Abstract
Background:
Ego-boundary disturbance (EBD) is a unique symptom cluster characterized by passivity experiences (involving thoughts, actions, emotions and sensations) attributed by patients to some external agency. The neurobiology of these “first rank” symptoms is poorly understood. Aberrant mirror neuron activation may explain impaired self-monitoring and agency attribution underlying these symptoms. We aim to study mirror neuron activity (MNA) in schizophrenia patients with and without EBD using transcranial magnetic stimulation (TMS).
Materials and Methods:
50 right-handed schizophrenia patients (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) were evaluated using the Mini-International Neuropsychiatric Interview and the Positive and Negative Syndrome Scale. They completed a TMS experiment to assess putative premotor MNA. Motor evoked potential (MEP) was recorded in the right first dorsal interosseous muscle (FDI) with (a) 120% of resting motor threshold (RMT), (b) stimulus intensity set to evoke MEP of motor threshold 1 mV amplitude (MT1), (c) two paired pulse paradigms (short- and long interval intra-cortical inhibition). These were done in three states: Actual observation of an action using the FDI, virtual-observation (video) of this action and resting state. The percent change of MEP from resting to action-observation states formed the measure of putative MNA.
Results:
MNA measured using MT1 and 120% RMT paradigms was significantly lower in the 18 patients with EBD (thought-broadcast/withdrawal/insertion, made-act/impulse/affect and somatic passivity) than the 32 patients without EBD (t = 2.431, P = 0.020; t = 2.051, P = 0.04 respectively for the two paradigms). The two groups did not differ on age, gender, education and total symptom scores.
Conclusion:
Schizophrenia patients with EBD have lower premotor MNA. This highlights the role of MNA dysfunction in the pathophysiology of this unique and intriguing symptom cluster in schizophrenia.
Keywords: Ego-boundary disturbances, first rank symptoms, mirror neuron activity, psychosis, transcranial magnetic stimulation
INTRODUCTION
First rank symptoms (FRSs) – a group of intriguing symptoms characterized by a striking breach of “self-versus nonself” boundaries, first described by Schneider,[1] have had a critical influence on the diagnosis of schizophrenia over the past many decades. This symptom cluster comprises of audible thoughts, voices arguing, voices commenting on one's action, influence playing on the body (somatic passivity), thought withdrawal, thought insertion, broadcasting of thoughts, made feelings, made impulses, made volitional acts, and delusional perception.[1,2]
Factor analytical studies have supported a two-dimensional structure of FRS that is stable over time: Symptoms characterized by ego-boundary disturbances (EBDs; delusions of alien control, thought alienation phenomena and somatic passivity) and those characterized by different auditory hallucinations.[3] Although FRS lack diagnostic specificity, these symptoms provide a well-defined template to study the neurobiological basis of psychotic symptoms from a phenomenological perspective.[4] The first symptom dimension characterized by EBDs is of particular interest as it represents an alienated feature of the sense of one's own mental or physical activity.[5]
The neural underpinnings of such EBDs are poorly understood. It has been hypothesized that a dysfunctional mirror neuron network (inferior frontal gyrus, ventral premotor cortex and inferior parietal lobule) leads to functional dissociations between the action imagination, and action enactment, thus resulting in misattribution of agency,[6] which is seen in symptoms of EBD. Mirror neurons are specialized neurons that are active not only when performing an action oneself, but also while observing someone else perform that action.[7] Interestingly, there is emerging evidence for dysfunctional mirror neuron activity (MNA) in patients with schizophrenia.[8,9]
However, there are no studies, which have empirically tested this hypothesis. Transcranial magnetic stimulation (TMS) is a unique, noninvasive, tool having both investigative and therapeutic applications.[10] It has been extensively used to derive a putative measure of MNA (henceforth referred to as MNA) in humans by detecting changes in the motor evoked potentials (MEP) of hand muscles recorded using electromyography. Enhancement in the MEP amplitude during action observation relative to rest states gives a measure of putative MNA.[11,12] When compared to functional neuroimaging, TMS has poorer spatial resolution, but better temporal resolution to measure MNA.[13] In this study, we aim to compare putative MNA, measured using TMS between schizophrenia patients with and without symptoms of EBDs. We hypothesize that patients with EBD will have reduced MNA than patients without EBD.
