockford, IL, USA).
Acknowledgments:
We would like to thank Keqin Guo, Xin Pan, Jun Liang, Wenchang Gao from Department of Neurosurgery, Affiliated Hospital of Xuzhou Medical College in China for their help with glioma sample collection.
Footnotes
Conflicts of interest: None declared.
Funding: This work was supported in part by the National Natural Science Foundation of China, No. 81072072, 31070933 and the guidance project of Xuzhou Science and Technology Bureau, No. X22D1056.
Ethical approval: The project received full ethical approval from the Ethics Committee of the Affiliated Hospital of Xuzhou Medical College in China.
(Edited by Zhang GX, Feng H/Yang Y/Song LP)
REFERENCES
- 1.Park DM, Rich JN. Biology of glioma cancer stem cells. Mol Cells. 2009;28(1):7–12. doi: 10.1007/s10059-009-0111-2. [DOI] [PubMed] [Google Scholar]
- 2.Gilg AG, Tye SL, Tolliver LB, et al. Targeting hyaluronan interactions in malignant gliomas and their drug-resistant multipotent progenitors. Clin Cancer Res. 2008;14(6):1804–1813. doi: 10.1158/1078-0432.CCR-07-1228. [DOI] [PubMed] [Google Scholar]
- 3.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100(25):15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu SP, Yang XJ, Zhang B, et al. Enhanced invasion in vitro and the distribution patterns in vivo of CD133+ glioma stem cells. Chin Med J (Engl) 2011;124(17):2599–2604. [PubMed] [Google Scholar]
- 5.Bleau AM, Hambardzumyan D, Ozawa T, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4(3):226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Wang H, Eyler CE, et al. Turning cancer stem cells inside out: an exploration of glioma stem cell signaling pathways. J Biol Chem. 2009;284(25):16705–16709. doi: 10.1074/jbc.R900013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denysenko T, Gennero L, Roos MA, et al. Glioblastoma cancer stem cells: heterogeneity, microenvironment and related therapeutic strategies. Cell Biochem Funct. 2010;28(5):343–351. doi: 10.1002/cbf.1666. [DOI] [PubMed] [Google Scholar]
- 8.Kim KJ, Lee KH, Kim HS, et al. The presence of stem cell marker-expressing cells is not prognostically significant in glioblastomas. Neuropathology. 2011;31(5):494–502. doi: 10.1111/j.1440-1789.2010.01194.x. [DOI] [PubMed] [Google Scholar]
- 9.Foong CS, Ng FS, Phong M, et al. Cryopreservation of cancer-initiating cells derived from glioblastoma. Front Biosci (Schol Ed) 2011;3:698–708. doi: 10.2741/s181. [DOI] [PubMed] [Google Scholar]
- 10.Gursel DB, Beyene RT, Hofstetter C, et al. Optimization of glioblastoma multiforme stem cell isolation, transfection, and transduction. J Neurooncol. 2011;104(2):509–522. doi: 10.1007/s11060-011-0528-2. [DOI] [PubMed] [Google Scholar]
- 11.Lindberg N, Kastemar M, Olofsson T, et al. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene. 2009;28(23):2266–2275. doi: 10.1038/onc.2009.76. [DOI] [PubMed] [Google Scholar]
- 12.Lathia JD, Hitomi M, Gallagher J, et al. Distribution of CD133 reveals glioma stem cells self-renew through symmetric and asymmetric cell divisions. Cell Death Dis. 2011;2:e200. doi: 10.1038/cddis.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 14.Qiang L, Yang Y, Ma YJ, et al. Isolation and characterization of cancer stem like cells in human glioblastoma cell lines. Cancer Lett. 2009;279(1):13–21. doi: 10.1016/j.canlet.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Guryanova OA, Wu Q, Cheng L, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell. 2011;19(4):498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shmelkov SV, St Clair R, Lyden D, et al. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005;37(4):715–719. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Yao XH, Liu Y, Chen K, et al. Chemoattractant receptors as pharmacological targets for elimination of glioma stem-like cells. Int Immunopharmacol. 2011;11(12):1961–1966. doi: 10.1016/j.intimp.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng H, Ying H, Yan H, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455(7216):1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broadley KW, Hunn MK, Farrand KJ, et al. Side population is not necessary or sufficient for a cancer stem cell phenotype in glioblastoma multiforme. Stem Cells. 2011;29(3):452–461. doi: 10.1002/stem.582. [DOI] [PubMed] [Google Scholar]
- 20.Wu P, Tarasenko YI, Gu Y, et al. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rat. Nat Neurosci. 2002;5(12):1271–1278. doi: 10.1038/nn974. [DOI] [PubMed] [Google Scholar]
- 21.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12(11):4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dell’Albani P. Stem cell markers in gliomas. Neurochem Res. 2008;33(12):2407–2415. doi: 10.1007/s11064-008-9723-8. [DOI] [PubMed] [Google Scholar]
- 24.Wu A, Oh S, Wiesner SM, et al. Persistence of CD133+ cells in human and mouse glioma cell lines: detailed characterization of GL261 glioma cells with cancer stem cell-like properties. Stem Cells Dev. 2008;17(1):173–184. doi: 10.1089/scd.2007.0133. [DOI] [PubMed] [Google Scholar]
- 25.Ignatova TN, Kukekov VG, Laywell ED, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 26.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 27.Setoguchi T, Taga T, Kondo T. Cancer stem cells persist in many cancer cell lines. Cell Cycle. 2004;3(4):414–415. doi: 10.4161/cc.3.4.799. [DOI] [PubMed] [Google Scholar]
- 28.Fan H, Guo H, Zhang IY, et al. The different HMGA1 expression of total population of glioblastoma cell line U251 and glioma stem cells isolated from U251. Brain Res. 2011;1384:9–14. doi: 10.1016/j.brainres.2011.01.105. [DOI] [PubMed] [Google Scholar]
- 29.Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci U S A. 2004;101(3):781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wulf GG, Wang RY, Kuehnle I, et al. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98(4):1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- 31.Yuan X, Curtin J, Xiong Y, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23(58):9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 32.State Council of the People's Republic of China. Administrative Regulations on Medical Institution. 1994 [Google Scholar]


