Abstract
Kainic acid can simulate excitatory amino acids in vitro. Neural stem cells, isolated from newborn Wistar rats, were cultured in vitro and exposed to 100–4 000 μM kainic acid for 7 days to induce neuronal cell differentiation, causing the number of astrocytes to be significantly increased. Treatment with a combination of 0.5 mg/L gastrodin and kainic acid also caused the number of differentiated neurons to be significantly increased compared with treatment with kainic acid alone. Experimental findings suggest that gastrodin reduces the excitability of kainic acid and induces neural stem cell differentiation into neurons.
Keywords: kainic acid, gastrodin, neural stem cells, differentiation
Abbreviations:
GFAP, glial fibrillary acidic protein; NSE, neuron-specific enolase
INTRODUCTION
Gastrodin is a safe sedative, which has analgesic, anti-inflammatory, anti-free radical effects and improved cardiovascular function[1]. An increasing number of studies show that gastrodin inhibits excessive proliferation of glial cells by inhibiting the role of excitatory amino acid α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors and reducing the fiber-like changes characteristic of glial cells[2,3]. Strong evidence suggests gastrodin could inhibit cell expression of glial fibrillary acidic protein (GFAP) after ischemia and reperfusion, diminish secondary damage to the nerve cells by inhibiting excessive proliferation of glial cells. Gastrodin can also weaken the extent of glial cell fiber-like changes[4,5]. Using a model of ischemic hypoxemia in neural stem cells, the percentage of neurons that formed following differentiation of neural stem cells was significantly decreased compared with control, while the ratio of glial cells, especially astrocytes, was increased[6,7,8]. When brain tissue is injured, glial cell hyperplasia may produce and release nerve toxic substances, such as tumor necrosis factor and nitric oxide, which promote development of damage and form scars, resulting in rehabilitation difficulties[9,10,11]. It is therefore essential to investigate how to induce neural stem cells to differentiate into neurons, thereby promoting rehabilitation of brain function in neural-related disorders, including ischemic cerebrovascular disease, neurodegenerative diseases and epilepsy. This study aimed to evaluate whether gastrodin can block neural stem cell differentiation into glial cells after treatment with excitatory amino acids.
RESULTS
Identification of neural stem cells
Nestin-positive antigen expression was observed in cultured cell clones at passage 3 (Figure 1). Nestin, detected by immunofluorescence, indicated that a large number of neurospheres, amplified by scattered single cell clones, were positive for nestin antigen expression (Figure 2).
Figure 1.
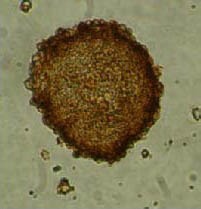
Immunohistochemistry staining showing a nestin-positive (brown) neurosphere at passage 3 (× 400).
Figure 2.
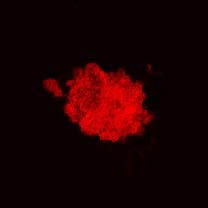
Immunofluroesent staining showing a nestin-positive (red, Cy3) neurosphere at day 7 at passage 3 (× 400).
Neurosphere formed from single cell clones were subjected to immunofluorescence staining, rather than cell clones at primary extraction.
Isolated cells had a strong self-renewal potential and maintained clonogenic capacity continuously throughout passage, maintaining the ability to form new clones after 20 generations (supplementary Figure 1 online). After culture of neural stem cells for three generations and induction of differentiation for 7 days, cells were observed using an inverted microscope. Neurospheres adhered quickly following growth factor removal and cell processes migrated around the neurospheres. The majority of nerve cells began to surround the neurospheres immediately. After culture for 10 days, most cells had migrated from the neurospheres and exhibited neuronal and glial-like cell morphologies. Neuronal cells were larger, irregular, oval or triangular with slender processes. Glial-like cells were flattened with flat processes and an absence of a sharp boundary between the cell bodies and processes (Figure 3). Positive nestin expression within the clone pellet, confirmed that the cells were embryo-derived[12,13].
Figure 3.

Cells migrating from the neurospheres (day 10) showed neuronal and glial-like cell morphology. Neuronal cells were larger, irregular, oval or triangular, with slender processes and glial-like cells were flattened.