MATERIALS AND METHODS
A total of 50 right-handed schizophrenia patients (18 with FRS and 32 without FRS) from the inpatient and outpatient services of the National Institute of Mental Health and Neurosciences, Bengaluru who were recruited as part of a larger study assessing MNA in schizophrenia[14] were recruited for this analysis. They were diagnosed independently by two qualified psychiatrists according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria, and confirmed using the Mini-International Neuropsychiatric Interview (MINI).[15] Symptoms were assessed using Positive and Negative Syndrome Scale (PANSS).[16] Presence or absence of EBD symptoms (though withdrawal, broadcast and insertion, made volition, impulse and affect; and somatic passivity) was determined initially during interviews with PANSS and MINI; which was then confirmed by a comprehensive clinical interview and mental status examination, based on the definitions given by Mellor.[2]
Patients with substance dependence in the previous 6 months (except nicotine), presence of co-morbid neurological or medical disorder, clinically diagnosable or self-reported visual or auditory impairment, current pregnant or postpartum state and a score of ≤19 on the Hindi Mental Status Examination[17] were excluded from the study. All subjects were assessed with (a) the Edinburgh inventory for handedness[18] and (b) TMS adult safety screen[19] to screen for their potential to develop complications. The Institute's Ethics Committee approved the study and all subjects provided written informed consent.
Transcranial magnetic stimulation experiment to assess putative mirror neuron activity
Subjects underwent an experiment to assess motor cortical excitability during goal-directed action observation, relative to rest states, to give a putative measure of MNA.
They were seated comfortably in a chair, in a silent room, 50 cm from the presentation monitor, with their elbows flexed at 90° and hands rested on the armrest of the chair in a prone position.[11] Single pulse TMS was applied using a 70-mm figure-of-eight coil (MagPro R30 with MagOption; MagVenture, Farum, Denmark) positioned tangentially over the hand area of the left motor cortex, with the handle pointing posterolaterally at a 45° to the sagittal plane.
For each subject, the optimal coil position was determined based on standard methods described in previous studies[20,21] for localizing the scalp area to stimulate the right first dorsal interosseous (FDI) muscle. This site was marked with a skin marker pen to ensure uniformity of coil positioning throughout the experiment. The coil was held with both hands bracing the coil against the head.[22] Magnetic pulses activate cortical pyramidal neurons, leading to corticospinal output that can be measured peripherally as an MEP using electromyography. Initially, all participants underwent a calibration session during which their motor thresholds were established. Resting motor threshold (RMT) was defined as the minimum stimulation intensity (measured in percentage of maximum machine output) required, to evoke a >50 μV MEP in the resting, right FDI muscle in at least 6 out of 10 consecutive trials, measured using electromyography.[23] Motor threshold of 1 mV (MT1) was defined as the minimum stimulation intensity, evoking 1 mV peak-to-peak amplitude in the resting, right FDI muscle in at least 6 out of 10 successive recordings.[23] Both RMT and MT1 were calculated using progressive reduction of the stimulator intensity from supra-threshold levels in 1% decrements as described in previous studies.[20,21]
Next, participants underwent the experiment session. Four stimulus paradigms (two single-pulse and two paired-pulse paradigms) were used to study cortical excitability while the participants watched three different action related visual displays (see below).
Single-pulse paradigms
120% of RMT: MEPs obtained with stimulus intensity equal to 120% of RMT were recorded. This stimulus intensity has been the most commonly implemented in studying putative mirror mechanisms using TMS.[9,22]
MT1: MEPs obtained with stimulus intensity equal to MT1 were recorded.[24]
Both 120% RMT and MT1 are a measure of membrane excitability of pyramidal neurons, being influenced by voltage-gated sodium channels.
