Abstract
Background:
Metabolic syndrome is the constellation of several cardiometabolic risk factors, and is associated with a heightened risk of coronary heart disease (CHD). The pro-oxidant–antioxidant balance (PAB) is a measure of factors that promote and control oxidative stress. PAB may also be associated with the risk factors of CHD.
Objectives:
This study aimed to explore the impact of supplementation with barberry, a fruit rich in antioxidants, on PAB in patients with metabolic syndrome.
Patients and Methods:
A total of 106 patients diagnosed with metabolic syndrome were randomized in two groups: case and control. The case group received three capsules of barberry and the control group received three capsules of placebo for 6 weeks. Serum PAB was measured in all patients before and after the intervention.
Results:
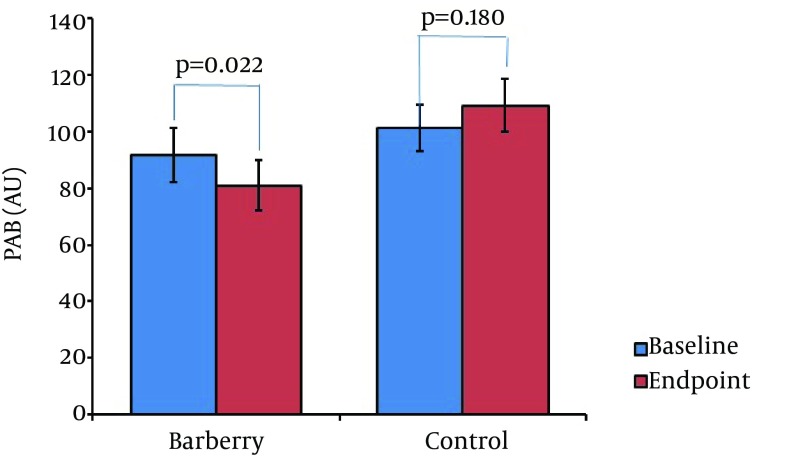
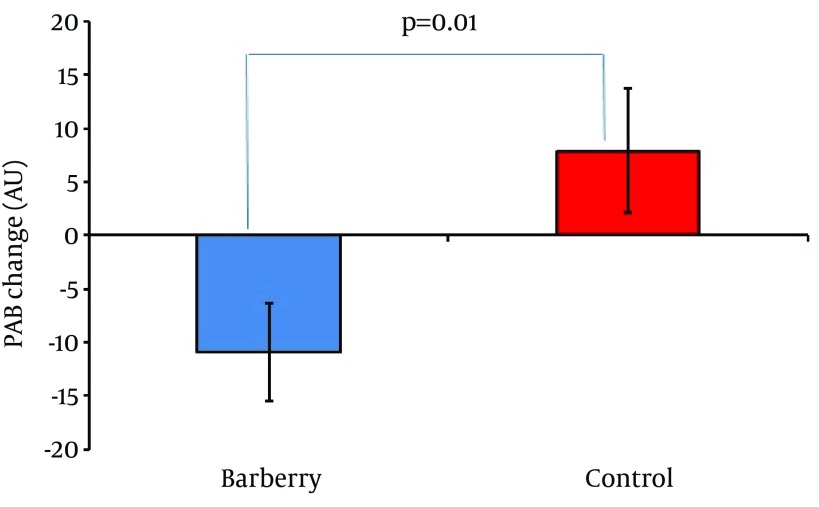
There was no significant difference between the groups regarding their baseline PAB values (P = 0.32). A significant decrease in PAB was observed in the barberry group (P = 0.022), whilst there was no significant change in the control group (P = 0.18). The magnitude of change in PAB during the study was significantly greater in the case group compared to the control group (P = 0.01).
Conclusions:
Barberry supplementation reduces oxidative burden in patients with metabolic syndrome.
