Abstract
Numerous investigations have definitively shown amygdalar involvement in delay and contextual fear conditioning. However, much less is known about amygdala contributions to trace fear conditioning, and what little evidence exists is conflicting as noted in previous studies. This discrepancy may result from selective targeting of individual nuclei within the amygdala. The present experiments further examine the contributions of amygdalar subnuclei to trace, delay, and contextual fear conditioning. Rats were trained using a 10-trial trace, delay, or unpaired fear conditioning procedure. Pretraining lesions targeting the entire basolateral amygdala (BLA) resulted in a deficit in trace, delay, and contextual fear conditioning. Immediate post-training infusions of the protein synthesis inhibitor, cycloheximide, targeting the basal nucleus of the amygdala (BA) attenuated trace and contextual fear memory expression, but had no effect on delay fear conditioning. However, infusions targeting the lateral nucleus of the amygdala (LA) immediately following conditioning attenuated contextual fear memory expression, but had no effect on delay or trace fear conditioning. In follow-up experiments, rats were trained using a three-trial delay conditioning procedure. Immediate post-training infusions targeting the LA produced deficits in both delay tone and context fear, while infusions targeting the BA produced deficits in context but not delay tone fear. These data fully support a role for the BLA in trace, delay, and contextual fear memories. Specifically, these data suggest that the BA may be more critical for trace fear conditioning, whereas the LA may be more critical for delay fear memories.
Pavlovian fear conditioning is one of the most extensively studied systems for investigating the neural mechanisms mediating learning and memory processes. It is a behavioral paradigm in which an organism learns to anticipate an aversive event by pairing that event (i.e., unconditioned stimulus; US) with a particular place or predictive stimulus (i.e., conditioned stimulus; CS). The amygdala serves a critical role in this fear learning; it receives both unimodal and multimodal sensory information and projects to a number of individual response circuits allowing for a coordinated fear response (e.g., Davis 1997, 2006; Fanselow and LeDoux 1999; Lee et al. 2001). More specifically, CS (e.g., tone, context) and US (e.g., footshock) sensory inputs converge in the basolateral amygdala (BLA) where the CS–US association is formed (Barot et al. 2009). Formation of this association requires protein synthesis in the amygdala (e.g., Bailey et al. 1999; Schafe and LeDoux 2000; Maren et al. 2003; Kwapis et al. 2011). Once formed, this BLA-dependent association permanently supports the expression of fear memory (LeDoux 1993; Fanselow and LeDoux 1999; Gale et al. 2004; Davis 2006; Amano et al. 2011). The BLA projects, both directly and indirectly, to the central nucleus of the amygdala (CeA), which in turn projects to brainstem and hypothalamic regions to trigger individual fear responses (LeDoux et al. 1988; Wilensky et al. 2006; Amano et al. 2011; Viviani et al. 2011).
Typically, fear conditioning to an auditory stimulus is performed using a delay procedure in which tone and footshock are temporally contiguous. There is a wealth of experiments that have demonstrated that this type of learning depends on the amygdala (e.g., Fanselow and LeDoux 1999). Trace fear conditioning differs from delay conditioning in that a stimulus-free trace interval is inserted between the termination of the tone and the onset of footshock. Unlike delay conditioning (but see Quinn et al. 2008, 2009; Maren 2008), acquisition of trace fear conditioning is critically dependent on several other structures, such as the medial prefrontal cortex and the hippocampus (e.g., McEchron et al. 1998; Quinn et al. 2002, 2005, 2008; Han et al. 2003; Chowdhury et al. 2005; Gilmartin and McEchron 2005a,b; Gilmartin and Helmstetter 2010). Surprisingly, little is known about amygdalar contributions to trace fear conditioning, and the few published studies are conflicting (Kwapis et al. 2011; Raybuck and Lattal 2011; Gilmartin et al. 2012). Further, no studies have addressed possible differential contributions of amygdalar subnuclei to trace fear conditioning.
