Abstract
Previous studies have demonstrated that pine pollen can inhibit cerebral cortical cell apoptosis in mice with arsenic poisoning. The present study sought to detect the influence of pine pollen on apoptosis-related proteins. Immunohistochemistry, western blotting and enzyme-linked immunosorbent assays were used to measure the levels of apoptosis-related proteins in the cerebral cortex of mice with arsenic poisoning. Results indicated that pine pollen suppressed cell apoptosis in the cerebral cortex of arsenic-poisoned mice by reducing Bax, Bcl-2 protein expression and increasing p53 protein expression.
Keywords: pine pollen, arsenic poisoning, apoptosis, cerebral cortex, neural regeneration
Abbreviations:
ELISA, enzyme-linked immunosorbent assay; MAPK, mitogen-activated protein kinase
INTRODUCTION
The mechanism of arsenic poisoning has attracted growing interest[1,2,3]. Pine pollen is effective in treating hyperplasia of the prostate gland, protecting the liver, reducing blood fat, improving immunity and relieving fatigue[4,5]. A previous study reported that pine pollen can reduce renal cell apoptosis in arsenic poisoned mice[6]. Studies focusing on arsenic-induced liver and renal cell apoptosis concluded that Bcl-2 is an anti-apoptotic factor[7], and morphological studies have been used to determine damage to the brain by arsenic[8,9]. In addition, the apoptosis-related factor, Fas and Bcl-2 are linked to immunological tolerance[9].
Pine pollen extract has significant anti-tumor effects in vivo and in vitro[10,11]. Inorganic arsenic can influence nerve reactions in mice[12], and can induce hippocampal nerve cell apoptosis by upregulating Bax and downregulating Bcl-2 proteins[13]. In addition, arsenate can selectively activate p38/mitogen-activated protein kinase 14 (MAPK14) and c-Jun N-terminal kinase 3, inducing cell apoptosis in the mouse cerebellum[14].
The present study utilized immunohistochemistry and western blotting techniques to detect cell apoptosis and determine expression of apoptosis-related factors, Bax, Fas, Bcl-2, p38 and p53 in the cerebral cortex of mice treated with pine pollen after arsenic poisoning.
RESULTS
Quantitative analysis of experimental animals
A total of 54 adult mice were randomly assigned to normal control, treatment and NaAsO2 control groups, with 18 animals in each group. The normal control group was treated with distilled water, whereas treatment and NaAsO2 control groups were injected with NaAsO2 every 2 days to establish arsenic poisoning. The treatment group was intragastrically perfused with pine pollen from day 15 after the initiation of arsenic poisoning until 45 days after the beginning of arsenic poisoning[6]. All mice were analyzed at 45 days after initiation of arsenic poisoning. All 54 mice were included in the final analysis.
Pine pollen suppressed cell apoptosis in the cerebral cortex of mice with arsenic poisoning
Flow cytometry showed the percentage of apoptotic cells (6.95 ± 0.01%) in the treatment group was significantly lower than in the NaAsO2 control group (21.64 ± 0.50%, P < 0.05), but similar to that of the normal control group (2.49 ± 0.02%; P > 0.05) at 30 days following pine pollen treatment.
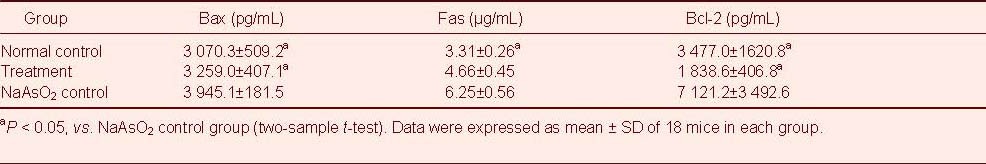
Influence of pine pollen on apoptosis-related factors Bax, Fas and Bcl-2 levels in the cerebral cortex of mice with arsenic poisoning
Enzyme-linked immunosorbent assay (ELISA) showed less Bax and Bcl-2 content in the treatment group compared with the NaAsO2 control group at 30 days following pine pollen treatment (P < 0.05; Table 1). Levels of Fas were slightly lower in the pollen treated group compared with the NaAsO2 control groups (Table 1).
Table 1.