Keywords: Metabolic Cardiovascular Syndrome, Berberidaceae, Randomized Controlled Trial
1. Background
Metabolic syndrome (MS) is a clustering of several cardiometabolic risk factors, including central obesity, hypertension, hyperglycemia, and dyslipidaemia. MS is an important health concern due to its relation with increased risk of coronary heart disease (CHD). MS is defined in various by different health organizations (1). Its pathogenesis has two potential causative factors: central obesity and insulin resistance. However, other factors such as genetic predisposition, ageing, and physical inactivity are also implicated in its development (1, 2).
Developing societies experience an increasing trend in the prevalence of MS with rising affluence and ageing of the population. Because of the high CHD death rate, effective methods are warranted to treat MS-associated disorders (3, 4). This means that identification and treatment of the MS is important for prevention and even treatment of CHD.
Oxidative stress is the consequence of increased generation of pro-oxidant species accompanied by a depletion of biological antioxidants (5). Oxidative stress is important because it plays a crucial role in the pathophysiology of several disorders, including atherosclerosis and subsequent CHD as well as MS. Moreover, there is a strong correlation between PAB and atherosclerosis (6). Previous studies have shown that PAB values may serve as an indicator of the CHD risk and other oxidative stress-associated disorders (7).
In traditional medicine, Berberis vulgaris (Berberidaceae family) is recommended in the treatment of heart diseases, including hypertension and arrhythmia (8). Barberry is a bush with yellow timber and obovate leaves. The plant, which grows in Asia and Europe, has yellow flowers succeeded by oblong red colored fruits. Barberry extract contains ingredients such as berberine, berbamine, palmatine, oxyacanthine, malic acid, and berberubin (9). Many studies have previously shown that Berberis vulgaris possesses various medicinal properties such as antipyretic, antihyperglycaemic, anti-bacterial, anticancer, antihistaminic, antimicrobial, antioxidant, anticholinergic and hypolipidaemic activities (9, 10), that support the traditional use of this plant in Ayurveda and Chinese medicine. It is reported that berberine, an isoquinoline alkaloid (present in barberry) can be used as therapeutics against a number of diseases like hyperlipidemia, diabetes, metabolic syndrome, obesity, and coronary artery disease. These compounds have antioxidant effects owing to their interaction with multiple molecular targets resulting in a synergistic and comprehensive pharmacological response (9, 11, 12).
2. Objectives
The present study aimed to investigate the effect of supplementation with barberry on PAB as a surrogate marker of systemic oxidative stress in patients with metabolic syndrome.
3. Patients and Methods
3.1. Subjects
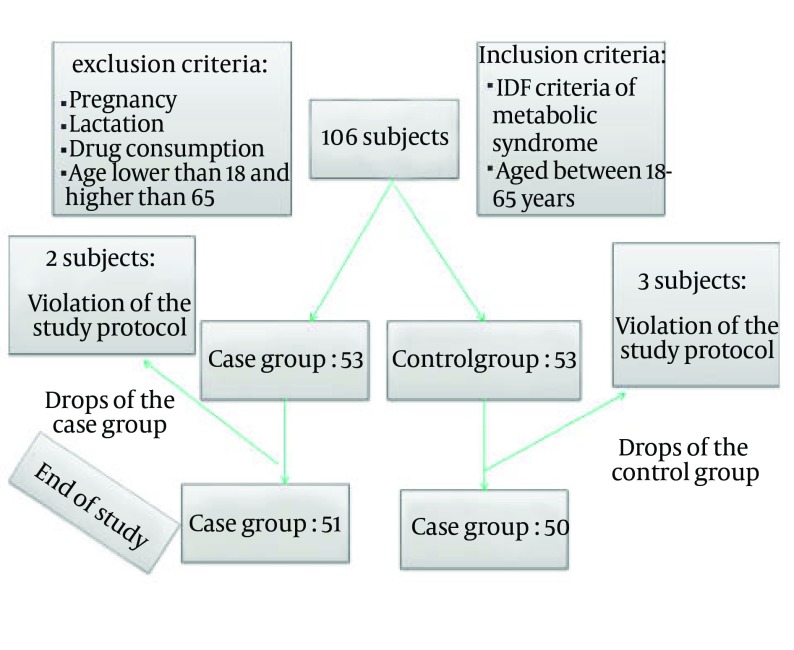
One hundred and six patients with metabolic syndrome (defined according to the International Diabetic Federation Criteria), aged 18-65 years old, and without diabetes were chosen sequentially from clinic and randomly recruited from those referring to the Nutrition Clinic of the Ghaem Hospital, Mashhad, Iran. The objectives and protocol of the study were explained to each participant prior to the study. Participants were provided with information about the study both by verbal explanation and written information sheets. Inclusion criteria were an age range of 18-65 years and having a metabolic syndrome according to IDF (International Diabetic Federation) criteria. Exclusion criteria included known systemic diseases; pregnancy; lactation; and consumption of the antidyslipidemic, antihypertensive and antidiabetic drugs. Written informed consent was obtained from all participants and the study protocol was approved by the Ethics Committee at the Mashhad University of Medical Sciences.