To further investigate the role of the amygdala in trace, delay, and contextual fear conditioning, we performed five experiments. In Experiment 1, rats received pretraining lesions of the basolateral amygdala (BLA) or sham surgery prior to 10-trial trace or delay fear conditioning. This allowed us to assess the collective contribution of the basal and lateral amygdalar nuclei to acquisition and/or expression of trace, delay, and simultaneously learned contextual fear conditioning. In Experiment 2, rats received bilateral infusions of the protein synthesis inhibitor, cycloheximide, or vehicle into the basal nucleus of the amygdala (BA) immediately following 10-trial trace or delay fear conditioning. This experiment allowed us to assess the role of de novo protein synthesis in BA in the consolidation of trace and delay fear conditioning, as well as simultaneously acquired contextual fear conditioning. Experiment 3 was identical to Experiment 2 except that infusions targeted the LA. In Experiment 4, rats received bilateral infusions of cycloheximide or vehicle into the BA immediately following three-trial delay conditioning. Experiment 5 was identical to Experiment 4 except that infusions targeted the LA. Experiments 4 and 5 allowed us to address the role of training strength/session duration in the effects of cycloheximide on the consolidation of delay fear conditioning.
Results
Experiment 1: basolateral amygdalar lesions disrupt tone and context fear memory in trace and delay conditioned rats
Prior to trace or delay fear conditioning, rats received bilateral neurotoxic lesions of the basolateral amygdala. Tests for freezing to both tone and context occurred across two consecutive days following training (see Fig. 1A).
Figure 1.
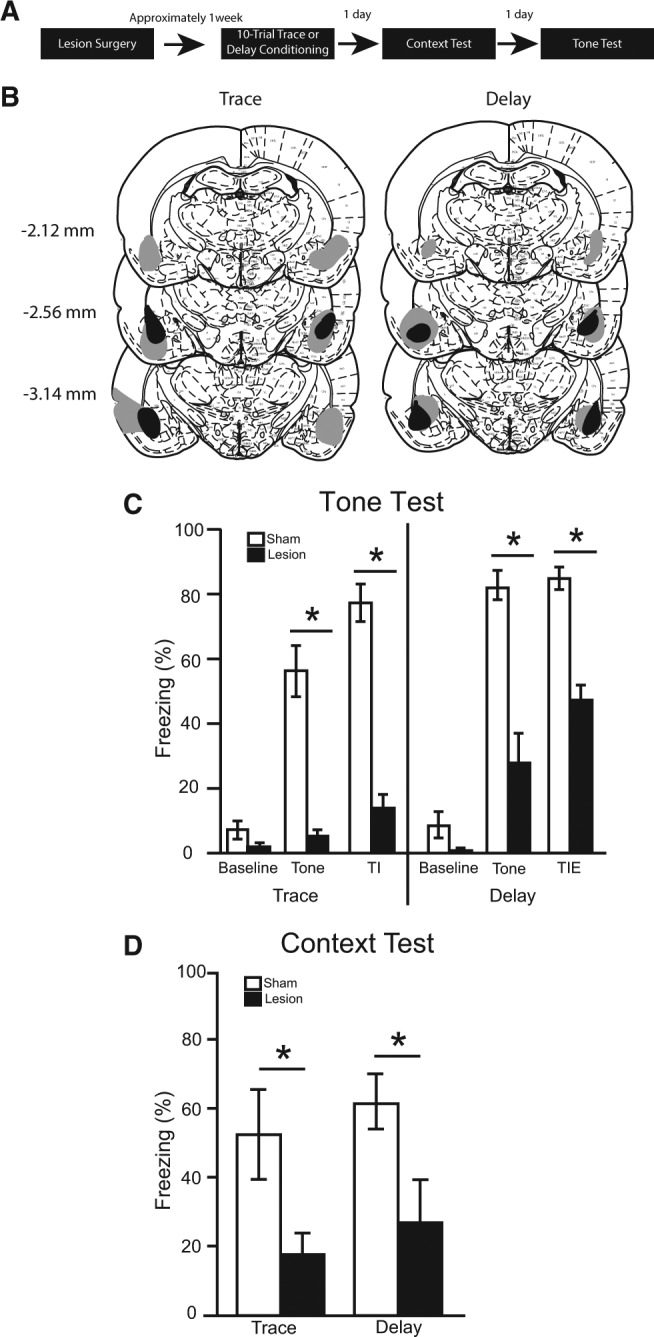
(A) Timeline for Experiment 1. (B) The minimum (black) and maximum (gray) extent of bilateral lesions in BLA (atlas images taken and modified from Paxinos and Watson 1998 with permission from Elsevier 1998). The number of animals in each group was as follows: trace sham, n = 7; trace lesion, n = 7; delay sham, n = 8; delay lesion, n = 9; N = 31. (C) The percentage of time spent freezing during the baseline period (first 3 min), tone, and trace interval or trace interval equivalent during the tone test. (D) Simultaneously learned contextual fear expressed during the context test.
