Abstract
At 8 weeks after intragastric administration of icariin to senescence-accelerated mice (P8 strain), Morris water maze results showed that escape latency was shortened, and the number of platform crossings was increased. Immunohistochemical staining and western blot assay detected significantly increased levels of cyclic adenosine monophosphate response element binding protein. These results suggest that icariin upregulates phosphorylated cyclic adenosine monophosphate response element binding protein levels and improves learning and memory functions in hippocampus of the senescence-accelerated mouse.
Keywords: icariin, Alzheimer's disease, hippocampus, phosphorylated cyclic adenosine monophosphate response element binding protein, neural regeneration
Abbreviations:
AD, Alzheimer's disease; CREB, cyclic adenosine monophosphate response element binding protein; SAMP8, senescence accelerated mouse P8; SAM, senescence accelerated mouse
INTRODUCTION
Alzheimer's disease (AD) mainly affects cerebral cortical neurons[1]. It induces dementia and is characterized by progressive decrease in learning and memory functions[1]. At present, the cause of AD remains unclear, and there is a lack of effective drugs. Icariin, an active constituent of herba epimedii, can invigorate the kidney.
Numerous studies have demonstrated that icariin reduces the number of senile plaques containing amyloid-beta, inhibits abnormal phosphorylation of tau protein, reduces oxidative damage, suppresses acetylcholine esterase activity, and improves the cognitive abilities of AD mice[2,3,4]. Senescence accelerated mice (SAM) exhibit some characteristics of aging through inbreeding over 20 generations, and these models show stable genetic backgrounds and aging characteristics. Senescence accelerated mouse models are divided into senescence accelerated mouse/resistant (SAM-R) and senescence accelerated mouse/prone (SAM-P) lines. The senescence-accelerated prone mouse strain 8 (SAMP8) mice are affected by a cognitive disorder. After 4–6 months, accelerated aging occurs spontaneously, with obvious beta-amyloid protein deposition, a granular PAS-positive pattern and spongy lesions. SAMP8 animal models are characterized by a homogenous genetic background and stable aging, similar to clinical occurrence of AD[5,6,7]. Moreover, SAM-R and SAMP8 belong to the same strain, and have similar physiological indexes and life spans to normal animals. Therefore, this study used SAM-R mice as a normal control group.
The transcription factor cyclic adenosine monophosphate response element binding protein (CREB) plays an important role in learning and memory processes[8]. Previous studies have confirmed that phosphorylated CREB exerts crucial effects on long-term memory formation, and that a decrease in CREB activity suppresses long-term memory formation, while enhanced CREB activity promotes long-term memory formation[9,10]. However, a functional impairment in the protein kinase A-CREB signaling pathway reduces phosphorylated CREB levels, and amyloid-beta at a low concentration can induce dysfunction of the protein kinase A-CREB signaling pathway and decreased levels of phosphorylated CREB (p-CREB)[11]. It is assumed that p-CREB is a target protein of icariin when used in the treatment of AD. In this study, we observed the effects of p-CREB on memory and learning functions in the senescence-accelerated mouse (SAM; P8 strain, SAMP8) using immunohistochemistry and western blot assay.
RESULTS
Quantitative analysis of experimental animals
A total of 20 male SAMP8 mice aged 6 months were equally and randomly assigned to an icariin group and a model control group. A total of 10 male SAM-R mice aged 6 months were included as a normal control group.
Mice in the icariin group were intragastrically administered icariin, and those in the model control and normal control groups were intragastrically administered double distilled water of equal volume. A total of 30 mice were included in the final analysis, and there was no death or infection.
Icariin increases CREB phosphorylation in the dentate gyrus of the SAMP8 mouse hippocampus
Under an optical microscope, p-CREB was observed in nuclei. Avidin-biotin-peroxidase complex immunohistochemistry revealed p-CREB-positive granule cells in the dentate gyrus of the hippocampus. Compared with the normal control group, the number of p-CREB-positive cells was significantly reduced in the model control group (Figure 1B). The number of p-CREB-positive cells in the icariin group was significantly higher than that in the model control group, but lower than that in the normal control group (Figure 1C). Compared with the absorbance value (198.12 ± 13.87) for p-CREB-positive cells in the normal control group, the level of CREB phosphorylation was significantly reduced in the hippocampal dentate gyrus in the model control group (128.46 ± 8.94) (P < 0.05). Compared with the model control group, the level of CREB phosphorylation was significantly increased in the dentate gyrus of the hippocampus in mice in the icariin group (157.12 ± 8.97) (P < 0. 05).