Paired-pulse paradigms
Short interval intra-cortical inhibition (SICI): A sub-threshold conditioning stimulus (80% of RMT) was given 3 ms before a supra-threshold test stimulus (MT1) with the right hand at rest. The sub-threshold conditioning stimulus excites only the cortical interneurons and, therefore, inhibits the MEP response to the test stimulus (conditioned MEP) by 50%-90%.[25] GABAA receptor agonists potentiate SICI, thus suggesting that SICI may be mediated by GABAA receptor-mediated neurotransmission.[26]
Long interval intra-cortical inhibition (LICI): A supra-threshold conditioning stimulus (MT1) is given 100 ms before a supra-threshold test stimulus (MT1).[27] The supra-threshold conditioning stimulus activates GABAB receptor-mediated inhibitory postsynaptic potentials, thus inhibiting the MEP response to the test stimulus (conditioned MEP).[28] Thus, LICI reflects cortical inhibition mediated through GABAB receptor-mediated neurotransmission, based on findings that baclofen, a specific GABAB receptor agonist enhances LICI.[29]
Cortical inhibition was expressed as a percentage of the ratio between the conditioned MEPs and the nonconditioned MEPs with stimulus intensity of MT1; that is, (conditioned MEP/nonconditioned MEP) × 100.[30]
Ten MEP recordings using each of these four stimulus paradigms (total of 40 recordings) were elicited in random sequence with 5-s intervals, while the subjects observed each of the following:
Actual observation of an action with active FDI: The subjects observed the experimenter's hand, holding a key in lateral pinch grip (grasping objects between the side of the index finger and the thumb) to perform locking/unlocking actions. This action requires contraction of the FDI to abduct the index finger.[31]
Virtual observation: The subjects observed a video showing the above action [Figure 1a].
Resting state: The subjects observed a still image of a hand and a lock displayed on the monitor [Figure 1b].
Figure 1.
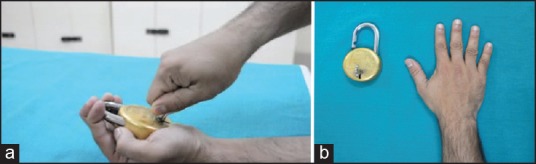
(a) Screen-shot and (b) Photograph of the video (depicting a goal-directed action using the right first dorsal interosseous muscle) and static images of a hand/lock and key, that were used in the experiment to assess putative mirror neuron activity during the virtual observation and rest states respectively
The sequence of displaying these experimental states to each subject was randomized. The percent change of the MEP or CI from resting to action observation states (average of virtual and actual observation) formed a measure of MNA. It was calculated using the following formula, where cortical reactivity refers to MEP (in mV) for single-pulse paradigms and CI (%) in paired-pulse paradigms:

Data acquisition and analysis were done using Signal-4 Software, (Cambridge Electronic Devices, Cambridge, UK).
Statistical analyses
Univariate statistics (independent samples t-test and Chi-square tests) were used to compare clinical and demographic parameters across the two groups (patients with and without FRS). MNA of the two groups was compared using independent samples t-test. All statistical tests were two-tailed, and significance was set at an error P – 0.05.
RESULTS
Demographic and clinical variables
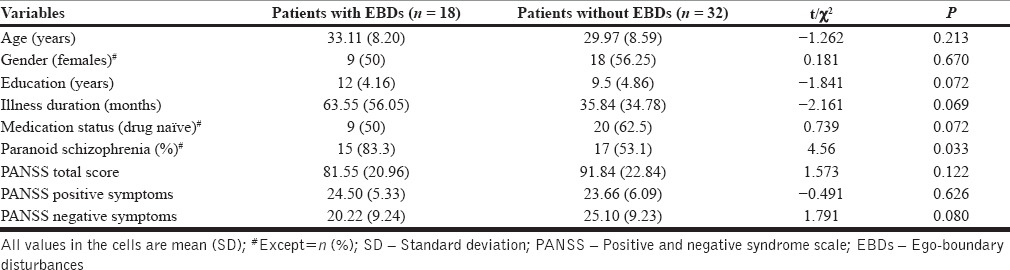
As shown in Table 1, the two groups did not significantly differ from each other in terms of age, gender and education, illness duration, medication status (drug-naïve v/s on medication) and symptom scores. Greater proportion of patients with symptoms of EBD had a diagnosis of paranoid schizophrenia. Twelve patients were receiving risperidone, 4 olanzapine, 3 risperidone + olanzapine, 1 olanzapine + amisulpride and 1 aripiprazole. Median duration of treatment with anti-psychotics was 60 days and mean chlorpromazine equivalent was 413.43 ± 226.95 mg/day. The rest were drug-naïve (never treated with any psychotropic medications).