Verification of lesions
Lesion extent was quantified in a manner similar to that described previously (Quinn et al. 2013). Briefly, three brain slices throughout the extent of the BLA were stained using immunofluorescence for NeuN and GFAP. Lesion extent was visualized via fluorescent microscopy, and was quantified using ImageJ (NIH) software. Five rats were excluded from statistical analyses; four cases were excluded due to unilateral lesions, and one case was excluded due to lesion misplacement. The lesion extents of the remaining 31 rats were deemed acceptable and included in all statistical analyses (see Fig. 1B). Overall, lesion extent covered 53% of the BLA, with trace animals averaging 59% and delay animals averaging 47%. Lesion extents were primarily confined to the BLA, but seven cases extended laterally into adjacent temporal cortices (five trace, two delay) and six cases extended medially into the lateral portion of the CeA (four trace, two delay). Additionally, eight cases had at least unilateral sparing of the most anterior portion of the BLA (four traces, four delays).
Tone test
Despite very low levels of freezing during the 180-sec baseline period of the tone test, there was a significant main effect of surgery [F(1,27) = 6.17, P < 0.05], but no main effect of training [F(1,27) > 0.01, P = 0.973] and no training × surgery interaction [F(1,27) = 0.40, P = 0.531]. However, pairwise comparisons within each training condition revealed no differences between lesion and sham rats (P > 0.05; Fig. 1C).
During the tone (averaged across the three presentations), there was a significant main effect of training [F(1,27) = 16.17, P < 0.001], a significant main effect of surgery [F(1,27) = 73.82, P < 0.001], but no training × surgery interaction [F(1,27) = 0.04, P = 0.844]. Delay conditioned animals froze significantly more than trace conditioned animals. Further, both trace and delay lesioned animals showed a significant deficit in freezing to tone compared with their corresponding sham controls (P < 0.05; Fig. 1C).
During the trace interval (or trace interval equivalent for delay animals), there was a significant main effect of surgery [F(1,27) = 195.02, P < 0.001], but no main effect of training [F(1,27) = 2.29, P = 0.142] and no training × surgery interaction [F(1,27) = 0.04, P = 0.841]. Following both trace and delay conditioning, lesion rats froze significantly less than shams during the 28-sec period following the tone (P < 0.05; Fig. 1C).
Context test
The average percentage of time spent freezing over the entire 8 min of the context test was calculated (Fig. 1D). There was a significant main effect of surgery [F(1,27) = 11.41, P < 0.01], but no main effect of training [F(1,27) = 0.82, P = 0.372] and no training × surgery interaction [F(1,27) < 0.01, P = 0.975]. Following both trace and delay conditioning, lesion rats froze significantly less than sham rats during the context test (P < 0.05).
Experiment 2: basal amygdalar protein synthesis is necessary for the consolidation of trace and contextual conditioned fear memory
Immediately following trace, delay, or unpaired fear conditioning, rats received bilateral infusions of either cycloheximide or vehicle targeting the basal nucleus of the amygdala. Over the next 2 d, rats were tested for freezing to both tone and context in separate sessions (see Fig. 2A).
Figure 2.