Influence of pine pollen treatment on Bax, Fas and Bcl-2 levels in the cerebral cortex of arsenic-poisoned mice
Influence of pine pollen on p38 protein expression in the cerebral cortex of mice with arsenic poisoning
p38 is a proapoptotic factor. p38 expression in cells of the cerebral cortex was similar between the treatment and NaAsO2 control groups at 30 days following pine pollen treatment (absorbance ratio to β-actin: 1.152 ± 0.050 vs. 1.644 ± 0.440), and slightly greater than the normal control group (0.423 ± 0.205; Figure 1).
Figure 1.

p38 protein expression in cells of cerebral cortex in mice. The experiment was performed in triplicate.
p53 protein expression levels in the cerebral cortex of mice with arsenic poisoning
p53 protein is an anti-apoptotic factor. Immunohistochemistry showed that p53 protein expression in the cerebral cortex of mice was present in the control and NaAsO2 control groups but was increased in the pollen treated group at 30 days following pine pollen treatment (Figure 2).
Figure 2.
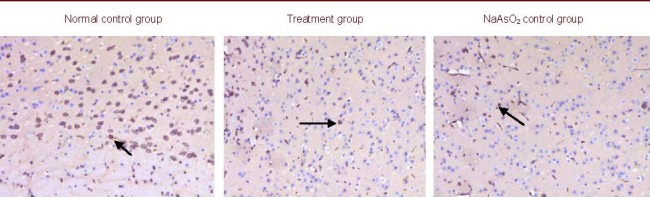
p53 protein expression in the cerebral cortex of mice (immunohistochemical staining, × 400). Positive p53 expression is represented as a brown yellow stain (arrows). High levels of p53-positive cells were observed in the normal and NaAsO2 control mice. There were increased numbers of p53-positive cells in the treatment group compared with NaAsO2 control group.
DISCUSSION
In the present study, flow cytometry showed that pine pollen reduced the cell apoptosis rate in the cerebral cortex of mice with arsenic poisoning. In addition, we detected the expression of apoptosis-related factors, Bax, Fas, Bcl-2 and p53. Arsenic has been shown to induce nerve cell apoptosis in the hippocampus, and Bcl-2 is an anti-apoptotic factor[13]. However, results from the present study suggested that cortical Bcl-2 and Fas content was higher in the NaAsO2 control group compared with normal control and treatment groups, indicating Bcl-2 may be an apoptosis-inducing factor. Bcl-2 may also be associated with the Fas pathway[15,16,17] during cell apoptosis in the cerebral cortex of mice with arsenic poisoning. However, the precise mechanism of how these pathways interact remains unknown. In conclusion, treatment with pine pollen can downregulate cortical Bax and Bcl-2 expression, suggesting that pine pollen may be a useful treatment for arsenic poisoning. However, levels of p53, a pro-apoptotic protein, were also increased, thus further studies are needed to confirm the precise mechanism of pine pollen treatment.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiment was performed at the Central Laboratory, Youjiang Medical College for Nationalities, China, in October 2011.
Materials
Animals
A total of 24 healthy, female Kunming mice, aged 12 weeks, weighing 23–25 g, were purchased from the Department of Laboratory Animal, Guangxi Medical University (license No. SCXK (Gui) 2009-0002). Experimental procedures were performed in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[18].
Drugs
Thirty packages × 3 g pine pollen disruption powder (Xinshidai Company, Yantai, Shandong, China; G20070244), were used to prepared 100% pine pollen powder following disruption at low temperature, with a disruption rate of 100%. Pine pollen was administrated to mice by intragastric perfusion (by placing the perfusion device through the mouth and directly into the stomach). Pine pollen is composed of over 200 nutrition elements, including 22 amino acids, 15 vitamins, 30 minerals, 100 enzymes, nucleic acids, unsaturated fatty acids, lecithin, flavonoids, monosaccharides and polysaccharides. NaAsO2 and analytical reagents were purchased from the Fourth Branch of Shanghai Chemical Reagent Company, China.
Methods
Establishment of arsenic poisoning model
The treatment and NaAsO2 control groups were injected with NaAsO2 (1 mg/kg), every 2 days to induce arsenic poisoning. Blood arsenic content in each group was determined 15 days after arsenic poisoning induction. Successful poisoning was represented by a significant difference in the blood arsenic content between normal and arsenic poisoning induction groups. In addition, the treatment group was intragastrically perfused with pine pollen, 1 g/kg, for 30 days.
