Abstract
Structures in the medial temporal lobe, including the hippocampus and perirhinal cortex, are known to be essential for the formation of long-term memory. Recent animal and human studies have investigated whether perirhinal cortex might also be important for visual perception. In our study, using a simultaneous oddity discrimination task, rats with perirhinal lesions were impaired and did not exhibit the normal preference for exploring the odd object. Notably, rats with hippocampal lesions exhibited the same impairment. Thus, the deficit is unlikely to illuminate functions attributed specifically to perirhinal cortex. Both lesion groups were able to acquire visual discriminations involving the same objects used in the oddity task. Patients with hippocampal damage or larger medial temporal lobe lesions were intact in a similar oddity task that allowed participants to explore objects quickly using eye movements. We suggest that humans were able to rely on an intact working memory capacity to perform this task, whereas rats (who moved slowly among the objects) needed to rely on long-term memory.
The importance of medial temporal lobe (MTL) structures (i.e., the perirhinal, entorhinal, and parahippocampal cortices and the hippocampus) for memory has been established in humans, monkeys, and rodents (Squire and Zola-Morgan 1991; Eichenbaum and Cohen 2001). The pattern of impaired and spared functions after damage to the MTL has been described since early studies of patient H.M., emphasizing the profound deficits in declarative memory together with intact perception, intellectual functions, and nondeclarative memory (Milner et al. 1968; Kensinger et al. 2001; Squire and Wixted 2011).
Some recent human and animal studies have challenged these descriptions by suggesting that perirhinal cortex is important for visual perception in addition to memory (Lee et al. 2005; Murray et al. 2007; Graham et al. 2010), particularly for making difficult visual discriminations between stimuli having high feature-overlap or ambiguity (Bussey et al. 2002; Barense et al. 2005; Bussey and Saksida 2005). Yet this interpretation has not been consistently supported (Shrager et al. 2006; Knutson et al. 2012; Kim et al. 2013), and several issues have been raised (Hampton 2005; Suzuki 2009, 2010), including the suggestion that the tasks have not effectively eliminated a role for memory in task performance.
To minimize the potential role of memory, the simultaneous oddity discrimination (SOD) task was developed for the rat (Bartko et al. 2007a). In this task, three objects constructed from Lego blocks are presented simultaneously (two identical and one altered object). Given the animal's natural tendency to prefer novelty, the objective was to create a perceptual task in which the rat would divide its exploration between the two identical objects and exhibit an overall preference for the altered object. In the critical condition, involving stimuli with a high degree of feature-overlap, control rats exhibited a preference to explore the odd object, but rats with perirhinal lesions did not (Bartko et al. 2007a). Because all stimuli were presented simultaneously, the task was expected to depend only on visual perception and not require memory. However, it is worth considering whether, given the physical separation of the stimuli, rats might need to hold the visual representation of an object in memory as they moved from exploring one object to the next (as suggested by Suzuki 2009).
We reasoned that if the task depends at all on memory, performance might be impaired in rats after either perirhinal or hippocampal lesions. We tested animals with each of these lesions on the SOD task. In addition, we tested three patients with damage limited to the hippocampus and one patient with extensive damage to the MTL that included perirhinal cortex. For the patients, we modified the SOD task so that exploration could be assessed by eye movements during visual search. If the task depends to some extent on maintaining objects in memory during exploration, then patients might succeed by relying on their well-developed working memory capacity, which is intact after MTL lesions (Milner 1972; Jeneson and Squire 2011), to hold information in mind as they quickly shift their gaze from object to object.
Results
Experiment 1: simultaneous oddity discrimination (rats)
Neurohistological findings
Figure 1 shows reconstructions of coronal sections through the hippocampus (A) and perirhinal cortex (B), showing the smallest (black) and largest (stippled) lesion. Numbers represent the distance (mm) posterior to bregma.
Figure 1.
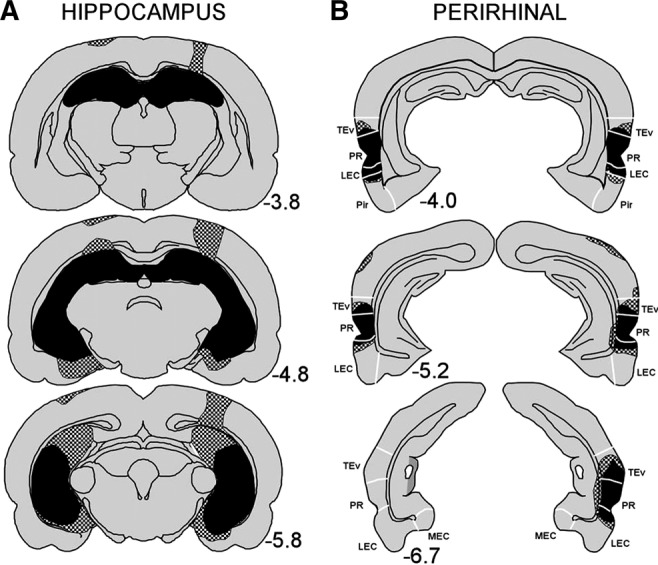
Reconstructions of coronal sections through the (A) hippocampus and (B) perirhinal cortex showing the smallest (black) and largest (stippled) lesion for the hippocampal and perirhinal lesion groups, respectively, in Experiment 1. Numbers represent the distance (millimeters) posterior to bregma. (TEv) ventral TE, (PR) perirhinal cortex, (LEC) lateral entorhinal cortex, (MEC) medial entorhinal cortex, (Pir) piriform area. White lines indicate approximate borders between these structures.
Hippocampal lesion
All rats sustained bilateral damage to all cell fields of the hippocampus. The damage included 88%–96% of the hippocampus (mean = 94% ± 0.6%). Sparing occurred most frequently in the most medial aspect of the dorsal dentate gyrus and CA1 cell field and in the ventral-most region of the hippocampus. In all rats, there was minor damage to the cortex and to the fimbria overlying the dorsal hippocampus, which was associated with the placement of the syringe during surgery and possibly with spread of the neurotoxin up the needle track. One animal had substantial unilateral damage to the cortical area overlying the dorsal hippocampus, but this animal's performance was consistent with the group. Five rats also had minor damage to the posterior aspect of the lateral entorhinal cortex and posterior subiculum. There was no evidence of damage to the amygdala or thalamus in any animal.
Perirhinal lesion
All rats sustained extensive bilateral damage to the perirhinal cortex (average damage 96% ± 1.9%; range 93%−100%). When perirhinal tissue was spared, it was located at the most anterior level. All rats sustained limited bilateral damage (e.g., <10% of the structure's total volume) to ventral temporal association areas, lateral entorhinal cortex, postrhinal cortex, and ventral TE. Four rats had unilateral damage to the ventral subiculum and four rats had unilateral damage to the ventral aspect of CA1 immediately adjacent to the rhinal sulcus.