Figure 1.
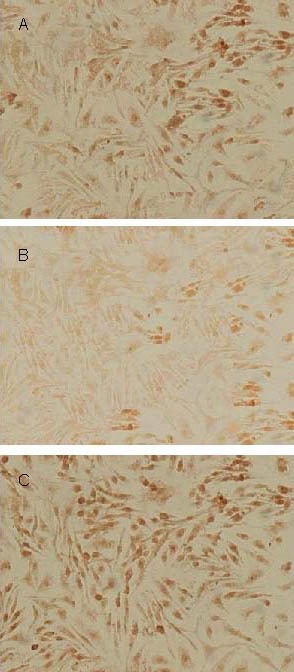
Immunohistochemical staining of phosphorylated cyclic adenosine monophosphate response element binding protein in the mouse hippocampal dentate gyrus in Icariin (A), model control (B), and normal control (C) groups, respectively (× 400).
Immunoblotting was also used to assess p-CREB levels in the mouse hippocampus (Figure 2), and the results were consistent with those from immunohistochemical staining. The level of p-CREB (average absorbance value) was significantly lower in the model control group (0.64 ± 0.19) than in the normal control group (1.35 ± 0.69) (P < 0.05), while it was significantly higher in the icariin group (0.82 ± 0.16) than in the model control group (P < 0.05).
Figure 2.
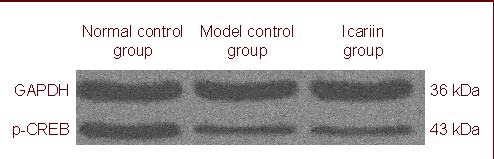
Western blot showing the presence of phosphorylated cyclic adenosine monophosphate response element binding protein (p-CREB) in the hippocampal dentate gyrus of mice in different groups.
GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Icariin improves learning and memory functions of SAMP8 mice
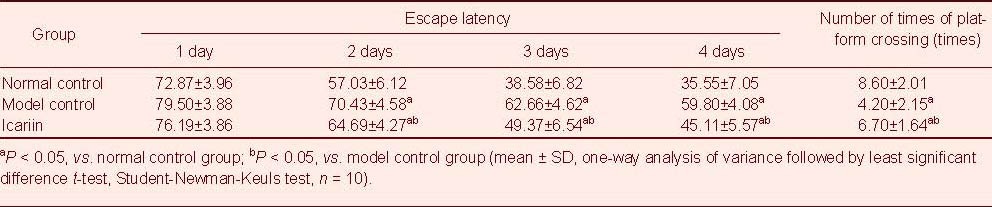
As shown in Table 1, in navigation tests, the average escape latency was significantly reduced in each group at 1 and 2 days, gradually becoming stable by days 3–4. The escape latency was significantly longer in the model control group compared with normal control group (P < 0.05). The escape latency was significantly shortened in the icariin group compared with the model control group (P < 0.05), but was longer than that in the normal control group (P < 0.05). In the spatial probe test, the swim paths of mice in the icariin group were mainly in the original quadrant, although some mice searched along the edge. Mice in the model control group showed a random swim path. The number of times test animals crossed the platform was significantly higher in the icariin group compared with the model control group (P < 0.05), but lower in the icariin group compared with the normal control group (P < 0.05).
Table 1.
Escape latency in the navigation test (second) and number of platform crossings in the spatial probe test (times) in mice from each group
DISCUSSION
Various AD animal models have been established based on different concepts of the processes underlying AD onset, such as cholinergic damage models, β-amyloid protein-induced models, and transgenic models[12]. These models cannot reflect the complexity of the mechanisms underlying AD onset. Thus, in this study, we used SAM mice that could reflect the complicated pathological process underlying AD.