Table 1.
Demographic and clinical variables
Symptoms of ego-boundary disturbances
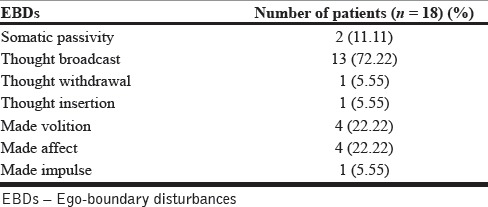
A total of 18 (36%) patients experienced symptoms of EBDs as defined. Thought broadcast was the most common symptom, being present in 72% of the patients who experienced symptoms of EBDs [Table 2].
Table 2.
Distribution of symptoms of EBDs
Mirror neuron activity in patients with and without symptoms of ego-boundary disturbances
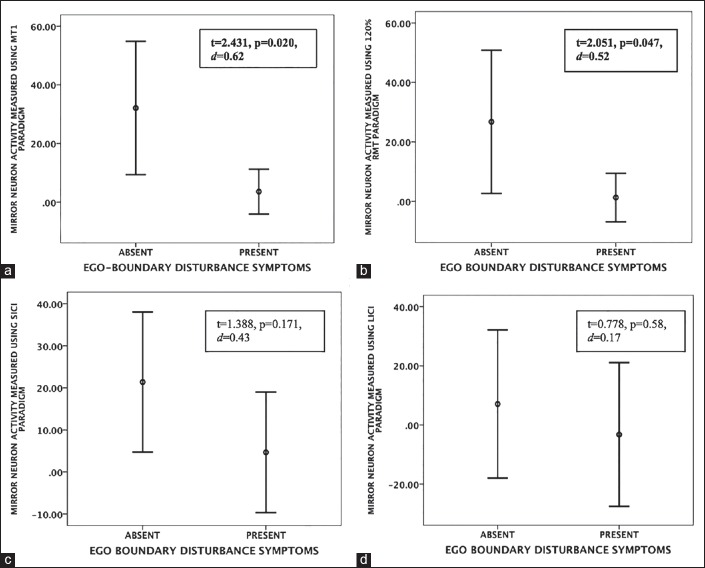
Independent samples t-test on the two groups (patients with and without ego-boundary disturbances) revealed significantly greater MNA in patients without ego-boundary disturbances than in patients with ego-boundary disturbances for MT1 and 120%RMT stimulus paradigms, indicating lesser mirror neuron activity in patients with symptoms of ego-boundary disturbances [Table 3, Figure 2]. Effect sizes (Cohen's d) ranged from 0.52 to 0.62. There was no significant difference in MNA measured using the two paired-pulse paradigms (SICI and LICI) across the 2 patient groups.
Table 3.
Putative MNA in both groups: The MEP difference between resting and action-observation states (average across virtual and actual action-observation) formed the measure of putative MNA
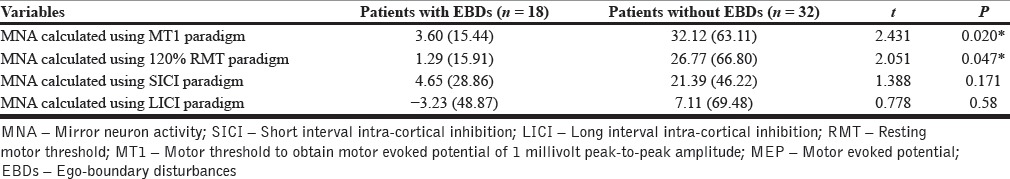
Figure 2.