Behavioral findings
Preference for the odd object
Rats with bilateral hippocampal lesions (H, n = 16), bilateral perirhinal lesion (PR, n = 16), and sham surgeries (Sham, n = 24) were tested on the simultaneous oddity discrimination (SOD) task (Fig. 2A). Objects consisted of six sets of three Lego objects constructed from LEGO blocks. Each set had two identical objects and one odd object, and sets were assigned to “low-ambiguity” or “high-ambiguity” conditions depending on the degree of feature overlap between the odd object and identical objects. Preference for the odd object was measured as the percent time that a rat explored the odd object out of the total time spent exploring all three objects (with chance taken to be 33%). Preference scores for the odd object were determined after each 1-min interval across the 5-min trial. The Sham group's preference for the odd object was 37.4% ± 1.3% after 1 min and 37.8% ± 0.8% after 5 min. There were no within group differences across the five, 1-min intervals for any of the groups (all Ps > 0.10). Accordingly, we report the preference scores obtained after the full 5-min trial because the variability was the lowest at this point for all three groups.
Figure 2.
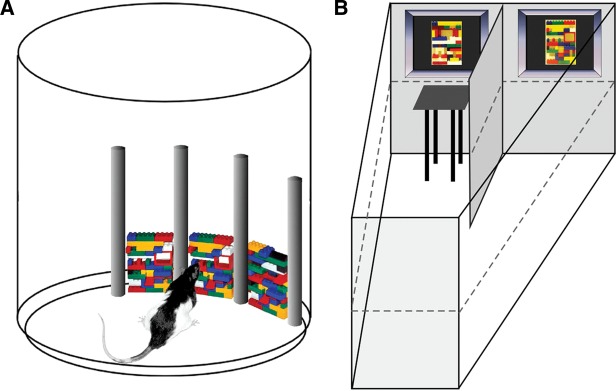
(A) Illustration of the apparatus used for the simultaneous oddity discrimination task. Objects constructed from Legos (two identical and one odd object) were placed in the apparatus. Rats could then explore for a total of 5 min. Here, the odd object is in the right-most location, and the most difficult of the two task conditions is illustrated. The bottom half of the odd object is identical to the bottom half of the other two objects. (B) Illustration of the apparatus used for the visual discrimination task. The tank was filled with opaque water (dashed line indicates water level), and the rat could escape by finding a hidden platform located in front of the correct stimulus. The two stimulus items were separated by a divider. Rats were first trained on a black–white discrimination, then on two different black/white patterns, and finally on two different Legos (shown here).
Low-ambiguity condition
The Sham group exhibited a 37.4% ± 1.5% preference for the odd object (chance = 33.3%, t(23) = 2.75, P < 0.05). The PR and H groups explored the odd object 34.3% ± 1.6% and 32.8% ± 1.0% of the time, respectively (Fig. 3A). Neither of these values was greater than the chance value of 33.3% (both t(15) values <0.63, P > 0.1). The Sham group exhibited a higher preference for the odd object than the H group (t(38) = 2.30, P < 0.05).
Figure 3.
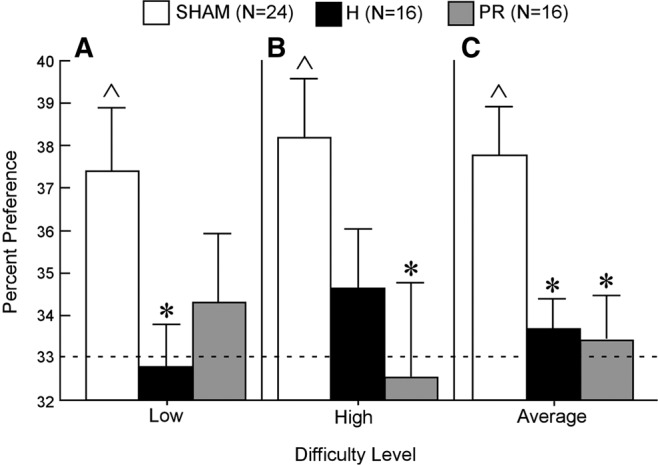
Simultaneous oddity discrimination by Sham (white), H (black), and PR (gray) groups (rats). Data are 5-min cumulative scores (time spent exploring the odd object divided by total exploration time for the odd and identical objects combined) in the (A) “low” condition, (B) “high” condition, and (C) average of the “low” and “high” conditions. The dotted line shows chance performance (33.33%). Error bars indicate SEM. Asterisks indicate a difference from the Sham group (P < 0.05). Carets indicate performance above chance (P < 0.05, chance = 33.3%).
High-ambiguity condition
The Sham group exhibited a 38.2% ± 1.4% preference for the altered object (t(23) = 3.47, P < 0.01). The PR and H groups explored the odd object 32.5% ± 2.2% and 34.6% ± 1.4% of the time, respectively (Fig. 3B). Neither of these values was greater than chance (both t(15) values <0.94, P > 0.1). The Sham group exhibited a higher preference for the odd object than the PR group (t(38) = 2.26, P < 0.05).
Low- and high-ambiguity condition combined
Because there was no reliable difference in the Sham group's preference for the odd object in the “low” and “high” conditions, the data were combined for the two conditions. The Sham group exhibited a 37.8% ± 1.1% preference for the odd object (t(23) = 4.00, P < 0.001). The PR and H groups explored the odd object 33.4% ± 1.0% and 33.1% ± 0.7% of the time, respectively (Fig. 3C). Neither of these values was greater than chance (both t(15) values <0.60, P > 0.1). The Sham group exhibited a higher preference for the odd object than either the PR group (t(38) = 2.69, P < 0.01) or the H group (t(38) = 2.74, P < 0.01). Finally, the subset of animals in the H group that sustained slight damage to the entorhinal cortex (n = 5) performed slightly better than the animals without entorhinal damage (n = 11) (mean 33.3% ± 0.8% versus 34.5% ± 1.0%, respectively), but this difference was not significant (t(114) = 0.73, P > 0.1). This finding indicates that the extra entorhinal cortex damage was not a contributor to the overall group impairment.
Time exploring the stimuli
The Sham, PR, and H groups spent on average 116 ± 4.4 sec, 85 ± 4.5 sec, and 121 ± 5.9 sec respectively, exploring the stimuli during the trials. The PR group explored the stimuli less than either the Sham (t(238) = 4.70, P < 0.0001) or H (t(238) = 4.87, P < 0.0001) group. The Sham and H groups were not different.
Transition times between stimuli
To determine how much time the rats tended to take between finishing exploration of one object and beginning to explore another object, we scored the time that rats spent between objects. The control group mean was 7.0 ± 0.7 sec (range 5.5–9.5 sec; median = 6.7 sec) and the lesion group mean was 9.5 ± 1.0 sec (range 7.4–13.9 sec; median = 8.7 sec). The groups were not different (t(9) = 1.94, P > 0.05).