In the present study, we used the Morris water maze test, which excludes effects of animal waste and secretory pheromones on other animals[13]. The Morris water maze assesses declarative memory, which is a form of hippocampus-dependent long-term memory[14]. Moreover, the significant clinical manifestation in AD patients is the damage to declarative memory. Therefore, it is appropriate to use the Morris water maze to detect AD and verify an effective treatment. Our study results showed that learning performance was obviously better in the treatment group than in the model group (short latency), and that memory performance was better in the treatment group compared with the model group (percentage of swim distance in platform quadrant/total distance was increased). These results show that the learning and memory abilities of SAMP8 mice were poorer than those of SAM-R mice, and that SAMP8 mice provide reliable AD models. These results also showed that icariin markedly improved learning and memory abilities in this AD mouse model.
Memory formation is associated with neuron production in the hippocampus. Abundant neural stem cells in the hippocampal dentate gyrus can proliferate and differentiate into various types of neurons. When they migrate to the granular cell layer, these neurons gain the function of synaptic transmission, which may contribute to the mechanism underlying memory formation. In the nervous system, CREB is an important component of intracellular diverse signaling pathways. Its downstream effects include an influence on neuronal survival and growth, synaptic plasticity and long-term memory formation[15]. Long-term synaptic potentiation is the manifestation of memory consolidation at the cellular level, and the establishment of long-term synaptic potentiation or facilitating effect not only requires new protein synthesis, but also requires gene transcription[16]. p-CREB-activated gene expression is strongly related to long-term memory.
A previous study showed that the distribution of p-CREB changes in different regions of the hippocampus after brain tissue injury, with the highest levels occurring in the hippocampal dentate gyrus granule cells, and only low levels being detected in CA1 region pyramidal cells[17]. Our preliminary experimental results show that p-CREB is detectable in pyramidal cells in the hippocampal CA1 and CA3 regions and in granule cells in the dentate gyrus in SAMP8 mice. They also show that the highest levels of p-CREB are present in dentate gyrus granule cells. On the basis of our preliminary results, we chose to focus on the dentate gyrus in this study. Protein immunoblotting analysis confirmed that p-CREB presented as a 43-kDa protein band, consistent with previously published results[18,19]. Icariin can therefore be used to elevate p-CREB levels in the mouse hippocampus. This study shows that icariin upregulates the phosphorylation of CREB in hippocampal neurons through the activation of genes related to transcription. These proteins induced by p-CREB strengthen long-term memory formation in the hippocampus by suppressing cell apoptosis and enhancing cell differentiation, regeneration and repair[4,19], resulting in improved orientation discrimination learning ability, memory consolidation and reappearance ability in AD mouse models.
Icariin is a major constituent of flavonoids isolated from Epimedium brevicornum Maxim (Berberidaceae), which is used in traditional Chinese medicine to nourish the kidney. Icariin was purified from extracts of Epimedium, and showed stable properties. Our studies used drugs of the same batch and exact drug doses to remove potential biases.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
Experiments were performed at the Laboratory of Neurology of Central South University, and Central Laboratory, First Affiliated Hospital, Hunan University of Chinese Medicine, China from January to May 2010.
Materials
A total of 20 healthy male SAMP8 mice aged 6 months and 10 SAM-R mice of the same age, weighing 24.0 ± 3.5 g, were supplied by the Animal Experimental Center, First Affiliated Hospital, Tianjin University of Traditional Chinese Medicine (No. 0001353). Animals were housed in a laminar flow room at 18–22°C with humidity of 55–58%. Drinking water and feed were sterilized by steam. The experiments were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[20].
The Traditional Chinese Medicine Epimedium was purchased from Shaanxi Jintai Biological Engineering Co., Ltd. Icariin was isolated as a light yellow crystal with the molecular formula C33H40O15 and a molecular weight of 676.65. The molecular structural formula is shown below:
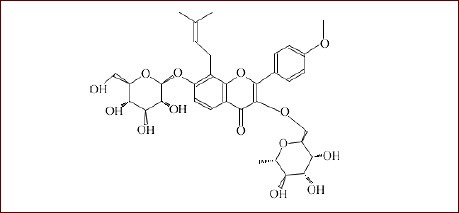
Icariin was purified in the Department of Biochemistry, College of Pharmacy, Hunan University of Chinese Medicine (purity 98%). Epimedium powder was extracted twice by reflux with as much as ten volumes of 70% alcohol, each time for one hour. The extract was filtrated, concentrated, and diluted three or four times using deionized water for 12-day stationary cultivation, followed by filtering. After being passed through a polyamide column, the filtrate was washed in deionized water until the eluate was colorless, eluted in 30% ethanol (eluent with > 25% ethanol was collected until it was colorless). The eluent was concentrated. The specimen was dissolved using 70% ethanol at a ratio of 1:20 for 8 hours without stirring. Crude icariin was obtained by filtration, and purified icariin was obtained by recrystallization using dehydrated alcohol at the ratio of 1:15.