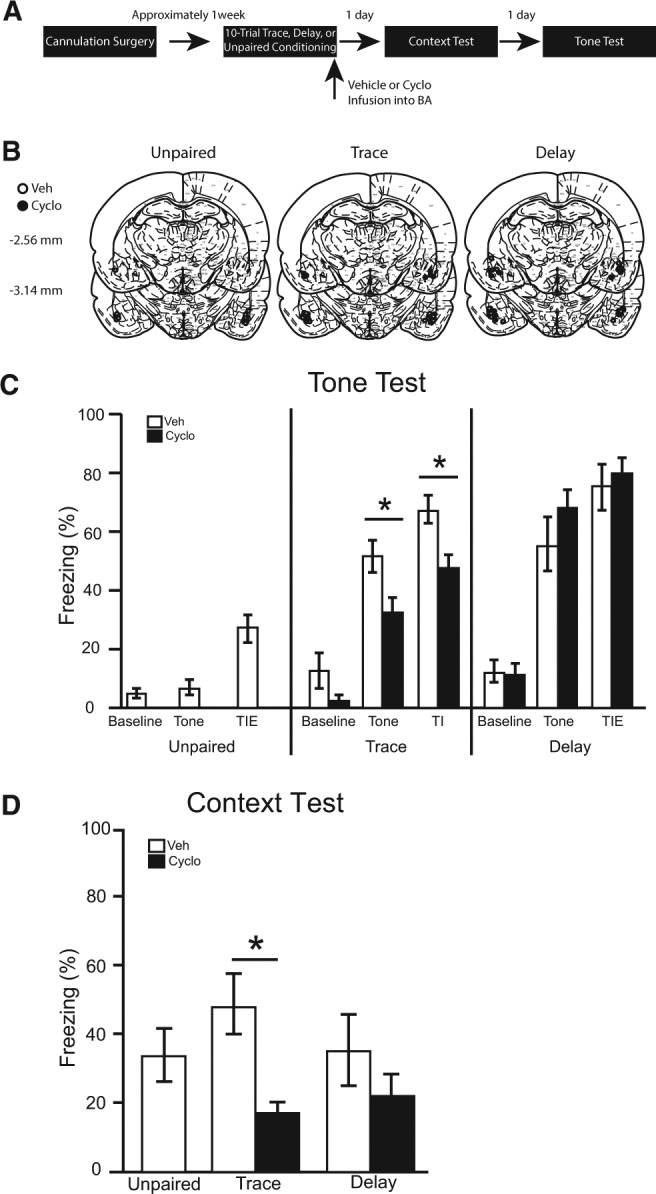
(A) Timeline for Experiment 2. (B) Cannula placement for all animals included in Experiment 2 (atlas images taken and modified from Paxinos and Watson 1998 with permission from Elsevier 1998). The number of animals in each group was as follows: unpaired veh, n = 9, trace veh, n = 11; trace cyclo, n = 11; delay veh, n = 10; delay cyclo, n = 10; N = 51. (C) The percentage of time spent freezing during baseline period (first 3 min), tone, and trace interval or trace interval equivalent during the tone test. (D) Simultaneously learned contextual fear expressed during the context test.
Verification of infusion location
Brains were sliced and stained with cresyl violet to verify cannulae placements. Three rats were excluded from statistical analysis due to misplaced cannulae. The cannulae placements in the remaining 51 rats were deemed acceptable and included in all statistical analyses (see Fig. 2B).
Tone test
During the 180-sec baseline period of the tone test, no differences were observed among groups [F(4,46) = 1.58, P = 0.195]. Further, among trace and delay conditioned animals, there were no main effects of training [F(1,38) = 1.01, P = 0.322] or infusion [F(1,38) = 1.88, P = 0.178] and no interaction [F(1,38) = 1.26, P = 0.268] (Fig. 2C).