Experiment 2: visual discrimination
Neurohistological findings
Figure 4 shows reconstructions of coronal sections through the hippocampus (A) and perirhinal cortex (B), showing the smallest (black) and largest (stippled) lesion. Numbers represent the distance (mm) posterior to bregma.
Figure 4.
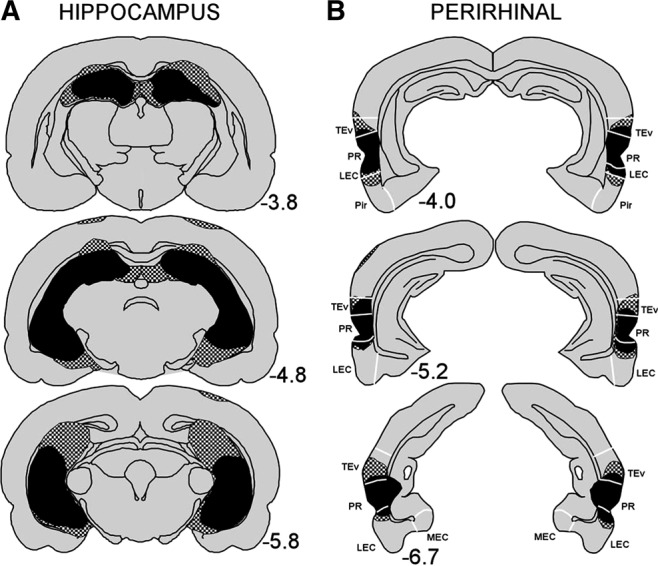
Reconstructions of coronal sections through the (A) hippocampus and (B) perirhinal cortex showing the smallest (black) and largest (stippled) lesion for the hippocampal and perirhinal lesion groups, respectively, in Experiment 2. Numbers represent the distance (mm) posterior to bregma. TEv (ventral TE), PR (perirhinal cortex), LEC (lateral entorhinal cortex), MEC (medial entorhinal cortex), Pir (piriform area). White lines indicate approximate borders between these structures.
Hippocampal lesion
The hippocampal lesions were highly similar to the lesions from Experiment 1. The damage included 83%–98% of the hippocampus (mean = 92% ± 1.9%). There was minor damage to the cortex and to the fimbria overlying the dorsal hippocampus and two rats had minor damage to the posterior aspect of the lateral entorhinal cortex.
Perirhinal lesion
The perirhinal lesions were highly similar to the lesions from Experiment 1. Here the average damage was 95% ± 2.1%; (range 92%–100%). Additionally, there was limited bilateral damage to ventral TE, ventral temporal association areas, postrhinal cortex, and lateral entorhinal cortex.
Behavioral findings
Figure 2B illustrates the visual discrimination task, adapted from Prusky et al. (2004). Rats with bilateral hippocampal lesions (H, n = 8), bilateral perirhinal lesion (PR, n = 8), and sham surgeries (Sham, n = 8) were tested on three discriminations, as stimulus pairs included a black screen versus a white screen (Black/White), two black and white high-contrast patterns (Pattern), and two photographs of “low-ambiguity” Lego objects from a pair used in Experiment 1 (Lego).
Discrimination 1 (black/white)
The Sham, PR, and H groups took 31.3 ± 2.3, 38.8 ± 4.4, and 43.8 ± 6.8 trials, respectively, to successfully reach the 17/20 criterion for the Black/White discrimination (Fig. 5A). There were no differences between groups (all ts < 1.8, all Ps > 0.1).
Figure 5.
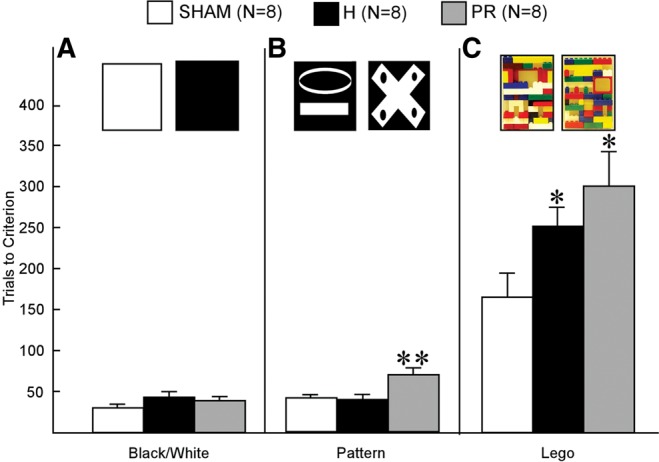
Discrimination performance by Sham (white), H (black), and PR (gray) groups (rats). Data are the mean number of trials to criterion (17/20 correct) on the (A) black–white discrimination, (B) pattern discrimination, and (C) Lego discrimination. At the top of each panel are illustrations of the stimuli used for each of the three discriminations. Error bars indicate SEM. Double asterisks indicate a difference from both the Sham and H groups (P < 0.05). Single asterisks indicate a difference from the Sham group (P < 0.05).
Discrimination 2 (pattern)
The Sham, PR, and H groups took 41.3 ± 3.0, 70.0 ± 8.2, and 38.3 ± 7.0 trials, respectively, to successfully reach the 17/20 criterion for the Pattern discrimination (Fig. 5B). The PR group required more trials to reach criterion than either the Sham (t(12) = 3.70, P < 0.01) or H (t(12) = 2.94, P < 0.05) group.
Discrimination 3 (Lego)
The Sham, PR, and H groups took 166.3 ± 29.4, 301.3 ± 42.2, and 251.3 ± 22.7 trials, respectively, to successfully reach the 17/20 criterion for the Lego discrimination (Fig. 5C). The PR group and the H group each required more trials to reach criterion than the Sham group (PR vs. Sham: t(14) = 2.63, P < 0.05; H vs. Sham: t(14) = 2.29, P < 0.05). The PR and H groups performed similarly (t(14) = 1.0, P > 0.1).
The results for the three discriminations did not significantly change when two rats with entorhinal cortex damage were excluded from the H group.
Experiment 3: eye tracking oddity discrimination (humans)
Human participants were tested on a modified version of the SOD task, viewing sets of Lego images with different levels of difficulty (Fig. 6). All three methods used for assessing viewing times yielded the same result: controls performed above chance and patients performed like controls. For the first method (chance = 8.3%), controls viewed the critical quadrant 16.1% ± 2.0%, 12.6% ± 1.7%, and 12.0% ± 1.2% of the time in the “low”, “medium,” and “high” conditions. For the H patients, the scores were 23.3% ± 7.5%, 16.2% ± 3.3%, and 19.1% ± 5.6% (MTL patient, overall mean = 25.3%). For the second method (chance = 33%), controls viewed the critical quadrant 41.0% ± 3.8%, 37.7% ± 3.1%, and 36.2% ± 1.7% of the time in the “low,” “medium,” and “high” conditions. For the H patients, the scores were 46.6% ± 7.9%, 45.9% ± 6.4%, and 47.4% ± 9.7% (MTL patient, overall mean = 55.4%). Despite their high scores and the fact that every patient scored numerically above chance in every condition, the scores of the three H patients were not significantly above chance with these two methods.