Methods
Drug administration
Mice in the icariin group were intragastrically administered icariin in a 0.5% sodium carboxymethyl cellulose suspension (0.01 mL/g). Conversions between mouse and adult doses were done according to body surface area ratio. The formula was D1: D2 = R1: R2 → D2 = D1 × R2/ R1 (D2: calculated dose; D1: known dose; R1 and R2 can be obtained by checking body surface area ratio tables for experimental animals and humans). Mice in the model control and normal control groups were intragastrically administered 1 mL of double distilled water. Intragastric administration was done once a day in each group, for eight consecutive weeks. To reduce intergroup bias, the grasping time, grasping and intragastric administration were maintained at identical levels among groups.
Spatial learning and memory abilities, as detected by Morris water maze test
A Morris water maze was purchased from the Institute of Materia Medica, Chinese Academy of Medical Sciences. A circular tank (150 cm diameter, 45 cm deep) was filled to a depth of 32 cm with water at about 26°C. Four entry points were marked on the wall of the pool, and the pool was divided into four quadrants. A platform (5.5 cm diameter, 30 cm high) was placed in the first quadrant. The platform and pool wall were dyed black to hide the platform. Fixed references (such as a triangle, quadrangle and circle) were placed on the pool wall for mice to use as a reference when finding the platform. A video camera was placed 3 m above the pool, and connected to a monitor and computer. The maze was placed in a laboratory with sufficient light. The references outside the maze remain unchanged. Tests included navigation test and spatial probe tests. Mouse's heads were dyed black to provide a contrast with the water in the pool. Tests began immediately after treatments.
Navigation test: mice swam freely for 2 days to orient themselves before the test. The platform was placed in the northeast quadrant. Each trial began by releasing the mouse into the water at one of the four placement points (east, south, west, and north) with its face toward the pool wall. The duration taken for the mice to search and climb the platform in the water was recorded and regarded as the escape latency. If an animal failed to climb onto the platform within 120 seconds, it was manually guided onto the platform and made to stay for 20 seconds. In such cases the escape latency was recorded as 120 seconds. The four periods of training for each mouse served as a session, and the training was separately performed in the morning and afternoon for four consecutive days, for a total of eight sessions. The daily changes in escape latency were compared in mice of each group.
Spatial probe test: the platform was removed from the pool on day 5, and mice were allowed to search for the platform for 120 seconds. Swim paths were recorded using a Canon FS49 video camera (Canon, Tokyo, Japan). The positions of references surrounding the pool were not changed. The numbers of platform crossings by each mouse were compared.
Detection of p-CREB in mouse brain tissue, as determined by immunohistochemistry
Five mice from each group were anesthetized with 10% chloral hydrate (300 mg/kg, i.p.) after the Morris water maze test, and then fixed using saline containing 4% paraformaldehyde. Brains were rapidly obtained, fixed overnight in the dark, and dehydrated using 15% and 30% sucrose solutions until the brain tissue sunk. Brain tissue samples were serially sliced into coronal sections using a cryostat (Leica, Wetzlar, Germany). One 25-μm-thick section of hippocampal dentate gyrus was collected every 13 sections. Four brain slices were collected from the same position in each mouse, and underwent avidin-biotin-peroxidase complex immunohistochemistry. The precise procedures were as follows: the slices were washed in phosphate-buffered saline (PBS, 0.01 M, pH 7.4) for 5 minutes, blocked in normal bovine serum at room temperature for 20 minutes, and incubated in rabbit anti-mouse IgG (p-CREB 1:100, specific recognition of p-CREB at Ser-133; Cell Signal Technology, MA, USA). Following three washes in PBS, each for 3 minutes, the slices were incubated overnight at 4°C, and then in diluted biotinylated goat anti-rabbit IgG (1:500; Cell Signal Technology) at 37°C for 30 minutes, in streptavidin-biotin-peroxidase complex (Maixin-Bio, Fuzhou, Fujian Province, China) at room temperature for 30 minutes, washed in PBS, and developed in 0.04% diaminobenzidine/0.03% H2O2 for 8–10 minutes. The specimens were dehydrated, permeabilized, and mounted. PBS, used instead of primary antibody, served as a negative control.