Neuron-specific enolase (NSE) is a neuron-specific enzyme, 2’,3’-cyclic-nucleotide 3’-phosphodiesterase is an enzyme characteristic of oligodendrocytes and GFAP is a protein characteristic of astrocytes[14]. Three types of nerve cells, positive for NSE, 2’,3’-cyclic-nucleotide 3’-phosphodiesterase or GFAP were detected using immunocytochemistry after induction in medium containing 10% fetal bovine serum after passage 3 (Figure 4).
Figure 4.
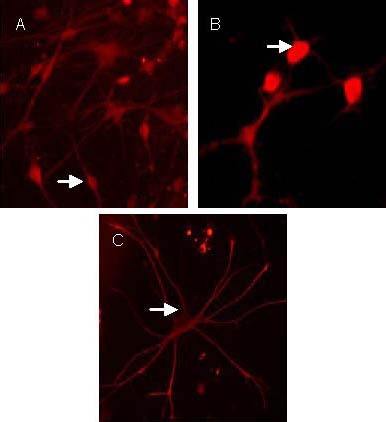
Neonatal rat neural stem cells differentiated into neuron-specific enolase-positive neurons (A), 2’,3’-cyclic-nucleotide 3’-phosphodiesterase-positive oligodendrocytes (B), glial fibrillary acidic protein-positive astrocytes (C) after induction by 10% fetal bovine serum (Cy3, × 400).
Arrows show positive neurons. The positive neuron in Cy3 staining is red.
Effect of gastrodin and kainic acid in neural stem cell differentiation
Neural stem cells at passage 3 were induced to differentiate for 7 days prior to observation with an inverted microscope. In the kainic acid treated group (100 μM), the number of astrocytes was greater in comparison to the control group, while numbers of neuron-like cells were fewer in comparison. Furthermore, the rate of differentiation was faster and the migration distance further in the kainic acid treated group compared with control group. In the combined gastrodin and kainic acid treated group, the number of neuron-like cells was greater than that of kainic acid treated group, but still lower than control group (Figure 5).
Figure 5.
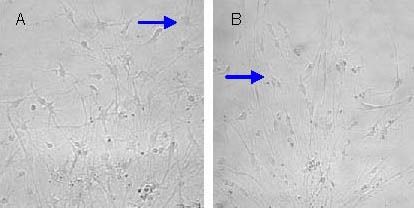
Neural stem cells (passage 3) were induced to differentiate for 7 days (× 200).
Astrocyte numbers in the kainic acid treated group were greater than those in the control group (A) and neuron-like cell numbers were greater in the gastrodin and 100 μM kainic acid treated groups (B).
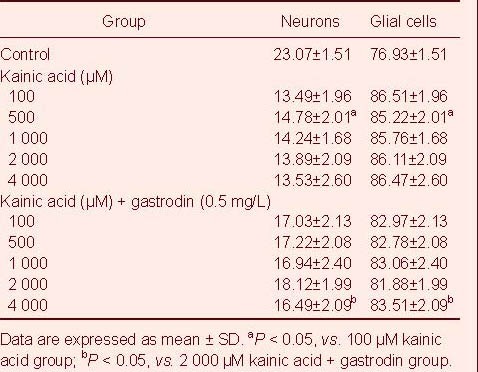
The effect of gastrodin and kainic acid on the differentiation of neural stem cells is shown in Table 1. No significant difference was found between the proportion of neurons and glial cells between kainic acid treated groups with different concentrations (F = 1.43, P > 0.05). The proportion of neurons in the combined gastrodin and kainic acid treated group was significantly higher than that in the kainic acid alone treated group (F = 115.6, P < 0.01).
Table 1.
The proportion (%) of neurons and glial cells formed by neural stem cell differentiation in different groups
DISCUSSION
Kainic acid can simulate the release of excitatory amino acids in cell culture[15]. This study found that: (1) After cell treatment with 5 mM kainic acid, a population of adherent cells retain the ability to differentiate. Their tolerance towards excitatory amino acids was much greater than neurons. When neurons were treated with 100 μM kainic acid, a large number of cells swelled and died[16].