Figure 6.
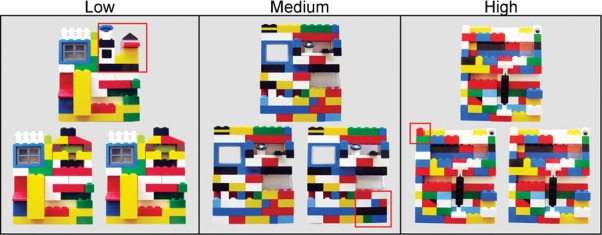
Examples of the Lego images used in the “low,” “medium,” and “high” conditions of Experiment 3. The red box outlines the altered quadrant in the odd image. In the “low” condition, 100% of the quadrant was altered, in the “medium” condition 50% of the quadrant was altered, and in the “high” condition 25% of the quadrant was altered.
The third method (chance = 25%) yielded the same result, though with less variability, and these results appear in Figure 7. With the third method, percent viewing time of the critical quadrants by the control group was 38.1% ± 2.3%, 32.5% ± 2.1%, and 32.6% ± 2.3% for the “low,” “medium,” and “high” conditions. All these values were well above the chance value of 25% (ts > 3.3, P < 0.01). The patients also exhibited a bias toward viewing the critical quadrants of the Lego images (H: “low,” 47.8% ± 6.8%; “medium,” 34.6% ± 2.2%; “high,” 39.2% ± 3.0%; MTL: “low,” 45.7%; “medium,” 42.9%; “high,” 33.2%). The H group scores were greater than chance (ts > 4.4, P < 0.05; Fig. 7), except in the “low” condition (t(2) = 3.4, P = 0.078), where the variability was unusually large. In addition, the H group performed similarly to or better than the control group (control versus H: “low,” t(13) = 1.8, P > 0.1; “medium,” t(13) = 0.5, P > 0.1; “high,” t(13) = 1.4, P > 0.1). The MTL patient scored better than controls in the “low” and “medium” conditions (single-sample ts > 4.9, P < 0.01) and similarly to controls in the “high” condition (t(11) = 0.3, P > 0.1).
Figure 7.
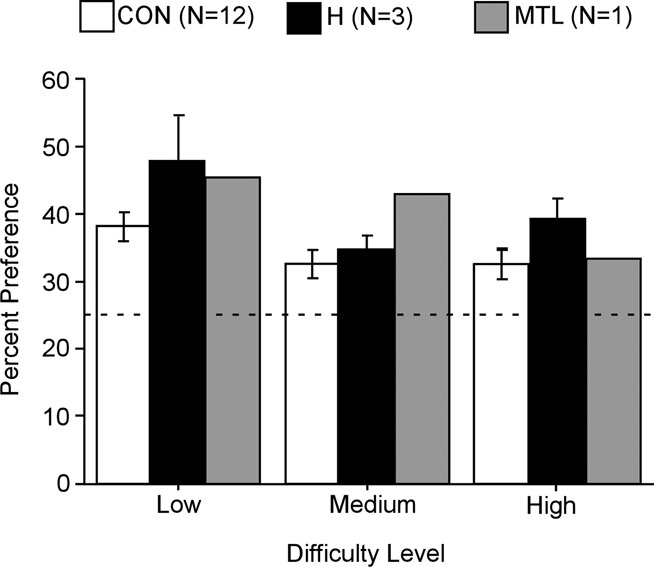
Simultaneous oddity discrimination by Control (white), H (black), and MTL (gray) groups (humans). Viewing preference for the altered critical quadrant (and the corresponding quadrant in the other two images) is shown as the percent of viewing time across the entire 20-sec trial. All participants showed a bias for the critical quadrants at all three levels of difficulty. The dotted line shows chance performance (25%). Error bars indicate SEM.
Discussion
Rats with PR lesions exhibited no preference for the odd object in the SOD task, failing to perform above chance in either the “low” or “high” condition (Fig. 3). Notably, rats with H lesions were similarly impaired on this task and also failed to perform above chance in any condition. When the “low” and “high” conditions were combined, the sham group exhibited a stronger preference for the odd object than either lesion group, and neither lesion group performed above chance. The important point is that an impairment was evident after hippocampal lesions, not only after perirhinal lesions. Accordingly, the deficit described here, and previously with a similar task (Bartko et al. 2007a), is not specific to perirhinal cortex.
Bartko et al. (2007a) designed the simultaneous oddity discrimination task to explore the possible perceptual functions of perirhinal cortex. They created Lego objects to test four levels of perceptual difficulty: “low,” “medium,” “medium-high,” and “high.” Rats with perirhinal lesions were marginally impaired in the “medium-high” condition and impaired in the “high” condition (Bartko et al. 2007a). In our study, rats with perirhinal lesions or hippocampal lesions were impaired in both the “low” and “high” conditions (our “low” condition was similar to the low condition in the earlier study). However, note that the objects in both our conditions shared many overlapping features (i.e., the same object size, shape, shades, and texture), and no single feature would have made the objects distinct from one another. As a result, the visual discriminations were difficult in both conditions.
The failure of the lesion groups to perform above chance could reflect an inability to perceptually discriminate between similar stimuli. Rats were first tested on a black–white discrimination, then on a pattern discrimination, and finally on a Lego discrimination that used photographs of the stimuli from the simultaneous oddity discrimination task. Although the PR group required more trials than the H or control groups on the pattern discrimination task, and both lesion groups required more trials than controls on the Lego discrimination task, all animals reached criterion (Fig. 5). Thus, rats with PR or H lesions have the perceptual ability to distinguish between the highly similar Lego stimuli used in the simultaneous oddity discrimination task. The complexity of the stimuli (Lego objects) likely accounts for the large number of trials needed to acquire the Lego discrimination task. All groups required fewer than 44 trials to reach criterion on a simple black–white discrimination. However, the Sham, PR, and H groups took 166, 301, and 251 trials, respectively, to reach criterion on the Lego discrimination task.