Detection of p-CREB in mouse brain tissue, as determined by western blot assay
Five mice from each group were anesthetized with 10% chloral hydrate (300 mg/kg, i.p.) after the Morris water maze test, and then sacrificed. Hippocampal dentate gyrus was dissociated and stored in liquid nitrogen. The specimen was split on ice, homogenized, and centrifuged at 4°C. The supernatant was obtained and made into protein samples. Protein contents were measured using Coomassie brilliant blue. Protein concentrations were adjusted prior to immunoblotting. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 12% gels, and then transferred to nitrocellulose membranes. Membranes were soaked in Tris-buffered saline, shaken and blocked at room temperature for 2 hours, and then incubated with rabbit anti-IgG (p-CREB 1:1 000, specific recognition of p-CREB at Ser-133; Cell Signal Technology) at 4°C overnight. The membranes were washed twice with Tris-buffered saline with Tween-20, each for 10 minutes, once with Tris-buffered saline for 10 minutes, and then incubated with a horseradish peroxidase-biotinylated goat anti-rabbit IgG (1:500; Cell Signal Technology) at room temperature for 2 hours. The membranes were washed twice with Tris-buffered saline containing Tween-20, each time for 10 minutes, and once with Tris-buffered saline for 10 minutes. They were then treated with enhanced chemiluminescence reagent (Amersham ECLplus Western Blotting Detection System), exposed, developed and fixed.
Result assessment
Results were analyzed using the Image Pro PlusMIS2000 image analysis system (Media Cybernetics, Silver Spring, MD, USA). Under a low power lens of an Olympus BX-50 optical microscope (Olympus, Tokyo, Japan), structures in brain tissue sections were identified; these were then photographed under a high power lens. Yellow or brown staining indicated positive staining. One section of hippocampal dentate gyrus was collected from every two sections. All sections were compared under the same strength and magnification. Five nonoverlapping visual fields (up, down, left, right, middle) were collected from each section. Integrated absorbance values in each visual field were measured. The average value was considered as the integrated absorbance. A high absorbance value represented a high level of p-CREB. The Eagle Eye 3000 gel image analysis system (Beijing Coldspring Science, Beijing, China) was utilized to measure protein absorbance for semiquantitative analysis. Absorbance values for target protein were compared with those for the internal reference glyceraldehyde-3-phosphate dehydrogenase to calculate the absorbance ratio.
Statistical analysis
Measurement data are expressed by means ± SD, and were analyzed utilizing SPSS 17.0 software (SPSS, Chicago, IL, USA). Intergroup differences in mean values were compared by one-way analysis of variance. Paired comparisons of intergroup differences in mean values were performed using the followed by lest significant difference-t test or Student-Newman-Keuls test at α = 0.05.
Acknowledgments:
We thank Liangying Liao from the Central Laboratory, First Affiliated Hospital, Hunan University of Chinese Medicine, China and Xiao Xing from the Central Laboratory, Third Xiangya Hospital, China for their guidance, as well as Yan Chen from the Laboratory of Tumor Proteomics, Xiangya Hospital, China for technical help.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, Central South University, China.