Average freezing during the test tones was significantly different in vehicle-infused rats as a function of training condition [F(2,27) = 16.04, P < 0.001]. Pairwise comparisons revealed that both trace and delay vehicle-infused rats froze significantly more than unpaired controls (P < 0.05), demonstrating that the freezing in trace and delay animals results from associative processes. Among trace and delay conditioned rats, there was a significant main effect of training [F(1,38) = 9.78, P < 0.01], and a significant training × infusion interaction [F(1,38) = 6.37, P < 0.05], but no main effect of infusion [F(1,38) = 0.28, P = 0.598]. Pairwise comparisons revealed a significant deficit in tone freezing for cycloheximide infusions in trace, but not delay, conditioned animals (Fig. 2C).
During the trace interval (or trace interval equivalent for unpaired and delay conditioned animals), freezing differed significantly in vehicle-infused rats as a function of training condition [F(2,27) = 17.42, P < 0.001]. Pairwise comparisons revealed that both trace and delay vehicle-infused rats froze significantly more than unpaired controls (P < 0.05), showing that the freezing during this period continues to be a result of associative learning. Among trace and delay conditioned rats, there was a significant main effect of training [F(1,38) = 12.61, P = 0.001], and a significant training × infusion interaction [F(1,38) = 4.77, P < 0.05], but no main effect of infusion [F(1,38) = 1.75, P = 0.194]. Pairwise comparisons revealed a significant deficit in trace interval freezing for cycloheximide infusions in trace, but not delay, conditioned animals (Fig. 2C).
Context test
The average percentage of time spent freezing over the entire 8 min of the context test was calculated (Fig. 2D). Among vehicle-infused rats, there were no significant differences in context freezing as a function of training [F(2,27) = 0.76, P = 0.478]. In trace and delay conditioned animals, there was a significant main effect of infusion [F(1,38) = 8.65, P < 0.05], but no main effect of training [F(1,38) = 0.26, P = 0.613] and no training × infusion interaction [F(1,38) = 1.31, P = 0.259]. A priori planned comparisons revealed that cycloheximide infusions following trace conditioning attenuated freezing compared with vehicle infusions [P < 0.05]. However, following delay conditioning, cycloheximide had no significant effect on context freezing [P > 0.05].
Experiment 3: lateral amygdalar protein synthesis is necessary for the consolidation of context conditioned fear memory
Immediately following trace or delay fear conditioning, rats received bilateral infusions of either cycloheximide or vehicle targeting the basal nucleus of the amygdala. Over the next 2 d, rats were tested for freezing to both tone and context in separate sessions (see Fig. 3A).
Figure 3.
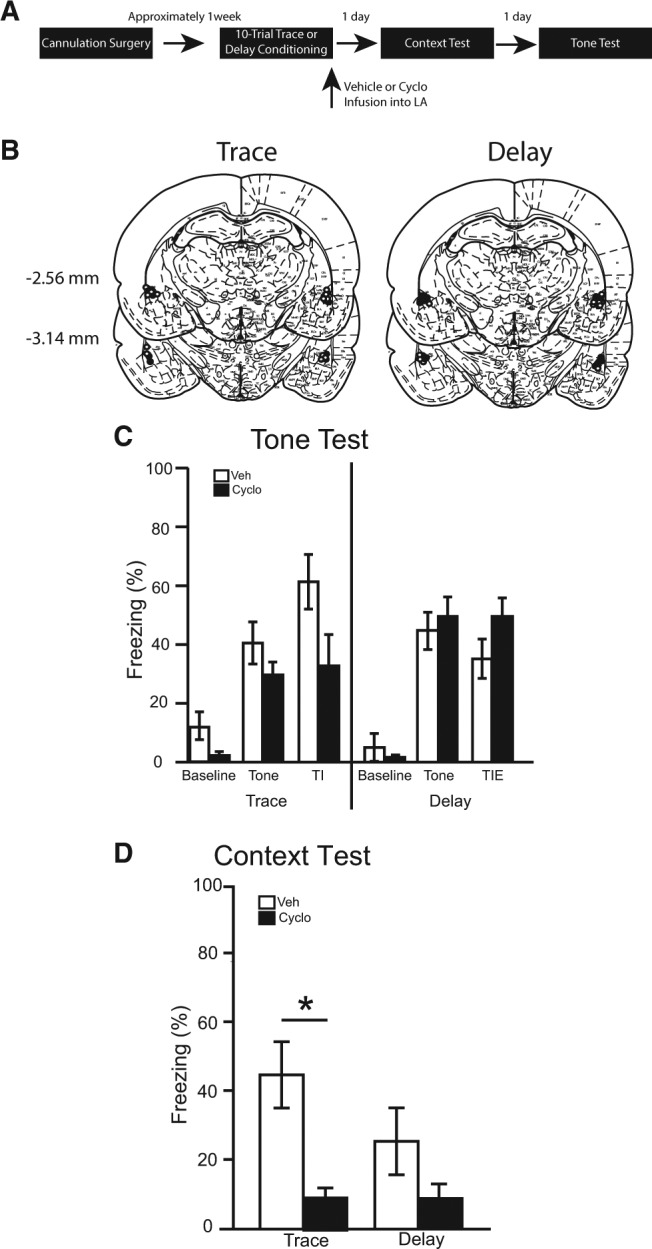