The results from the two-choice discrimination task cannot definitively decide between a perceptual impairment and a mnemonic impairment. Nonetheless, the results are informative. First, the impairment on discrimination tasks in animals with hippocampal lesions has been reported previously (Driscoll et al. 2005; Broadbent et al. 2006). Yet these discrimination tasks are also critically dependent on the caudate nucleus and are thought to be a form of habit memory (e.g., Broadbent et al. 2006). Thus, it appears that having an intact hippocampus (and perirhinal cortex; current study) confers some detectable advantage to the rodent. We suggest that this advantage comes in the form of providing the rodent information about which aspects of the stimuli are important for making the discrimination and which are not. In the case of pattern discrimination, the rodent learns that the details of the stimuli are important. The same line of reasoning holds for the very difficult Lego discrimination problem. If the PR group was impaired only on the Lego discrimination task, it would be difficult to decide whether the impairment was perceptual or mnemonic. However, because the PR group was also impaired on the more perceptually simple pattern discrimination task, a discrimination problem that should not present any obvious perceptual challenge to the rats, we suggest that the mnemonic deficit present in both groups is the more likely explanation of the observed impairments.
The oddity task was designed as a perceptual task with all the test objects available at the same time (Bartko et al. 2007a). However, rats did not of course explore all three objects at once. Instead, the rats approached and explored each object independently before moving to explore another object or another part of the environment. Because rats explored only one object at a time, performance on the task necessarily required memory to some extent. Earlier studies in rats with H or PR lesions that used variable retention intervals to test recognition memory showed that the same tasks that were performed poorly at long retention delays could nevertheless be performed well at short delays, presumably because animals could rely on intact working memory (e.g., Mumby and Pinel 1994; Ennaceur et al. 1996; Buffalo et al. 1999; Clark et al. 2000, 2001; Málková et al. 2001; Nemanic et al. 2004; Winters and Bussey 2005). In the present study, we suggest that the H and PR groups needed to depend on long-term memory to guide performance, perhaps because the stimuli were too complex to maintain in working memory. This interpretation is supported by the finding that hippocampal lesions, not just perirhinal lesions, impaired performance. Hippocampal lesions are known to impair memory, but we are unaware of suggestions that hippocampal lesions impair visuoperceptual tasks involving objects.
In contrast to the findings for rats, patients with hippocampal damage or larger MTL lesions performed normally on the oddity discrimination task. There were no differences between groups, and all groups performed above chance (Fig. 7). We suggest that the human subjects’ approach to the simultaneous oddity discrimination task is different than the rats’ approach to the task. Rats slowly moved from one object to the next, often exploring other parts of the environment as they moved (mean interobject interval = 7–9.5 sec). Humans spent the entire test period exploring only the objects and using saccades to move quickly within and between the objects. Accordingly, humans may have been able to rely on their working memory capacity to perform the task. We suggest that patients with hippocampal or perirhinal lesions would be impaired on this kind of task if the task were constructed so as to depend on long-term memory. For example, an impairment would be expected if the stimuli were arranged some distance apart, as on different walls of a room, with multiple similar stimuli and one or two odd stimuli. In this case, long-term memory would be required to take notice of the odd stimuli and to exhibit a viewing preference.
The conclusion that rats with PR lesions were impaired on the oddity task because of impaired long-term memory, and not impaired perceptual ability, differs from the conclusion of earlier studies of perirhinal lesions and visual discrimination performance (Bussey et al. 2002; Barense et al. 2005, 2012a,b; Bussey and Saksida 2005; Lee et al. 2005; Bartko et al. 2007a,b; Murray et al. 2007; Graham et al. 2010;; Erez et al. 2013). However, many of the tasks that have been used to assess perceptual abilities have not excluded a role for learning and memory (Hampton 2005; Suzuki 2009,2010; Squire and Wixted 2011).When visual perception was studied in circumstances designed to minimize the influence of learning and memory, monkeys with perirhinal lesions performed normally, even on very difficult discriminations where the stimuli were rotated, enlarged, shrunk, or degraded by masks (Hampton and Murray 2002). Additionally, in another study, rats with perirhinal lesions were intact at making difficult, feature-ambiguous discriminations despite being impaired on a standard recognition memory task (Clark et al. 2011). In this study, rats were given 10,500 training trials on an automated, two-choice discrimination task in order to establish consistent performance at a high level. Then, probe trials were interpolated to assess visual perceptual ability. The probe trials systematically varied the degree of feature ambiguity between the stimuli, and visual discrimination performance was tested across 14 different levels of difficulty. Sham rats and rats with perirhinal lesions were indistinguishable at every feature-ambiguity level.
Another example is informative based on work with humans. Patients with MTL lesions that included the perirhinal cortex were tested for their ability to identify the unique object among twin pairs of objects that had a high degree of feature ambiguity (Knutson et al. 2013). When the number of pairs were small (difficulty levels 1–4), the patients performed as well as controls despite the high levels of ambiguity between the objects. When the number of pairs were larger (difficulty levels 5–8), the patients performed more poorly than the controls. This deficit might be interpreted either as a memory deficit (because the number of elements to compare exceeded the working memory capacity), or as a perceptual impairment (because the larger number of elements increased the feature ambiguity beyond what the patients could perceive). However, in another condition, the same difficulty levels that had revealed an impairment (levels 5–8) were tested again, yet the participants were allowed to use a pencil to draw lines between the twin pairs. This procedure eliminated the need to hold material in memory as participants worked at each problem. In this condition, use of the pencil entirely rescued performance. Note that the perceptual demands of the task were the same with or without this memory aid. Accordingly, these results suggest that the deficit on this and similar tasks, which involve comparisons across stimuli with overlapping features, is better understood as impaired memory rather than impaired perception.
Our findings are consistent with the known role of the perirhinal cortex and hippocampus in the formation of long-term memory. In particular, the finding in rats that hippocampal lesions caused the same impairment as perirhinal lesions counts against the idea that the impairment in the oddity task is related to functions specific to perirhinal cortex, such as its proposed role in visual perception.
Materials and Methods
Experiment 1: simultaneous oddity discrimination (rats)
Subjects
Subjects were 67 male, Long-Evans rats weighing between 300 and 350 g at the beginning of the study. Rats were individually housed and maintained on a 12:12 h light:dark cycle. Food and water were freely available. Rats were randomly assigned to receive bilateral lesions of the hippocampus (H = 16), bilateral lesions of perirhinal cortex (PR = 16), or sham surgeries (Sham = 24). Calculations of the amount of time rats spend moving from one object to another object were based on data from 11 rats (PR = 6, Sham = 5).
Apparatus
The SOD task was based on the task developed by Bartko et al. (2007a). Testing was conducted in a round container, open on the top and measuring 40.2 cm in diameter and 51.1 cm high. Four vertical columns measuring 2.2 cm in width and 30.5 cm in height separated a portion of the wall into three sections 10.2 cm in width (Fig. 2A). Each of the three sections had Velcro along the bottom of the wall and on the floor in order to secure stimulus objects during testing. A video camera mounted on the wall directly above the box recorded each testing session for later analysis. Overhead fluorescent lighting illuminated the box.