(Edited by Li GC, Yu JB/Qiu Y/Wang L)
REFERENCES
- 1.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature. 2009;461(7266):916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urano T, Tohda C. Icariin improves memory impairment in Alzheimer's disease model mice (5xFAD) and attenuates amyloid β-induced neurite atrophy. Phytother Res. 2010;24(11):1658–1663. doi: 10.1002/ptr.3183. [DOI] [PubMed] [Google Scholar]
- 3.Zeng KW, Ko H, Yang HO, et al. Icariin attenuates β-amyloid-induced neurotoxicity by inhibition of tau protein hyperphosphorylation in PC12 cells. Neuropharmacology. 2010;59(6):542–550. doi: 10.1016/j.neuropharm.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 4.He XL, Zhou WQ, Bi MG, et al. Neuroprotective effects of icariin on memory impairment and neurochemical deficits in senescence- accelerated mouse prone 8 (SAMP8) mice. Brain Res. 2010;2(1334):73–83. doi: 10.1016/j.brainres.2010.03.084. [DOI] [PubMed] [Google Scholar]
- 5.Nomura Y, Okuma Y. Age-elated defects in life span and learning ability in SAMP8 mice. Neurobiol Aging. 1999;20(2):111–115. doi: 10.1016/s0197-4580(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 6.Takeda T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem Res. 2009;34(4):639–659. doi: 10.1007/s11064-009-9922-y. [DOI] [PubMed] [Google Scholar]
- 7.Tomobe K, Nomura Y. Neurochemistry, neuropathology, and heredity in SAMP8: a mouse model of senescence. Neurochem Res. 2009;34(4):660–669. doi: 10.1007/s11064-009-9923-x. [DOI] [PubMed] [Google Scholar]
- 8.Mouravlev A, Dunning J, Young D, et al. Somatic gene transer of cAMP response element-binding protein attenuates memory impairment in aging rats. Proc Natl Acad Sci U S A. 2006;103(12):4705–4710. doi: 10.1073/pnas.0506137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pittenger C, Huang YY, Paletzki RF, et al. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron. 2002;34(3):447–462. doi: 10.1016/s0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- 10.Hinoi E, Balcar VJ, Kuramoto N, et al. Nuclear transcription factors in the hippocampus. Progr Neurobiol. 2002;68(2):145–165. doi: 10.1016/s0301-0082(02)00078-3. [DOI] [PubMed] [Google Scholar]
- 11.Gong B, Vitolo OV, Trinchese F, et al. Persistent improvement in synaptic and cognitive functions in an Alzheimer mouse model after rolipram treatment. J Clin Invest. 2004;114(24):1624–1634. doi: 10.1172/JCI22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shekhawat GS, Lambert MP, Sharma S, et al. Soluble state high resolution atomic force microscopy study of Alzheimer's beta-amyloid oligomers. Appl Phys Lett. 2009;95(18):183701–183710. doi: 10.1063/1.3251779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunomura A, Tamaoki T, Tanaka K, et al. Intraneuronal amyloid beta accumula- tion and oxidative damage to nucleic acids in Alzheimer disease. Neurobiol Dis. 2010;37(3):731–737. doi: 10.1016/j.nbd.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebeck GW, Hoe HS, Moussa CE. Beta-amyloid1-42 gene transfer model exhib its intraneuronal amyloid, gliosis, tau phosphorylation, and neuronal loss. J Biol Chem. 2010;285(10):7440–7446. doi: 10.1074/jbc.M109.083915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayward P. Presenilin dysfunction leads to memory and plastic it defects. Lancet Neurol. 2004;3(6):327. doi: 10.1016/s1474-4422(04)00784-7. [DOI] [PubMed] [Google Scholar]
- 16.Kande lE. The molecular biology of memory storage: a dialogue between “genes and synapses”. Science. 2001;94(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 17.Hu BR, Fux CM, Martone ME, et al. Persistent phosphorylation of cyclic AMP responsive element binding protein and activating transcription factor-2 transcription factors following transcient cerebral ischemia in rat brain. Neuroscience. 1999;89(2):437–452. doi: 10.1016/s0306-4522(98)00352-2. [DOI] [PubMed] [Google Scholar]
- 18.Pcrcz J, Tinclli D, Brunello N, et al. cAMP-dependent phosphorylalion of soluble and crude microtubule fractions of rat cerebral cortex after prolonged desmethylimipramine treatment. Eur J Pharmacol. 1989;172(3):305–316. doi: 10.1016/0922-4106(89)90060-6. [DOI] [PubMed] [Google Scholar]
- 19.Chen AC, Shirayama Y, Shin K, et al. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry. 2001;49(9):753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- 20.The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]