(a) Error bars representing differences in means (95% confidence interval) of mirror neuron activity (y axis) assessed using motor threshold-1, (b) 120% resting motor threshold, (c) Short interval intra-cortical inhibition and (d) Long interval intra-cortical inhibition stimulus paradigms, across patients with and without symptoms of ego boundary disturbances (x axis). Note: d represents effect size (Cohen's d)
DISCUSSION
This study aimed to explore MNA of a subset of schizophrenia patients with symptoms of EBDs like thought alienation phenomena, made phenomena and somatic passivity. A common link across these symptoms is a breach in the barrier separating the self from one's environment. A dysfunctional mirror neuron system has been proposed to underlie deficits in self-awareness and agency attribution, which may lead to symptoms of EBD.[6,32] We used TMS to derive a putative measure of mirror mechanisms of the brain as has been used in earlier studies.[9,11,22] MNA was measured in schizophrenia patients with and without symptoms of EBD.
Thrity-six percent of the patients experienced symptoms of EBDs and thought broadcast was the most common symptom as has been reported in previous studies.[2,33] We found greater enhancement of MEP during action observation relative to rest states in schizophrenia patients without EBD than those with EBD. That is to say, schizophrenia patients with symptoms of EBD had significant deficits in MNA when compared to patients without symptoms of EBD. The effect sizes ranged from 0.52 to 0.62 indicating moderate to good magnitude of difference across the two groups. The two patient groups did not differ in terms of their age, education, and illness duration or symptom scores assessed using PANSS. Hence the observed differences in MNA may be attributed to presence or absence of EBD symptoms. Not surprisingly, more patients with symptoms of EBD had a diagnosis of paranoid sub-type of schizophrenia. Results were consistent when MNA was measured using single-pulse TMS paradigms (MT1 and 120% RMT). However, when MNA was measured using the paired pulse paradigms (SICI and LICI), there was no significant difference between the two groups, though the findings were in the expected direction (i.e., patients with EBD had poorer MNA than those with EBD). This indicates a possible type-2 error as the sub-group with EBD had a small sample size (n = 18).
While an aberrant hyperactive MNA may underlie catatonic[34] or affective[35] symptoms, there is emerging evidence for MNA deficits in patients with schizophrenia[8,9] and its association with deficits in social information processing[36] and negative symptoms.[37] To the best of our knowledge this is the first study to demonstrate specific associations of premotor MNA deficits with specific symptom dimensions (EBD) of schizophrenia. Our findings indirectly support existing theoretical formulations of the neurobiological substrates of Schneiderian “FRSs of schizophrenia. Aberrant hyperactivity,[38] deficient cortical thickness[39] and reduced volume[40] of the inferior parietal lobule have been demonstrated in schizophrenia patients with FRSs. Interestingly, the inferior parietal lobule is one of the brain regions most consistently shown to have mirror mechanisms in humans.[41] Our study showing deficient putative MNA in schizophrenia patients with EBD indirectly supports these findings.
According to Frith et al.[42] whenever an action is intended an “efference copy” of the intended action diminishes responses to sensations of limb movements because of which the action is anticipated, the response does not come as a surprise to the person and his self-monitoring is, therefore, intact. When this attenuation does not happen, it leads to parietal cortex hyperactivity; the person perceives the action as an event not under his/her control and goes further to confabulate the agency as external. Mirror neurons could provide a physiological substrate of the “efference copy,”[43,44] thus playing an integral role in self-monitoring. In schizophrenia patients with EBD, deficient MNA may result in the absence of this attenuation of the responses to sensations of limb movements, thus resulting in a sense of alien control.