Objects
Objects were constructed from blocks purchased from LEGO (LEGO Systems, Inc.) and were rectangular in shape (width = 7.9 cm; height = 11.7 cm) (Fig. 2A). Six sets of three Lego objects were constructed. Each set consisted of two identical objects and one odd object. Three of the sets were assigned to the “low-ambiguity” condition. For these sets, the odd object was entirely distinct from the two identical objects, i.e., no part of the odd object matched the pattern of the other two objects. The other three sets were assigned to the “high-ambiguity” condition. For these sets, 50% of the odd object matched either the top or bottom half of the two identical objects. Once the objects were constructed, the Lego pieces comprising each object were glued together, and Velcro was secured to each lower back side and bottom.
Surgery
Surgery was designed to remove the entire hippocampus or the entire perirhinal cortex bilaterally. Anesthesia was maintained throughout surgery with isoflurane gas (0.8%–2.0% isoflurane delivered in O2 at 1 L/min). The rat was placed in a Kopf stereotaxic instrument, and the incisor bar was adjusted until bregma was level with Lambda. For the lesion groups, bilateral excitotoxic hippocampal (H) or perirhinal (PR) lesions were produced by local microinjections of ibotenic acid (IBO; Biosearch Technologies). IBO was dissolved in 0.1 M phosphate-buffered saline to provide a solution with a concentration of 10 mg/mL, pH 7.4. IBO was injected at a rate of 0.1 μL/min with a 10 μL Hamilton syringe mounted on a stereotaxic frame and held with a Kopf Microinjector (model 5000). The syringe needle was lowered to the target coordinate and left in place for 1 min before beginning the injection. Following the injection, the syringe needle was left in place for an additional 2 min to reduce the spread of IBO up the needle tract. For the H lesion group, a total of 0.51 μL of IBO was injected into 18 sites within each hippocampus (all coordinates are in millimeters and relative to bregma): anteroposterior (AP) −2.4, mediolateral (ML) ±1.0, dorsoventral (DV) −3.5; AP −3.2, ML ±1.4, DV −3.1, −2.3; AP −3.2, ML ±3.0, DV −2.7; AP −4.0, ML ±2.5, DV −2.8, −1.8; AP −4.0, ML ±3.7, DV −2.7; AP −4.8, ML ±4.9, DV −7.2, −6.4; AP −4.8, ML ±4.3, DV −7.7, −7.1, −3.5; AP −5.4, ML ±4.2, DV −4.4, −3.9; AP −5.4, ML ±5.0, DV −6.6, −5.9, −5.2, −4.5. For the PR lesion group, a total of 0.105 μL of IBO was injected into five sites within each hemisphere of perirhinal cortex: AP −3.0, ML ±6.4, DV −7.7; AP −4.0, ML ±6.5, DV −7.7; AP −5.0, ML ±6.8, DV −7.5; AP −6.6, ML ±6.8, DV −7.0; AP −7.68, ML ±6.3, DV −6.7. The procedure for the sham-operated control (Sham) group was the same as for the lesion groups, but the dura was not punctured, the syringe needle was not lowered into the cortex, and IBO was not injected. Once awake and responsive, each rat was returned to its home cage for a 14-d recovery period.
Habituation
Rats were acclimated to the testing room and apparatus for two consecutive days prior to testing (45 min in the testing room and 5 min to explore the empty apparatus).
Simultaneous oddity discrimination
Rats were given three trials in the “low-ambiguity” condition and three trials in the “high-ambiguity” condition (one trial/day). The order of the conditions was counterbalanced across rats. Three objects (two identical objects and one odd object) were first placed inside the empty container and secured to the floor and wall by Velcro. A different set of objects were used on each day of testing. The position of the odd object (left, middle, or right) and which rat received which set of objects were counterbalanced across rats and trials. To begin testing, rats were placed inside the container facing the wall opposite the objects and were then allowed to explore for 5 min. After the 5-min trial, rats were returned to their home cage. The container and the objects were cleaned with 95% ethanol between every trial to minimize olfactory cues. Object exploration was later scored from video recordings of each trial by an experimenter blind to the group membership of the rat. An object was identified as being explored when the rat's nose was within 1 cm of the object and the vibrissae were moving (see Clark et al. 2000). Object exploration was not scored when the rat used the object to rear upward such that the nose of the rat faced the ceiling. Preference for the odd object was expressed as the percent time that a rat explored the odd object (out of the total time exploring all three objects). Cumulative preference scores were determined at the end of each 1-min interval across the 5-min trial. Chance was taken to be 33%.
Transition times
The mean transition time from one object to the next was determined by recording the length of every transition interval (time between the end of exploring one object and beginning of exploring the next) during each 5-min test (three trials at each of two difficulty levels).
Histology
At completion of testing, the rats were administered an overdose of sodium pentobarbital and perfused transcardially with buffered 0.9% NaCl solution followed by 10% formaldehyde solution (in 0.1 M phosphate buffer). The brains were then removed and cryoprotected in 20% glycerol/10% formaldehyde. Coronal sections (50 μm) were cut with a freezing microtome ranging from the anterior commissure through the length of the hippocampus. Every fifth section was mounted and stained with thionin to assess the extent of the lesions. Quantification of the perirhinal lesion was based on previous work showing that the extent of damage along the anterior–posterior axis is a good predictor of the lesion's efficacy (Bucci and Burwell 2004; Burwell et al. 2004). Accordingly, we quantified the proportion of 14 sections along the anterior–posterior extent of the perirhinal cortex (AP range: −2.45 to −6.65 from bregma) that contained damaged tissue (Burwell et al. 2004). Quantification of the hippocampal lesion was obtained by calculating the percent damage in 1-mm increments through the anterior–posterior extent of the hippocampus (four sections, from −2.80 to −5.80 mm from bregma; Paxinos and Watson 1998).
Each section was assessed under magnification, and the tissue was considered damaged if it was absent or necrotic (i.e., hippocampal or perirhinal tissue was present, but there was no evidence of Nissl staining, or the tissue was gliotic). The region damaged was drawn onto a control template for each section, and the area of damage was calculated using an automated tool in a computer graphics program (Canvas 8, Deneba). The area of hippocampal or perirhinal damage was then summed across all the template sections and calculated as a percentage of the total control hippocampal area or total perirhinal area, respectively.
Experiment 2: visual discrimination
Subjects
Subjects were 24 male, Long-Evans rats weighing between 300 and 350 g at the beginning of the study. Housing, feeding, and assignment to lesion groups (hippocampus, H = 8, perirhinal cortex, PR = 8, or sham surgeries, Sham = 8) were as in Experiment 1.