Interestingly, neural stem cells exposed to concentrations 50 times greater than those mentioned above, continued to survive. In summary, increased concentrations of excitatory amino acid resulted in neuron cell death, but enabled survival of neural stem cells, which may continue to differentiate and thus promote recovery of neurological function. The mechanisms underlying neural stem cell tolerance towards excitatory amino acids remain unclear, thus further research is required. (2) Kainic acid at concentrations of 100 μM–5 mM significantly affected the differentiation of neural stem cells, especially the percentage of astrocytes and neurons. Glial cell numbers were not significantly affected in different kainic concentrations. Under normal conditions, glial cells secrete various cytokines and neurotrophic factors, which play important roles in forming and maintaining the neuronal survival microenvironment: (1) To promote differentiated neurons to migrate: once neurospheres have undergone differentiation, astrocytes migrate faster than other cell types, acting as a migration guidance scaffold for other differentiated cell types. (2) To promote cell survival and differentiation: the micro-environment formed by the astrocytes is not only conducive to the survival of neural stem cells, but also promotes their differentiation and the differentiation and survival of mature neurons[17,18]. However, when brain tissue is damaged, over proliferation of glial cells results in excessive production and release of nerve toxic substances, such as nitric oxide and tumor necrosis factor, which promote the development of damage and form scar tissue, making recovery more difficult following brain injury[19]. The present study indicates that gastrodin could prevent neural stem cells from differentiating toward glial cells after treatment with kainic acid to some extent. It is supposed that gastrodin may function to prevent kainic acid binding with its receptor. In this study, gastrodin was found to block kainic acid's effect and induce neural stem cell differentiation into neurons, to some degree. Gastrodin can block channels of excitatory amino acids in the nervous system, whereby conductivity relies on neural stem cell differentiation into neurons to form the electrical activity of nerves, highly consistent with normal cortex[20]. These findings provide a new research direction for treating ischemic cerebrovascular disease and also bring hope in treating key degenerative diseases in the central nervous system.
MATERIALS AND METHODS
Design
A comparative observation at cellular level.
Time and setting
Experiments were performed on March 2009 in the Henan Institute of Medical Sciences, China.
Materials
Animals
Two newborn (1 day old), specific free grade Wistar rats, were provided by the Experimental Animal Center of Zhengzhou University, China, with Certificate of Conformity Number, 410116. All protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[21].
Drugs
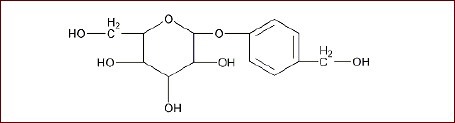
Kainic acid was purchased from Sigma, St. Louis, MO, USA. Gastrodin was provided by Shanghai Huayi Bio-Technology Company, Ltd, Shanghai, China. Gastrodin's chemical name and formula are 4-hydroxy phenyl-D-β-glucopyranoside[22,23] and C13H18O7 respectively and the molecular structure is:
Gastrodin's chemical Abstracts Service Number is 62499-27-8 and purity level is 99.9%.
Methods
Isolation, culture and induced differentiation of rat neural stem cells
According to previously described methods[24,25,26,27] with some modifications, newborn Wistar rat brain tissue was harvested, washed three times with cold D-Hank's solution and cut with eye scissors into approximately 1 mm3 tissue blocks. Specimens were trypsinized with 0.25% trypsin for 10 minutes and digestion was terminated with fetal bovine serum. Cells were centrifuged three times for 5 minutes each at 1 000 r/min and incubated in serum-free medium containing 20 μg/L basic fibroblast growth factor. Supernatant was discarded. Cells were prepared into a single-cell suspension using a 200-mesh screen filter and seeded at 5 × 105 cells/mL into culture flasks and cultured in saturated humidity at 37°C at 5% CO2 for 7 days. Cells were separated with a pipette and passaged according to previously described methods[24,28]. Each passage was subcultured for 7 days. Primary cultured cell clones at passage 3 were inoculated in 24-well culture plates pre-equipped with Thermanox plastic cell culture chips, in medium without fetal bovine serum and basic fibroblast growth factor for 10 days. Neurosphere morphology was observed under an inverted phase contrast microscope (Olympus, Tokyo, Japan).
Identification of neural stem cells by immunohistochemistry and immunofluorescence
Neural stem cells were fixed in paraformaldehyde and incubated with goat serum, rabbit anti-nestin, NSE (1:100), 2’,3’-cyclic-nucleotide 3’-phosphodiesterase (1:200), GFAP (1:200; Santa Cruz, CA, USA) at 37°C for 60 minutes. Secondary antibody goat anti-rabbit IgG (1:1 000; Boster, Wuhan, China) was used and immunofluorescence was detected with Cy3-labeled goat anti-rabbit IgG (1:1 000; Boster). Cells were incubated at 37°C for 60 minutes and observed microscopically (Leica Camera AG, Canada).