3.2. Study Design
The study was designed as a randomized double-blind and placebo-controlled trial. The research was conducted in the Nutrition Clinic of the Ghaem Hospital, Mashhad, Iran. Figure 1 shows the flowchart of the study design. Patients were randomly assigned to the treatment group receiving capsules of barberry (600 mg/day) for a period of 6 weeks, and control group receiving a placebo capsule for the same period. All participants were given dietary advice on the basis of the AHA (American Heart Association) guidelines during the study. Compliance was monitored during a three-weekly visit, assessing by counting capsules. Those subjects who refused to take their capsules regularly or were intolerant to the medication were excluded from the study. PAB were determined in all patients at baseline and at the end of the sixth week.
Figure 1. Flowchart of the Trial.
3.3. Blood Sampling
Twelve-hour fasting blood samples were collected from each subject at baseline as well as at the end of study (week 6). Hemolyzed samples were excluded from analysis. Separation of serum from blood samples was conducted by centrifugation at 10000g for 15 minutes.
3.4. Anthropometric Measurements
Anthropometric indices, including height, waist and hip circumference (cm) and weight (kg) were determined for each subject. Circumferences were measured with a plastic tape measure at the following levels: smallest waist circumference, umbilicus level, widest hip circumference, hips at the level of the anterior superior iliac spine of the iliac crest, and the highest thigh circumference.
3.5. Barberry Capsule Preparation
Barberry juice was provided in Ghaem, Khorasan Province, Iran. It was formulated as a capsule containing 200 mg of dried barberry. Barberry and placebo capsules were matched for size, shape, and color and manufactured by the same company (Khoosheh Sorkhe Shargh Agro Industrial Co., Tehran, Iran).
3.6. Pro-oxidant–antioxidant Assay
Measurement of PAB was performed using a previously described method (7). In brief, the standard solutions were prepared by mixing 250 μM hydrogen peroxide with uric acid (3 mM in 10 mM NaOH) in varying proportions (0-100%). In order to prepare TMB cation, 400 μL of TMB/DMSO solution (60 mg TMB in 10 mL DMSO) was added to 20 mL of acetate buffer (0.05M buffer, pH = 4.5), and then this solution was mixed with 70 μL of fresh chloramine T (100 mM), followed by incubation for 2 hours at room temperature in a dark place. Afterwards, 25 U of peroxidase enzyme solution was added to 20 mL of the TMB cation solution, dispensed in 1 mL and stored at -20°C. The working solution was prepared by mixing TMB cation solution (1 mL) with the TMB solution (10 mL), incubated for 2 minutes at room temperature in a dark place and immediately used. Ten microliter of each sample, standard or blank (distilled water), was mixed with the working solution (200 µL), in each well of a 96 well plate, which was then incubated in a dark place at 37°C for 12 minutes. At the end of the incubation time, the colorimetric reaction was stopped by adding 100 μL of 2 M HCl to each well. Then, the optical density of plates were read at 450 nm using an ELISA reader with a reference wavelength of 620 or 570 nm. A standard curve was generated from the values relative to the standard samples. The values of the PAB were expressed in an arbitrary unit, which represents the percentage of hydrogen peroxide in the standard solution.