Apparatus
The visual discrimination task was conducted in a trapezoidal-shaped tank made of clear Perspex measuring 140 cm long × 55 cm high × 25 cm wide (at the start wall) and 80 cm wide (at the finish wall) (adapted from Prusky et al. 2004; Fig. 1B). A 40-cm-high × 46-cm-long Perspex divider was placed in the tank, extending back from the middle of the finish wall so as to create two arms at the end of the tank. The tank was filled with room-temperature water to a depth of 15 cm, and a transparent Plexiglas escape platform (37 × 13 × 14 cm) was submerged at the end of one of the arms. The water was made opaque by the addition of powdered milk. The location of the platform could be moved to either arm for a given trial. Room lights were off during testing. Two computer monitors (26 × 42 cm) were located at the end of each arm facing the inside of the tank. The bottoms of the monitors were aligned to the level of the water. The stimulus that served as the correct object was counterbalanced across animals, and the position of the correct object (left/right monitor) varied from trial to trial following a pseudorandom sequence. The stimulus pairs included a black screen versus a white screen, two black and white high-contrast patterns, and two photographs of “low-ambiguity” Lego objects from a pair used in Experiment 1.
Surgery
The surgical procedures and coordinates for the hippocampal and perirhinal lesions were the same as in Experiment 1.
Visual discrimination training
Rats were first trained on the black–white discrimination. Rats were placed in the tank starting area with the stimuli on display at the far wall. The rats could see both stimuli from the start of the trial and all the way to the choice point. Most rats adopted a strategy of swimming directly to the choice point and holding onto the center divider for a few seconds before selecting an arm. When the correct stimulus was approached, the rat encountered the platform, escaped the water, and remained there for 10 sec before being returned to a holding cage for a 1-min intertrial interval (ITI). When the incorrect stimulus was approached, the rat was confined to that location by blocking the exit from the arm for 10 sec. The rat was then allowed to swim to the other arm to find the platform. In this case, too, the rat remained on the platform for 10 sec before being returned to the holding cage for a 1 min ITI. Rats were given 10 trials per day until reaching a learning criterion of 17 correct out of 20 trials (i.e., 85% correct across two testing days). After reaching the learning criterion for the black–white discrimination, rats were trained on a second discrimination involving two different black and white pattern images. After this discrimination was learned to criterion (17 correct out of 20 trials), rats were trained to discriminate between two displays of Lego objects used in Experiment 1 (the “low-ambiguity” condition).
Histology
The histological procedures were the same as in Experiment 1.
Experiment 3: eye tracking oddity discrimination (human)
Participants
Four memory-impaired patients participated. Of these, three had damage believed to be limited to the hippocampus (CA fields, dentate gyrus, and subicular complex). K.E. became amnesic after an episode of ischemia associated with kidney failure and toxic shock syndrome. L.J. (the only female) became amnesic during a 6-mo period in 1988 with no known precipitating event. Her memory impairment has been stable since that time. G.W. became amnesic after drug overdose and associated respiratory failure. Estimates of MTL damage were based on quantitative analysis of magnetic resonance (MR) images compared with data from 19 controls (11 for L.J.) (Gold and Squire 2005). K.E., L.J., and G.W. have an average bilateral reduction in hippocampal volume of 49%, 46%, and 48%, respectively (all values >3 SDs from the control mean). The volume of the parahippocampal gyrus (temporopolar, perirhinal, entorhinal, and parahippocampal cortices) for K.E., L.J., and G.W. were reduced by 11%, −17%, and 10%, respectively. The minus value indicates a volume larger for the patient than for controls (all values within 2 SDs of the control mean).
The fourth patient (G.P.) had a severe memory impairment resulting from viral encephalitis in 1987. G.P. has demonstrated virtually no new learning since the onset of his amnesia and, during repeated testing over many weeks, does not recognize that he has been tested before (Bayley et al. 2005). G.P. has a bilateral reduction in hippocampal volume of 96%. The volume of the parahippocampal gyrus is reduced by 94%, with sparing limited to the parahippocampal cortex. Eight coronal MR images from each patient, together with detailed descriptions of the lesions, can be found in Kim et al. (2013).
Twelve healthy individuals (8 males) served as controls for the memory-impaired patients. Controls averaged 63 ± 2.5 years of age and had 15.2 ± 0.7 years of education (patients: 62.3 ± 9.6 years of age; 13.4 ± 1.9 years of education). All procedures were approved by the Institutional Review Board at the University of California at San Diego, and participants gave written informed consent before participation.
Apparatus
Eye movements were recorded at 30 Hz with a ViewPoint eye tracker (Arrington Research) and PC-60 software (version 2.8.3) for detecting pupillary position. A fixation was scored when at least 100 msec elapsed without a saccade. A saccade was defined as an eye movement of at least 0.7° within 33 msec (0.25 in on the 20-in computer screen). Head motion and position were maintained with a bite bar, forehead rest, and chin rest. Viewing was binocular, although only movements of the left eye were tracked. The eye tracker was adjusted for each participant before the test session. Correction for head motion was performed as needed before each trial. A separate computer was used to control image presentation and record behavioral responses using E-prime software (version 1.2; Psychology Software Tools, Pittsburgh, PA, RRID:nlx_155747).
Materials and procedure
On each 20-sec trial, participants viewed a set of three Lego images (image size 12.7 cm tall × 10.2 cm wide; Fig. 6). Two of the images were identical, and one of the images was altered by changing one of the quadrants of the image. Three levels of difficulty were created by varying the percentage of the quadrant that was altered. In the “low-ambiguity” condition 100% of the quadrant was altered, in the “medium-ambiguity” condition 50% of the quadrant was altered, and in the “high-ambiguity” condition 25% of the quadrant was altered. Twelve sets of Lego images were assigned to each condition. The alterations occurred equally often in each of the four image quadrants and in each of the three image locations. The trials were presented in a pseudorandom order for each participant with the restriction that no more than three trials in a row could occur with the same difficulty level, the same altered image quadrant, or the same location of the altered image.
Participants were told that they would be viewing a set of images and were to explore the images the entire time they were presented. They were invited to view the images in a natural way: “Imagine how you would view images on the pages of an art book and form impressions.” Before each trial began, a central fixation point was presented. After the participants viewed the fixation point for 2–5 sec, three Lego images appeared for 20 sec. Trials were presented in three 12-trial blocks (36 trials total), and participants took a short break after each block of 12 trials (∼1 min).
Data analysis
We considered three different ways to assess viewing of the Lego images. First, we calculated the percent time that participants viewed the altered quadrant relative to the 11 other quadrants in the three images (chance = 8.3%). Second, we calculated the percent time participants viewed the altered quadrant relative to the corresponding quadrant in each of the other two Lego images (chance = 33%). Third, we reasoned that if the participants were able to detect the altered portion of the Lego, they would spend time viewing the altered area of one image and also compare that area to the corresponding areas of the other two unaltered (i.e., identical) Lego images. Accordingly, we calculated how much viewing time was directed to the three critical quadrants (i.e., the one altered quadrant and the two corresponding quadrants in the other two Lego images) and how much viewing time was directed to the nine other unaltered quadrants (three quadrants in each image). In this case, chance was 25% (three critical quadrants out of 12 total quadrants).