Flow cytometry for cell apoptosis in the cerebral cortex of mice
Following pine pollen treatment for 30 days, the mice were anesthetized and sacrificed. The cerebral cortex was harvested, dispersed by pipetting, filtrated, centrifuged, rinsed, and adjusted to density of 1 × 106 cells/L. Cells were treated according to a cell apoptosis kit (Boster, Wuhan, China), and the apoptosis rate was determined using BD FACScant II automatic flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA).
ELISA for Bax, Fas and Bcl-2 levels in cell supernatant of the cerebral cortex
The cerebral cortex was harvested, centrifuged, mixed with protein extraction cell lysate, suspended and centrifuged to extract protein samples. Mouse apoptosis factors Bax and Fas ELISA kit (Invitrogen Life Technologies, Carlsbad, CA, USA) and ELISA Bcl-2 kit (Shanghai Westang Bio-Tech Inc., Ltd., Shanghai, China) were used according to the manufacturer's instructions. Cells were incubated with primary antibodies, mouse anti-Bax, Fas, Bcl-2 monoclonal antibody, at 37°C for 1 hour, followed by secondary antibodies, biotin labeled goat anti-mouse Fas polyclonal antibody at 37°C for 30 minutes. Absorbance at 450 nm was determined using RT-60000 microplate reader (Rayto Life and Analytical Sciences Co., Ltd., Guangdong, China). A standard curve was drawn according to the absorbance values and corresponding concentration of standard samples, and a linear equation was obtained. Sample concentrations were calculated with the linear equation using the absorbance value of each group sample.
Western blot for p38 protein expression in cortical cells of mice
p38 protein expression was detected by western blot assay (Daan Gene Co., Ltd., Guangzhou, China). Briefly, cerebral cortex tissues, 0.3 g, were lysed using radioimmunoprecipitation assay buffer and cut into pieces, followed by 22% amplitude ultrasound. The tissue solution was then rotated and extracted in the refrigerator for 30 minutes, and centrifuged at 16 000 r/min, 4°C for 30 minutes. The supernatant was harvested, electrophoresed and stained with Coomassie brilliant blue to quantify sample amount. The samples were stored at −80°C, followed by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The protein was transferred to polyvinylidene fluoride membrane (Beijing Neuron Biotechnology, Beijing, China) at 350 mA for 1 hour. The membrane was blocked in Tris-buffered saline Tween-20 with 5% non-fat milk powder for 2 hours, washed with Tris-buffered saline Tween-20 three times, for 5 minutes each. The membrane was incubated with primary antibody, rabbit anti-MAPK14 (p38MAPK) polyclonal antibodies (1:1 000; Beijing Biosynthesis Biotechnology, Beijing, China) overnight at 4°C, washed with Tris-buffered saline Tween-20 three times, for 5 minutes each, followed by incubation with secondary antibody horseradish peroxidase-labeled goat anti-mouse antibody (1:5 000; Shanghai Yiboju, Shanghai, China) at room temperature for 1 hour. The membrane was washed with Tris-buffered saline Tween-20, incubated with chemiluminescence reagent A and B solution at equal volume for 1 minute, and imaged using gel imaging system (Bio-Rad, Hercules, CA, USA). Absorbance ratio of samples to internal reference (Mouse Anti-β-actin antibody, Boster) was calculated.
Immunohistochemistry for p53 protein expression in the cerebral cortex
Cerebral cortex paraffin sections were prepared, dewaxed, hydrated, washed with phosphate buffered saline (PBS), treated with high pressure to retrieve antigen, washed with PBS, incubated with primary antibody rabbit anti-p53 polyclonal antibody (200 μg/mL) overnight at 4°C, followed by incubation with secondary antibody, horseradish peroxidase-labeled anti-rabbit antibody (10 μg/mL), at 30°C for 30 minutes. The sections were washed with PBS, stained, washed with PBS, dehydrated, cleared and mounted. Positive p53 staining was represented by brown yellow color of sections, and observed by light microscopy (Olympus, Tokyo, USA).
Statistical analysis
Data were analyzed using SPSS version 13.0 (SPSS, Chicago, IL, USA) and was expressed as mean ± SD. Intragroup mean differences were compared utilizing two-sample t-test. A value of P < 0.05 was considered statistically significant.
Acknowledgments:
We thank the staff of Department of Pathology, Affiliated Hospital of Youjiang Medical College for Nationalities, for section preparation and p53 protein immunohistochemical staining. We also thank the Guangzhou Dayin Company for assisting analysis of p38 protein in cortical cells of each group.