3.7. Statistical Analysis
All statistical analyses were performed using SPSS statistical software package. All data were presented as mean (SD) in each group. Data were assessed for normality by using the Kolmogorov-Smirnov test. Paired sample t-test and independent sample t-test were used for comparison of normally distributed data, whilst non-normally distributed data were compared using. A P value of < 0.05 was considered to be statistically significant.
4. Results
Table 1 presents the comparison of the baseline characteristics between 2 groups. They were comparable regarding their baseline characteristics, including age, gender distribution, BMI, smoking, and serum PAB levels (P > 0.05). Figure 2 shows the comparison of the PAB between 2 groups. PAB values were comparable between the 2 groups at baseline (P = 0.324). A significant reduction of PAB from baseline by the end of trial was observed in the barberry group (P = 0.022), whilst no significant change occurred in the control group (P = 0.180). The magnitude of changes in PAB levels was also significantly different between the 2 groups (P = 0.01; Figure 3).
Table 1. Comparison of the Baseline Characteristics Between Case and Control Groups (n = 53) a, b, c.
| Variable | Barberry Group | Control Group | P Value |
|---|---|---|---|
| Gender | 0.44 | ||
| Female | 41 | 38 | |
| Male | 12 | 15 | |
| Smokers | 6 (11.3) | 5 (9.4) | 0.96 |
| Age, y | 38.96 ± 9.04 | 40.89 ± 9.61 | 0.30 |
| BMI, kg/m 2 | 31.54 ± 3.92 | 32.37 ± 5.01 | 0.35 |
a Abbreviation: BMI, body mass index.
b Data are presented as or No. (%) or Mean ± SD.
c Mann-Whitney and independent sample t-test were used to compare non-parametric and parametric variables between two groups, respectively.
Figure 2. Comparison of Pretreatment vs. Posttreatment PAB Values in the Study Groups.
Figure 3. Comparison of PAB Changes in the Barberry and Control Group During the Course of Study.
4.1. Precision and Storage
Intra-assay and inter-assay coefficients of variation (CV%) were determined for the PAB assay. The mean CV% of the intra-assay for 28 samples (analyzed in triplicate) was found to be 2.1%. The CV% of the inter-assay for 20 samples was between 4.1% and 8.5% with a mean of 6.1% (analyzed over 3 days).
5. Discussion
Results of the present study indicated that using barberry supplementation for six weeks in patients with metabolic syndrome decreased significantly PAB levels. To our knowledge, the present study is the first one to investigate the effect of barberry on PAB in patients with metabolic syndrome. Compelling evidence supports the antioxidant functions of barberry. Previous studies have shown that berberine prevents the ROS formation in several cell types like macrophages. Berberine has also the ability to chelate Fe2+ (13, 14). Moreover, berberine effectively scavenges different classes of free radicals, in particular O2- and OH• (15). It also regulates the activity of colonic antioxidant enzymes, including glutathione S-transferase, catalase, SOD, and glutathione peroxides in rats exposed to azoxymethane (16). Surveswaran et al. has reported that roots of Berberis aristata contain 3.59 mmol/100 g antioxidants and fruits of Berberis vulgaris contain a significantly higher amount of antioxidants compared with other fruits (17).