Acknowledgments
We thank Susan Davis, Laura Johnson, Ashley Knutson, Brittany Masatsugu, Kristin Mauldin, Mark Starr, and Anna van der Horst for technical assistance. This work was supported by the Medical Research Service of the Department of Veterans Affairs, the NIMH, and a NSF Temporal Dynamics of Learning Center grant.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.035840.114.
References
- Barense MD, Bussey TJ, Lee AC, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS 2005. Functional specialization in the human medial temporal lobe. J Neurosci 25: 10239–10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Groen II, Lee AC, Yeung LK, Brady SM, Gregori M, Kapur N, Bussey TJ, Saksida LM, Henson RN 2012a. Intact memory for irrelevant information impairs perception in amnesia. Neuron 75: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Ngo JK, Hung LH, Peterson MA 2012b. Interactions of memory and perception in amnesia: the figure-ground perspective. Cereb Cortex 22: 2680–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ 2007a. Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. J Neurosci 27: 2548–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ 2007b. Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learn Mem 14: 821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, Frascino JC, Squire LR 2005. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature 436: 550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE 2006. Rats depend on habit memory for discrimination learning and retention. Learn Mem 14: 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Burwell RD 2004. Deficits in attentional orienting following damage to the perirhinal or postrhinal cortices. Behav Neurosci 118: 1117–1122. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Clark RE, Teng E, Squire LR, Zola SM 1999. Dissociation between the effects of damage to perirhinal cortex and area TE. Learn Mem 6: 572–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Bucci DJ, Sanborn MR, Jutras MJ 2004. Perirhinal and postrhinal contributions to remote memory for context. J Neurosci 24: 11023–11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM 2005. Object memory and perception in the medial temporal lobe: an alternative approach. Curr Opin Neurobiol 15: 730–737. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA 2002. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. Eur J Neurosci 15: 365–374. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR 2000. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci 20: 8853–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, West AN, Zola SM, Squire LR 2001. Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus 11: 176–186. [DOI] [PubMed] [Google Scholar]
- Clark RE, Reinagel P, Broadbent NJ, Flister ED, Squire LR 2011. Intact performance on feature-ambiguous discriminations in rats with lesions of the perirhinal cortex. Neuron 70: 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Prusky GT, Rudy JW, Sutherland RJ 2005. Seahorse wins all races: hippocampus participates in both linear and nonlinear visual discrimination learning. Behav Brain Res 164: 29–35. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ 2001. From conditioning to conscious recollection: memory systems of the brain. Oxford University Press, Oxford. [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP 1996. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav Brain Res 80: 9–25. [DOI] [PubMed] [Google Scholar]
- Erez J, Lee AC, Barense MD 2013. It does not look odd to me: perceptual impairments and eye movements in amnesic patients with medial temporal lobe damage. Neuropsychologia 51: 168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JJ, Squire LR 2005. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus 15: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee AC 2010. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia 48: 831–853. [DOI] [PubMed] [Google Scholar]
- Hampton RR 2005. Monkey perirhinal cortex is critical for visual memory, but not for visual perception: reexamination of the behavioural evidence from monkeys. Q J Exp Psychol B 58: 283–299. [DOI] [PubMed] [Google Scholar]
- Hampton RR, Murray EA 2002. Learning of discriminations is impaired, but generalization to altered views is intact, in monkeys (Macaca mulatta) with perirhinal cortex removal. Behav Neurosci 116: 363–377. [DOI] [PubMed] [Google Scholar]
- Jeneson A, Squire LR 2011. Working memory, long-term memory, and medial temporal lobe function. Learn Mem 19: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger E, Ullman MT, Corkin S 2001. Bilateral medial temporal lobe damage does not affect lexical or grammatical processing: evidence from amnesic patient H.M. Hippocampus 11: 347–360. [DOI] [PubMed] [Google Scholar]
- Kim S, Sapiurka M, Clark RE, Squire LR 2013. Contrasting effects on path integration after hippocampal damage in humans and rats. Proc Natl Acad Sci 110: 4732–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson AR, Hopkins RO, Squire LR 2012. Visual discrimination performance, memory, and medial temporal lobe function. Proc Natl Acad Sci 109: 13106–13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson AR, Hopkins RO, Squire LR 2013. A pencil rescues impaired performance on a visual discrimination task in patients with medial temporal lobe lesions. Learn Mem 20: 607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Barense MD, Graham KS 2005. The contribution of the human medial temporal lobe to perception: bridging the gap between animal and human studies. Q J Exp Psychol B 58: 300–325. [DOI] [PubMed] [Google Scholar]
- Málková L, Bachevalier J, Mishkin M, Saunders RC 2001. Neurotoxic lesions of perirhinal cortex impair visual recognition memory in rhesus monkeys. Neuroreport 12: 1913–1917. [DOI] [PubMed] [Google Scholar]
- Milner B 1972. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg 19: 421–466. [DOI] [PubMed] [Google Scholar]
- Milner B, Corkin S, Teuber HL 1968. Further analysis of the hippocampal amnesic syndrome: 14-year followup study of H.M. Neuropsychologia 6: 215–234. [Google Scholar]
- Mumby DG, Pinel JP 1994. Rhinal cortex lesions and object recognition in rats. Behav Neurosci 108: 11–18. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM 2007. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu Rev Neurosci 30: 99–122. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J 2004. The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. J Neurosci 24: 2013–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C 1998. The rat brain in stereotaxic coordinates, 4th ed Academic, San Diego, CA. [Google Scholar]
- Prusky GT, Douglas RM, Nelson L, Shabanpoor A, Sutherland RJ 2004. Visual memory task for rats reveals an essential role for hippocampus and perirhinal cortex. Proc Natl Acad Sci 101: 5064–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Gold JJ, Hopkins RO, Squire LR 2006. Intact visual perception in memory-impaired patients with medial temporal lobe lesions. J Neurosci 26: 2235–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT 2011. The cognitive neuroscience of human memory since H.M. Annu Rev Neurosci 34: 259–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S 1991. The medial temporal lobe memory system. Science 253: 1380–1386. [DOI] [PubMed] [Google Scholar]
- Suzuki WA 2009. Perception and the medial temporal lobe: evaluating the current evidence. Neuron 61: 657–666. [DOI] [PubMed] [Google Scholar]
- Suzuki WA 2010. Untangling memory from perception in the medial temporal lobe. Trends Cogn Sci 14: 195–200. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ 2005. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci 25: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]