Gastrodin and kainic acid treatment
Passage 3 cell clones were diluted and suspended at 1 × 105 cells/mL, prior to seeding in 96-well plates containing 200 μL/well at 37°C in 5% CO2 at 100% humidity for 4 hours. Cells were divided into three groups: control group in absence of drug; kainic acid group with different concentrations of kainic acid (100, 500, 1 000, 2 000 and 4 000 μM) for 30 minutes; combined gastrodin + kainic acid group: treatment with kainic acid for 30 minutes, followed by physiological saline rinse (8 g NaCl, 0.4 g KCl, 0.18 g Na2HPO4·7H2O, 0.03 g KH2PO4, 3 g sucrose, 7.5 g glucose, 2.34 g Hepes and distilled water to 1 000 mL), then culture with 0.5 mg/L gastrodin for 30 minutes, prior to physiological saline rinse. Each concentration group was repeated in eight wells. The proportion of NSE and GFAP positive cells to total cell number was calculated, to determine the proportion of neuronal and glial-like cells.
Statistical analysis
SPSS 13.0 software (SPSS, Chicago, IL, USA) was used to analyze the variance (randomized block design) in conjunction with a two-sample t-test. A test for normality and homogeneity of variance were performed. The test standard significance level was 0.05.
Acknowledgments:
We would like to thank Jinghong Li from the Laboratory of Neurology Department, the First Affiliated Hospital, Zhengzhou University, Zhengzhou, Henan Province, China for guiding the experiment.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30770758.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, Zhengzhou University, Zhengzhou, Henan Province, China.
Supplementary information: Supplementary data associated with this article can be found in the online version, by visiting www.nrronline.org, and entering Vol. 7, No. 12, 2012 after selecting the “NRR Current Issue” button on the page.
(Edited by Gu PF, Huang H/Yang Y/Song LP)
REFERENCES
- 1.Wang Q, Chen G, Zeng S. Distribution and metabolism of gastrodin in rat brain. J Pharm Biomed Anal. 2008;46(2):399–404. doi: 10.1016/j.jpba.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Tian AG, Deng WM. Par-1 and Tau regulate the anterior-posterior gradient of microtubules in Drosophila oocytes. Dev Biol. 2009;327(2):458–464. doi: 10.1016/j.ydbio.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchida K, Nakajima H, Takamura T, et al. Gene expression profiles of neurotrophic factors in rat cultured spinal cord cells under cyclic tensile stress. Spine (Phila Pa 1976) 2008;33(24):2596–2604. doi: 10.1097/BRS.0b013e31818917af. [DOI] [PubMed] [Google Scholar]
- 4.Dvoriantchikova G, Barakat D, Brambilla R, et al. Inactivation of astroglial NF-kappa B promotes survival of retinal neurons following ischemic injury. Eur J Neurosci. 2009;30(2):175–185. doi: 10.1111/j.1460-9568.2009.06814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu JJ, Hong QT, Tang YP, et al. Protective effects of gastrodine against lesions in cultured astrocytes caused by simulated cerebral ischemia and reperfusion, and its influence on the activity of nitric oxide synthase. Beijing Zhongyiyao Daxue Xuebao. 2001;24(5):11. [Google Scholar]
- 6.Yu XH, Yang YJ, Zhong L, et al. Effect of hyperbaric oxygen on NSCs in the neonatal rat with hypoxic- ischemic brain damage. Zhongguo Bingli Shengli Zazhi. 2006;22(5):960. [Google Scholar]
- 7.Shin YJ, Choi JS, Choi JY, et al. Induction of vascular endothelial growth factor receptor-3 mRNA in glial cells following focal cerebral ischemia in rats. J Neuroimmunol. 2010;229(1-2):81–90. doi: 10.1016/j.jneuroim.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Benesova J, Hock M, Butenko O, et al. Quantification of astrocyte volume changes during ischemia in situ reveals two populations of astrocytes in the cortex of GFAP/EGFP mice. J Neurosci Res. 2009;87(1):96–111. doi: 10.1002/jnr.21828. [DOI] [PubMed] [Google Scholar]
- 9.Dunning MD, Lakatos A, Loizou L, et al. Superparamagnetic iron oxide-labeled Schwann cells and olfactory ensheathing cells can be traced in vivo by magnetic resonance imaging and retain functional properties after transplantation into the CNS. J Neurosci. 2004;24(44):9799–9810. doi: 10.1523/JNEUROSCI.3126-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porzionato A, Macchi V, Parenti A, et al. Trophic factors in the carotid body. Int Rev Cell Mol Biol. 2008;269:1–58. doi: 10.1016/S1937-6448(08)01001-0. [DOI] [PubMed] [Google Scholar]