Berberine hydrochloride has large reductive ability and can scavenge different radicals, especially ABTS radicals, hydroxyl radicals, DPPH (2, 2-diphenyl 1-picrylhydrazyl) radicals, and superoxide anion (18). The antioxidant content of Berberis vulgaris (Crni Lug origin) fruit has been determined by Zovko Koncic et al. using DPPH assay. The results indicated that the antioxidant contents of the roots, branches, and leaves were 1292.86 μg/mL, 691.57 μg/mL, and 65.09 μg/mL, respectively (19). PAB values have been previously shown to be elevated in patients with angiographically documented CAD and ACS (7). Alamdari et al. indicated that PAB may be a potential cardiovascular risk predictor (7). A number of trials have also demonstrated that PAB values may serve as an indicator of oxidative stress (20-23).
Ji-Yin Zhou et al. (2011) investigated protective effect of berberine on antioxidant enzymes in diabetic rat liver. Berberine reduced malondialdehyde concentrations and enhanced superoxide dismutase, catalase, glutathione peroxidase, and glutathione activities in liver tissue and serum of diabetic rats (24). Zhou has used purified berberine, which its content is higher compared to the berberine in barberry of our study. This study was conducted on animals but ours on MS patients. In a clinical trial, Cheng et al. (25) have shown that berberine improves endothelial function in humans via inhibiting endothelial microparticles-mediated oxidative stress in vascular endothelium. In their study, 12 healthy subjects consumed a 1-month berberine therapy and 11 healthy subjects were selected as control. Serum malondialdehyde (MDA) and circulating endothelial microparticles were measured before and after therapy. Circulating CD31+/CD42- MPs and the levels of serum MDA are significantly decreased in the berberine group compared to the control group. Berberine also caused a remarkable decline in MDA levels suggesting the efficacy of this phytochemical in reducing lipid peroxidation (25).
In one study, berberine was shown to ameliorate renal injury in streptozotocin-induced diabetic rats by attenuating oxidative stress and down-regulating the activity of aldose reductase. In the aforementioned study, berberine was orally administered at a dose of 200 mg/kg/day and was shown to significantly increase serum SOD activity and decrease the content of MDA compared to diabetic model group (P < 0.05) (26). This study also used purified berberine. The present trial had a number of limitations. For example, the trial recruited a relatively small number of patients and its results should be considered as pilot. In addition, although PAB is an overall oxidative stress biomarker, it would be useful to measure other measures of oxidative stress to confirm the pattern of PAB changes found in this study.
Overall, although PAB assay still needs to be further explored in terms of sensitivity and specificity for the assessment of oxidative burden in diseases, the current body of evidence clearly indicates that PAB increases in oxidative stress-related disorders and antioxidant can effectively reduce this measure of oxidation (21-30). PAB values are also known to be significantly and linearly associated with the concentration of pro-oxidant and antioxidant species, and correlate with standard carbonyl assay, advanced glycation end products assay and advanced oxidative protein products assay as standard measures for oxidative stress.
The findings of the present study indicated that supplementation with barberry (600 mg/day for 6 weeks) is associated with the suppression of systemic oxidative stress (as assessed by PAB). Current available data suggests that modulation of systemic oxidative stress is a plausible mechanism that can explain, at least in part, the well-known cardioprotective effects of barberry. Nevertheless, future large-scale trials with a longer duration of follow-up are warranted to confirm the present findings, and also compare the antioxidant effects of barberry with those of standard compounds e.g. α-tocopherol, ascorbic acid, and N-acetyl cysteine. Moreover, the present findings need to be validated by measuring additional standard pro-oxidant/antioxidant biomarkers including F2-isoprostanes, MDA and oxidized low-density lipoprotein.
Acknowledgments
The authors gratefully acknowledge the Research Council at Mashhad University of Medical Sciences (Mashhad, Iran) for financial support of this work.