(A) Timeline for Experiment 3. (B) Cannula placement for all animals included in Experiment 3 (atlas images taken and modified from Paxinos and Watson 1998 with permission from Elsevier 1998). The number of animals in each group was as follows: trace veh, n = 8; trace cyclo, n = 8; delay veh, n = 9; delay cyclo, n = 10; N = 35. (C) The percentage of time spent freezing during the baseline period (first 3 min), tone, and trace interval or trace interval equivalent during the tone test. (D) Simultaneously learned contextual fear expressed during the context test.
Verification of infusion location
Brains were sliced and stained with cresyl violet to verify cannulae placements. Fifteen rats were excluded from statistical analysis due to misplaced cannulae. The cannulae placements in the remaining 35 rats were deemed acceptable and included in all statistical analyses (see Fig. 3B).
Tone test
During the 180-sec baseline period of the tone test, no differences were observed among groups [F(3,31) = 2.08, P = 0.123]. Further, among trace and delay conditioned animals, there were no main effects of training [F(1,31) = 1.34, P = 0.255] or infusion [F(1,31) = 4.06, P = 0.053] and no interaction [F(1,31) = 1.09, P = 0.304] (Fig. 3C).
During the tone period of the tone test, no differences were observed among groups [F(3,31) = 1.99, P = 0.137]. Further, there were no main effects of training [F(1,31) = 3.83, P = 0.059] or infusion [F(1,31) = 0.36, P = 0.555] and no interaction [F(1,31) = 1.80, P = 0.189] (Fig. 3C).
Similarly, during the trace interval or trace interval equivalent period of the tone test, no differences were observed among groups [F(3,31) = 2.5, P = 0.077]. Further, there were no main effects of training [F(1,31) = 0.36, P = 0.551] or infusion [F(1,31) = 0.77, P = 0.338]. However, a significant training × infusion interaction was revealed [F(1,31) = 6.78, P < 0.05] (Fig. 3C). An a priori planned comparison revealed that trace conditioned animals infused with cycloheximide trended toward differing from vehicle controls, but did not reach significance [P = 0.062].
Context test
The average percentage of time spent freezing over the entire 8 min of the context test was calculated (Fig. 3D). Among vehicle-infused rats, there were no significant differences in context freezing as a function of training [F(1,15) = 1.98, P = 0.180]. In trace and delay conditioned animals, there was a significant main effect of infusion [F(1,31) = 12.98, P < 0.001], but no main effect of training [F(1,31) = 1.81, P = 0.198] and no training × infusion interaction [F(1,31) = 1.76, P = 0.195]. A priori planned comparisons revealed that cycloheximide infusions following trace conditioning attenuated freezing compared with vehicle infusions [P < 0.01]. However, following delay conditioning, cycloheximide had no significant effect on context freezing [P > 0.05].
Experiment 4: basal amygdalar protein synthesis is necessary for the consolidation of contextual, but not 3-trial delay, conditioned fear memory
Immediately following 3-trial delay fear conditioning, rats received bilateral infusions of either cycloheximide or vehicle targeting the basal nucleus of the amygdala. Over the next 2 d, rats were tested for freezing to both tone and context in separate sessions (see Fig. 4A).