- 11.Ide C, Nakai Y, Nakano N, et al. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 2010;1332:32–47. doi: 10.1016/j.brainres.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 12.Rietze RL, Valcanis H, Brooker GF, et al. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412(6848):736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- 13.Fariha MM, Chua KH, Tan GC, et al. Human chorion-derived stem cells: changes in stem cell properties during serial passage. Cytotherapy. 2011;13(5):582–593. doi: 10.3109/14653249.2010.549121. [DOI] [PubMed] [Google Scholar]
- 14.Liu CJ, Yin F, Zheng XR, et al. The isolating culture of neural stem cells from rat's fetus brain and transfection by electroporation. Zhonghua Yi Xue Za Zhi. 2009;89(42):3007–3009. [PubMed] [Google Scholar]
- 15.Levchenko-Lambert Y, Turetsky DM, Patneau DK. Not all desensitizations are created equal: physiological evidence that AMPA receptor desensitization differs for kainate and glutamate. J Neurosci. 2011;31(25):9359–9367. doi: 10.1523/JNEUROSCI.6761-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rangel A, Burgaya F, Gavín R, et al. Enhanced susceptibility of Prnp-deficient mice to kainate-induced seizures, neuronal apoptosis, and death: Role of AMPA/kainate receptors. J Neurosci Res. 2007;85(12):2741–2755. doi: 10.1002/jnr.21215. [DOI] [PubMed] [Google Scholar]
- 17.Faijerson J, Tinsley RB, Apricó K, et al. Reactive astrogliosis induces astrocytic differentiation of adult neural stem/progenitor cells in vitro. J Neurosci Res. 2006;84(7):1415–1424. doi: 10.1002/jnr.21044. [DOI] [PubMed] [Google Scholar]
- 18.Mena MA, García de Yébenes J. Glial cells as players in parkinsonism: the “good,” the “bad,” and the “mysterious” glia. Neuroscientist. 2008;14(6):544–560. doi: 10.1177/1073858408322839. [DOI] [PubMed] [Google Scholar]
- 19.Zeng J, Yao J, Wang Q, et al. Effect of propofol on spinal excitatory amino acid accumulation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(6):723–726. [PubMed] [Google Scholar]
- 20.Lynch M, Sutula T. Recurrent excitatory connectivity in the dentate gyrus of kindled and kainic acid-treated rats. J Neurophysiol. 2000;83(2):693–704. doi: 10.1152/jn.2000.83.2.693. [DOI] [PubMed] [Google Scholar]
- 21.The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- 22.Zeng X, Zhang S, Zhang L, et al. A study of the neuroprotective effect of the phenolic glucoside gastrodin during cerebral ischemia in vivo and in vitro. Planta Med. 2006;72(15):1359–1365. doi: 10.1055/s-2006-951709. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Han FM, Du P, et al. Pharmacokinetics of gastrodin from compound Tianma granule in rats. Yao Xue Xue Bao. 2010;45(4):484–488. [PubMed] [Google Scholar]
- 24.Jia YJ, Yang YJ, Song JH, et al. Isolation and cultivation of neural stem cells in neonatal rats. Zhongguo Dangdai Erke Zazhi. 2002;4(2):81–83. [Google Scholar]
- 25.Pagani L, Cenciarelli C, Casalbore P, et al. Identification and early characterization of genetically modified NGF-producing neural stem cells grafted into the injured adult rat brain. Neurol Res. 2008;30(3):244–250. doi: 10.1179/016164107X230667. [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedi RK, Shukla S, Seth K, et al. Zuckerkandl's organ improves long-term survival and function of neural stem cell derived dopaminergic neurons in Parkinsonian rats. Exp Neurol. 2008;210(2):608–623. doi: 10.1016/j.expneurol.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Yang P, He X, Li H, et al. Isolation and culture of neural stem cells in injured region of compressive spinal cord injury in adult rat. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(2):151–155. [PubMed] [Google Scholar]
- 28.Deng H, Zou F, Luo HJ. Differentiation of neonatal rat striatal neural stem cells induced by all-trans retinoic acid. Di Yi Jun Yi Da Xue Xue Bao. 2005;25(11):1357–1360. 1374. [PubMed] [Google Scholar]