Footnotes
Funding: This study was supported by the Guangxi Bureau of Traditional Chinese Medicine, No. [2009]73; the Department of Science and Technology, Guangxi Zhuang Autonomous Region, No. 0640203.
Conflicts of interest: None declared.
Ethical approval: This study received permission from the Animal Ethnics Committee of Youjiang Medical College for Nationalities, China.
(Edited by Wang D, Duan HB/Su LL/Wang L)
REFERENCES
- 1.Argos M, Kalra T, Rathouz PJ, et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet. 2010;376(9737):252–258. doi: 10.1016/S0140-6736(10)60481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin YL, Liang CK, He GL, et al. Study on distribution of endemic arsenism in China. Weisheng Yanjiu. 2003;32(6):519–540. [PubMed] [Google Scholar]
- 3.Uddin R, Huda NH. Arsenic poisoning in bangladesh. Oman Med J. 2011;26(3):207. doi: 10.5001/omj.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He XY, Sun XY, Yu ZY. Effective components and pharmacological function of pine pollen. Dongbei Linye Daxue Xuebao. 2007;35(9):78–79. [Google Scholar]
- 5.Meng SX. Effects of ruangan pill on express of bcl-2 and bax in hepatic stellate cells. Guangming Zhongyi. 2010;25(9):1588–1589. [Google Scholar]
- 6.Luo YH, Wang TZ, Wang JL, et al. The influence of Pine Pollen to the apoptosis of cerebrum cortex cells of arsenic poisoning mouses. Linchuang Yiyao Shijian. 2010;19(10):1502–1505. [Google Scholar]
- 7.Li YF, Kang CS, Zang GY, et al. Effect of chronic arsenism on ultra-structure of rats’ hippocampus CA3. Huanjing yu Jiankang Zazhi. 2008;25(6):517–519. [Google Scholar]
- 8.Jiang D, Sun BF. Effect of chronic arsenic poisoning on astrocyte in hippocampal CA1 area of mouse. Jujie Shoushuxue Zazhi. 2011;20(3):239–241. [Google Scholar]
- 9.Xiong XP, Ren MS, Yang Yi, et al. The role of nasal immune tolerance on the expression of Fas and Bcl-2 in the thymus of experim ental autoimmune myasthenia gravis rats. Zhongguo Jingshen Shenjing Zazhi. 2009;35(1):11–14. [Google Scholar]
- 10.Chen DH, Quyang H. A review of flavonoids against cancer and its mechanism. Zhongguo Nongyexue Tongbao. 2005;21(4):91–96. [Google Scholar]
- 11.Li WW, Chen CF, Li Y. A preliminary study on the anti-tumor activity of alcohol extracts of pini pollen. Jiefangjun Yaoxue Xuebao. 2011;27(3):199–201. [Google Scholar]
- 12.Xi S, Sun W, Wang F, et al. Transplacental and early life exposure to inorganic arsenic. Arch Toxicol. 2009;83(6):549–556. doi: 10.1007/s00204-009-0403-5. [DOI] [PubMed] [Google Scholar]
- 13.Yang DY, Liang CK, Jin YL, et al. Study on the apoptosis of rat hippocampal neurons in primary culture due to sodium arsenite. Zhongguo Difangbing Xue Zazhi. 2003;22(3):204–206. [Google Scholar]
- 14.Namgung UK, Xia Z. Arsenicinduces apoptosis in rat cerebellarneurous via activation of JNK3 and P38 mapkinase. Toxicol Appl Pharmacol. 2001;174(2):130–138. doi: 10.1006/taap.2001.9200. [DOI] [PubMed] [Google Scholar]
- 15.Beyersmann D, Hartwig A. Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol. 2008;82(8):493–512. doi: 10.1007/s00204-008-0313-y. [DOI] [PubMed] [Google Scholar]
- 16.Frankel B, Longo SL, Ryken TC. Human astrocytomas co-expressing Fas and Fas ligand also produce TGF beta2 and Bcl-2. J Neurooncol. 1999;44(3):205–212. doi: 10.1023/a:1006311231189. [DOI] [PubMed] [Google Scholar]
- 17.Jia J, Wang MD, Liu SX, et al. Expression and significance of Fas and Bcl-2 proteins in human astrocytoma. Xi’an Jiaotong Daxue Xuebao: Yixue Ban. 2004;25(3):275–278. [Google Scholar]
- 18.The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]