Footnotes
Authors’ Contributions:Majid Ghayour Mobarhan and Tayyebeh Kermani developed the original idea; Majid Ghayour Mobarhan and Marzieh Zhilaee conducted the clinical evaluations; Akram Mohammadi and Shima Tavallaie did the experimental work; Amirhossein Sahebkar and Akram Mohammadi interpreted the data and performed statistical analysis; Akram Mohammadi and Amirhossein Sahebkar prepared and submitted the manuscript.
Funding/Support:This work was financially supported by the Research Council at Mashhad University of Medical Sciences, Mashhad, Iran.
References
- 1.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629–36. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 4.Gale EA. Should we dump the metabolic syndrome? Yes. BMJ. 2008;336(7645):640. doi: 10.1136/bmj.39477.500197.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–82. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 6.Ashok BT, Ali R. The aging paradox: free radical theory of aging. Exp Gerontol. 1999;34(3):293–303. doi: 10.1016/s0531-5565(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 7.Alamdari DH, Ghayour-Mobarhan M, Tavallaie S, Parizadeh MR, Moohebati M, Ghafoori F, et al. Prooxidant-antioxidant balance as a new risk factor in patients with angiographically defined coronary artery disease. Clin Biochem. 2008;41(6):375–80. doi: 10.1016/j.clinbiochem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Gundogdu M. Determination of Antioxidant Capacities and Biochemical Compounds of Berberis vulgaris L. Fruits. Adv Environ Biol. 2013;7(2):344–8. [Google Scholar]
- 9.Hajzadeh M, Rajaei Z, Shafiee S, Alavinejhad A, Samarghandian S, Ahmadi M. Effect of barberry fruit (Berberis vulgaris) on serum glucose and lipids in streptozotocin-diabetic rats. Pharmacol online. 2011;1:809–17. [Google Scholar]
- 10.Potdar D, Hirwani RR, Dhulap S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia. 2012;83(5):817–30. doi: 10.1016/j.fitote.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Hayden MR, Tyagi SC. Homocysteine and reactive oxygen species in metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: the pleiotropic effects of folate supplementation. Nutr J. 2004;3:4. doi: 10.1186/1475-2891-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao J, Kong W, Jiang J. Learning from berberine: Treating chronic diseases through multiple targets. Sci China Life Sci. 2013 doi: 10.1007/s11427-013-4568-z. [DOI] [PubMed] [Google Scholar]
- 13.Jang MH, Kim HY, Kang KS, Yokozawa T, Park JH. Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Arch Pharm Res. 2009;32(3):341–5. doi: 10.1007/s12272-009-1305-z. [DOI] [PubMed] [Google Scholar]
- 14.Shirwaikar A, Shirwaikar A, Rajendran K, Punitha IS. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol Pharm Bull. 2006;29(9):1906–10. doi: 10.1248/bpb.29.1906. [DOI] [PubMed] [Google Scholar]
- 15.Choi DS, Kim SJ, Jung MY. Inhibitory activity of berberine on DNA strand cleavage induced by hydrogen peroxide and cytochrome c. Biosci Biotechnol Biochem. 2001;65(2):452–5. doi: 10.1271/bbb.65.452. [DOI] [PubMed] [Google Scholar]
- 16.Yokozawa T, Ishida A, Kashiwada Y, Cho EJ, Kim HY, Ikeshiro Y. Coptidis Rhizoma: protective effects against peroxynitrite-induced oxidative damage and elucidation of its active components. J Pharm Pharmacol. 2004;56(4):547–56. doi: 10.1211/0022357023024. [DOI] [PubMed] [Google Scholar]
- 17.Surveswaran S, Cai Y, Corke H, Sun M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007;102(3):938–53. doi: 10.1016/j.foodchem.2006.06.033. [DOI] [Google Scholar]