It is crucial to note that TMS is an indirect measurement of mirror neuron processes in the brain. Intracranial depth electrodes[45] give the most direct evidence of MNA, but their use in humans, especially in those with psychiatric disorders, is extremely challenging. Further, MNA mediation using TMS presumably reflects mirror properties of the premotor cortex, which through corticocortical connections to the motor cortex[11] or cortico-spinal connections to the spinal cord,[46] result in greater MEPs from the hand muscle during action observation when compared to rest states. Measuring MNA from the motor cortex limits the generalizability of these experiments to possible premotor/inferior frontal gyrus mirror properties. Functional neuroimaging studies are better suited to produce an indirect measure of MNA in other parts of the core mirror system (inferior parietal lobule) and the extended mirror system (insular cortex, superior temporal sulcus, primary, and secondary motor and somatosensory cortices).[47]
Our results need to be interpreted in the light of them being preliminary findings from a larger study.[14] The results indicate possible mirror neuron dysfunction in schizophrenia patients with symptoms of EBD, and are not definitive. Replication of these findings in studies with larger sample sizes is necessary to derive conclusive deductions. Future studies should focus on assessing MNA in a trans-diagnostic group of patients with symptoms of EBDs using combined TMS-functional neuroimaging methods to achieve optimal spatial and temporal resolution. FRSs of schizophrenia have been associated with a heightened social threat perception during emotion processing tasks.[48] Assessing the relationship between mirror neuron mechanisms, social cognition and EBDs would certainly enhance our understanding of this complex and intriguing symptom cluster of psychosis.
CONCLUSION
Schizophrenia patients with EBD symptoms showed lower MEP enhancement during action observation relative to rest states, than patients without EBD. This reflects a possible deficit in mirror neuron activation in schizophrenia patients who experience symptoms characterized by a breach in their ego-boundary. Given the emerging evidence for the role of mirror neurons in psychiatric disorders in general,[49] these findings contribute to the already existing understanding of the neurophysiology of FRS in schizophrenia patients, thus providing novel treatment targets[50] to be explored in future research.
ACKNOWLEDGMENTS
This study was supported by a grant to Dr. Urvakhsh Meherwan Mehta from the Department of Biotechnology, Ministry of Science and Technology, Government of India, (Grant number BT/PR14311/Med/30/470/2010).
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Schneider K. Clinical Psychopathology. In: Hamilton MW, editor. 5th ed. New York: Grune & Stratton; 1959. [Google Scholar]
- 2.Mellor CS. First rank symptoms of schizophrenia. I. The frequnncy in schizophrenics on admission to hospital. II. Differences between individual first rank symptoms. Br J Psychiatry. 1970;117:15–23. [PubMed] [Google Scholar]
- 3.Heering HD, van Haren NE, Derks EM GROUP Investigators. A two-factor structure of first rank symptoms in patients with a psychotic disorder. Schizophr Res. 2013;147:269–74. doi: 10.1016/j.schres.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 4.Nordgaard J, Arnfred SM, Handest P, Parnas J. The diagnostic status of first-rank symptoms. Schizophr Bull. 2008;34:137–54. doi: 10.1093/schbul/sbm044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waters FA, Badcock JC. First-rank symptoms in schizophrenia: Reexamining mechanisms of self-recognition. Schizophr Bull. 2010;36:510–7. doi: 10.1093/schbul/sbn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbib MA, Mundhenk TN. Schizophrenia and the mirror system: An essay. Neuropsychologia. 2005;43:268–80. doi: 10.1016/j.neuropsychologia.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, et al. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp Brain Res. 1996;111:246–52. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- 8.Kato Y, Muramatsu T, Kato M, Shibukawa Y, Shintani M, Mimura M. Magnetoencephalography study of right parietal lobe dysfunction of the evoked mirror neuron system in antipsychotic-free schizophrenia. PLoS One. 2011;6:e28087. doi: 10.1371/journal.pone.0028087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enticott PG, Hoy KE, Herring SE, Johnston PJ, Daskalakis ZJ, Fitzgerald PB. Reduced motor facilitation during action observation in schizophrenia: A mirror neuron deficit? Schizophr Res. 2008;102:116–21. doi: 10.1016/j.schres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 10.McClintock SM, Freitas C, Oberman L, Lisanby SH, Pascual-Leone A. Transcranial magnetic stimulation: A neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry. 2011;70:19–27. doi: 10.1016/j.biopsych.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: A magnetic stimulation study. J Neurophysiol. 1995;73:2608–11. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- 12.Strafella AP, Paus T. Modulation of cortical excitability during action observation: A transcranial magnetic stimulation study. Neuroreport. 2000;11:2289–92. doi: 10.1097/00001756-200007140-00044. [DOI] [PubMed] [Google Scholar]
- 13.Gangitano M, Mottaghy FM, Pascual-Leone A. Phase-specific modulation of cortical motor output during movement observation. Neuroreport. 2001;12:1489–92. doi: 10.1097/00001756-200105250-00038. [DOI] [PubMed] [Google Scholar]
- 14.Mehta UM, Thirthalli J, Basavaraju R, Gangadhar BN, Pascual-Leone A. Reduced Mirror Neuron Activity in Schizophrenia and its Association With Theory of Mind Deficits: Evidence From a Transcranial Magnetic Stimulation Study. Schizophr Bull. 2013 doi: 10.1093/schbul/sbt155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 16.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 17.Ganguli M, Ratcliff G, Chandra V, Sharma S, Gilby J, Pandav R, et al. A Hindi version of the MMSE: The development of a cognitive screening instrument for a largely illiterate rural elderly population in India. International Journal of Geriatric Psychiatry. 1995;10:367–77. [Google Scholar]
- 18.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 19.Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald PB, Brown TL, Daskalakis ZJ, Kulkarni J. A transcranial magnetic stimulation study of inhibitory deficits in the motor cortex in patients with schizophrenia. Psychiatry Res. 2002;114:11–22. doi: 10.1016/s0925-4927(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 21.Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002;59:347–54. doi: 10.1001/archpsyc.59.4.347. [DOI] [PubMed] [Google Scholar]
- 22.Maeda F, Kleiner-Fisman G, Pascual-Leone A. Motor facilitation while observing hand actions: Specificity of the effect and role of observer's orientation. J Neurophysiol. 2002;87:1329–35. doi: 10.1152/jn.00773.2000. [DOI] [PubMed] [Google Scholar]
- 23.Wasserman E. Oxford Handbook of Transcranial Magnetic Stimulation. Oxford: Oxford University Press; 2008. Inter-and intra-individual variation in the responses to TMS. [Google Scholar]
- 24.Trevillion L, Howells J, Bostock H, Burke D. Properties of low-threshold motor axons in the human median nerve. J Physiol. 2010;588:2503–15. doi: 10.1113/jphysiol.2010.190884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–19. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: A transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–78. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- 27.Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–64. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- 28.Daskalakis ZJ, Farzan F, Barr MS, Maller JJ, Chen R, Fitzgerald PB. Long-interval cortical inhibition from the dorsolateral prefrontal cortex: a TMS-EEG study. Neuropsychopharmacology. 2008;33:2860–9. doi: 10.1038/npp.2008.22. [DOI] [PubMed] [Google Scholar]
- 29.McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp Brain Res. 2007;180:181–6. doi: 10.1007/s00221-006-0849-0. [DOI] [PubMed] [Google Scholar]
- 30.Patuzzo S, Fiaschi A, Manganotti P. Modulation of motor cortex excitability in the left hemisphere during action observation: A single- and paired-pulse transcranial magnetic stimulation study of self-and non-self-action observation. Neuropsychologia. 2003;41:1272–8. doi: 10.1016/s0028-3932(02)00293-2. [DOI] [PubMed] [Google Scholar]