- 18.Luo A, Fan Y. Antioxidant activities of berberine hydrochloride. J Med Plants Res. 2011;5(16):3702–7. [Google Scholar]
- 19.Zovko Koncic M, Kremer D, Karlovic K, Kosalec I. Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food Chem Toxicol. 2010;48(8-9):2176–80. doi: 10.1016/j.fct.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Ghayour-Mobarhan M, Alamdari DH, Moohebati M, Sahebkar A, Nematy M, Safarian M, et al. Determination of prooxidant--antioxidant balance after acute coronary syndrome using a rapid assay: a pilot study. Angiology. 2009;60(6):657–62. doi: 10.1177/0003319709333868. [DOI] [PubMed] [Google Scholar]
- 21.Yazdanpanah MJ, Ghayour-Mobarhan M, Taji A, Javidi Z, Pezeshkpoor F, Tavallaie S, et al. Serum zinc and copper status in Iranian patients with pemphigus vulgaris. Int J Dermatol. 2011;50(11):1343–6. doi: 10.1111/j.1365-4632.2011.04968.x. [DOI] [PubMed] [Google Scholar]
- 22.Parizadeh MR, Azarpazhooh MR, Mobarra N, Nematy M, Alamdari DH, Tavalaie S, et al. Prooxidant-antioxidant balance in stroke patients and 6-month prognosis. Clin Lab. 2011;57(3-4):183–91. [PubMed] [Google Scholar]
- 23.Parizadeh SM, Azarpazhooh MR, Moohebati M, Nematy M, Ghayour-Mobarhan M, Tavallaie S, et al. Simvastatin therapy reduces prooxidant-antioxidant balance: results of a placebo-controlled cross-over trial. Lipids. 2011;46(4):333–40. doi: 10.1007/s11745-010-3517-x. [DOI] [PubMed] [Google Scholar]
- 24.Zhou JY, Zhou SW. Protective effect of berberine on antioxidant enzymes and positive transcription elongation factor b expression in diabetic rat liver. Fitoterapia. 2011;82(2):184–9. doi: 10.1016/j.fitote.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Cheng F, Wang Y, Li J, Su C, Wu F, Xia WH, et al. Berberine improves endothelial function by reducing endothelial microparticles-mediated oxidative stress in humans. Int J Cardiol. 2013;167(3):936–42. doi: 10.1016/j.ijcard.2012.03.090. [DOI] [PubMed] [Google Scholar]
- 26.Alamdari DH, Paletas K, Pegiou T, Sarigianni M, Befani C, Koliakos G. A novel assay for the evaluation of the prooxidant-antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patients. Clin Biochem. 2007;40(3-4):248–54. doi: 10.1016/j.clinbiochem.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Liu WH, Hei ZQ, Nie H, Tang FT, Huang HQ, Li XJ, et al. Berberine ameliorates renal injury in streptozotocin-induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin Med J (Engl). 2008;121(8):706–12. [PubMed] [Google Scholar]
- 28.Sahebkar A, Mohammadi A, Atabati A, Rahiman S, Tavallaie S, Iranshahi M, et al. Curcuminoids modulate pro-oxidant-antioxidant balance but not the immune response to heat shock protein 27 and oxidized LDL in obese individuals. Phytother Res. 2013;27(12):1883–8. doi: 10.1002/ptr.4952. [DOI] [PubMed] [Google Scholar]
- 29.Sahebkar A, Pourghadamyari H, Moohebati M, Parizadeh SM, Falsoleiman H, Dehghani M, et al. A cross-sectional study of the association between heat shock protein 27 antibody titers, pro-oxidant-antioxidant balance and metabolic syndrome in patients with angiographically-defined coronary artery disease. Clin Biochem. 2011;44(17-18):1390–5. doi: 10.1016/j.clinbiochem.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Tara F, Rayman MP, Boskabadi H, Ghayour-Mobarhan M, Sahebkar A, Alamdari DH, et al. Prooxidant-antioxidant balance in pregnancy: a randomized double-blind placebo-controlled trial of selenium supplementation. J Perinat Med. 2010;38(5):473–8. doi: 10.1515/JPM.2010.068. [DOI] [PubMed] [Google Scholar]