- 31.Geere J, Chester R, Kale S, Jerosch-Herold C. Power grip, pinch grip, manual muscle testing or thenar atrophy - which should be assessed as a motor outcome after carpal tunnel decompression? A systematic review. BMC Musculoskelet Disord. 2007;8:114. doi: 10.1186/1471-2474-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arbib MA. Other faces in the mirror: A perspective on schizophrenia. World Psychiatry. 2007;6:75–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Peralta V, Cuesta MJ. Diagnostic significance of Schneider's first-rank symptoms in schizophrenia. Comparative study between schizophrenic and non-schizophrenic psychotic disorders. Br J Psychiatry. 1999;174:243–8. doi: 10.1192/bjp.174.3.243. [DOI] [PubMed] [Google Scholar]
- 34.Mehta UM, Basavaraju R, Thirthalli J. Mirror neuron disinhibition may be linked with catatonic echo-phenomena: A single case TMS study. Brain Stimul. 2013;6:705–7. doi: 10.1016/j.brs.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Mehta UM, Basavaraju R, Thirthalli J. Mirror Neuron Activity and Symptom Severity in Drug-naïve Mania - A Transcranial Magnetic Stimulation Study. Brain Stimul. 2014 doi: 10.1016/j.brs.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Mehta UM, Basavaraju R, Thirthalli J, Gangadhar BN. Mirror neuron dysfunction-a neuro-marker for social cognition deficits in drug naïve schizophrenia. Schizophr Res. 2012;141:281–3. doi: 10.1016/j.schres.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Lee JS, Chun JW, Yoon SY, Park HJ, Kim JJ. Involvement of the mirror neuron system in blunted affect in schizophrenia. Schizophr Res. 2014;152:268–74. doi: 10.1016/j.schres.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 38.Franck N, O’Leary DS, Flaum M, Hichwa RD, Andreasen NC. Cerebral blood flow changes associated with Schneiderian first-rank symptoms in schizophrenia. J Neuropsychiatry Clin Neurosci. 2002;14:277–82. doi: 10.1176/jnp.14.3.277. [DOI] [PubMed] [Google Scholar]
- 39.Venkatasubramanian G, Jayakumar PN, Keshavan MS, Gangadhar BN. Schneiderian first rank symptoms and inferior parietal lobule cortical thickness in antipsychotic-naïve schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:40–6. doi: 10.1016/j.pnpbp.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 40.Danivas V, Kalmady S, Arasappa R, Behere RV, Rao NP, Venkatasubramanian G, et al. Inferior parietal lobule volume and schneiderian first-rank symptoms in antipsychotic-naïve schizophrenia: A 3-tesla MRI study. Indian J Psychol Med. 2009;31:82–7. doi: 10.4103/0253-7176.63578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev. 2012;36:341–9. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Frith CD, Blakemore S, Wolpert DM. Explaining the symptoms of schizophrenia: Abnormalities in the awareness of action. Brain Res Brain Res Rev. 2000;31:357–63. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 43.Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. Eur J Neurosci. 2001;13:400–4. [PubMed] [Google Scholar]
- 44.Pacherie E, Dokic J. From mirror neurons to joint actions. Cogn Syst Res. 2006;7:101–12. [Google Scholar]
- 45.Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Curr Biol. 2010;20:750–6. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–89. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pineda JA. Sensorimotor cortex as a critical component of an ‘extended’ mirror neuron system: Does it solve the development, correspondence, and control problems in mirroring? Behav Brain Funct. 2008;4:47. doi: 10.1186/1744-9081-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Behere RV, Venkatasubramanian G, Arasappa R, Reddy NN, Gangadhar BN. First rank symptoms and facial emotion recognition deficits in antipsychotic naïve schizophrenia: Implications for social threat perception model. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1653–8. doi: 10.1016/j.pnpbp.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 49.Buccino G, Amore M. Mirror neurons and the understanding of behavioural symptoms in psychiatric disorders. Curr Opin Psychiatry. 2008;21:281–5. doi: 10.1097/YCO.0b013e3282fbcd32. [DOI] [PubMed] [Google Scholar]
- 50.Meherwan Mehta U, Agarwal SM, Kalmady SV, Shivakumar V, Kumar CN, Venkatasubramanian G, et al. Enhancing putative mirror neuron activity with magnetic stimulation: A single-case functional neuroimaging study. Biol Psychiatry. 2013;74:e1–2. doi: 10.1016/j.biopsych.2013.02.009. [DOI] [PubMed] [Google Scholar]