Figure 4.
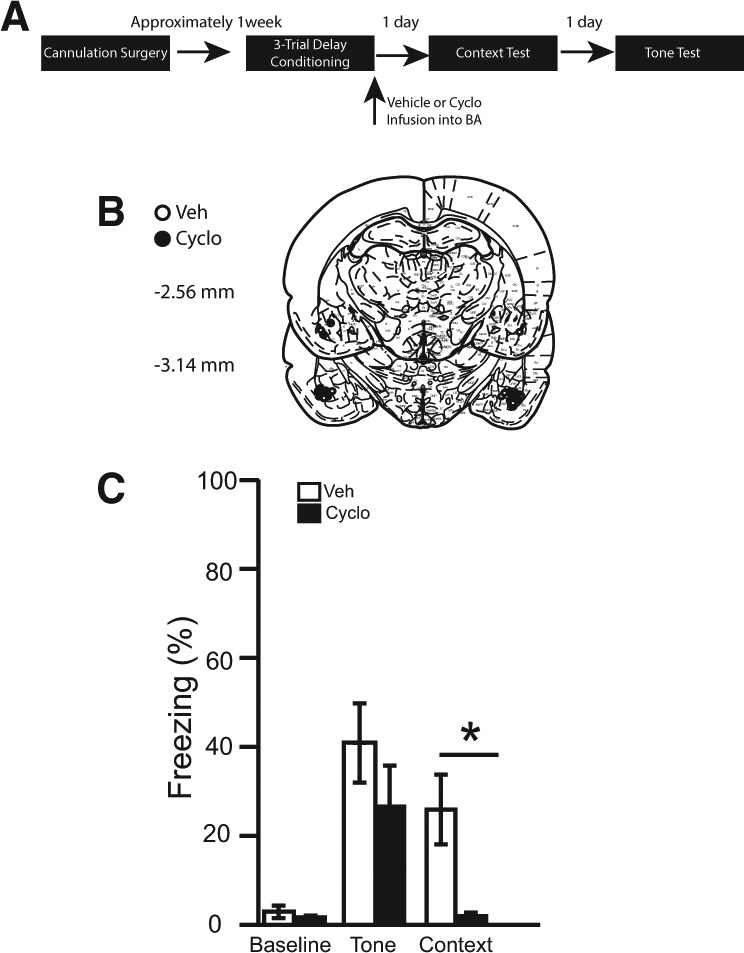
(A) Timeline for Experiment 4. (B) Cannula placement for all animals included in Experiment 4 (atlas images taken and modified from Paxinos and Watson 1998 with permission from Elsevier 1998). The number of animals in each group was as follows: delay veh, n = 11; delay cyclo, n = 11; N = 22. (C) The percentage of time spent freezing during the baseline period (first 3 min), tone, and context.
Verification of infusion location
Brains were sliced and stained using cresyl violet to verify cannulae placements. Two rats were excluded from statistical analysis due to misplaced cannulae. The cannulae placements in the remaining 22 rats were deemed acceptable and included in all statistical analyses (see Fig. 4B).
Tone test
During the 180-sec baseline period of the tone test, no differences were observed among infusion groups [t(20) = 0.90, P = 0.38]. In addition, no differences were observed between infusion groups during the tone [t(20) = 1.11, P = 0.28] (see Fig. 4C).
Context test
The average percentage of time spent freezing over the entire 8 min of the context test was calculated (Fig. 4C). It was revealed that post-training infusions of cycloheximide significantly attenuated freezing relative to controls [t(20) = 3.04, P < 0.01].
Experiment 5: lateral amygdalar protein synthesis is necessary for the consolidation of three-trial delay and contextual conditioned fear memory
Immediately following three-trial delay fear conditioning, rats received bilateral infusions of either cycloheximide or vehicle targeting the lateral nucleus of the amygdala. Over the next 2 d, rats were tested for freezing to both tone and context in separate sessions (see Fig. 5A).
Figure 5.
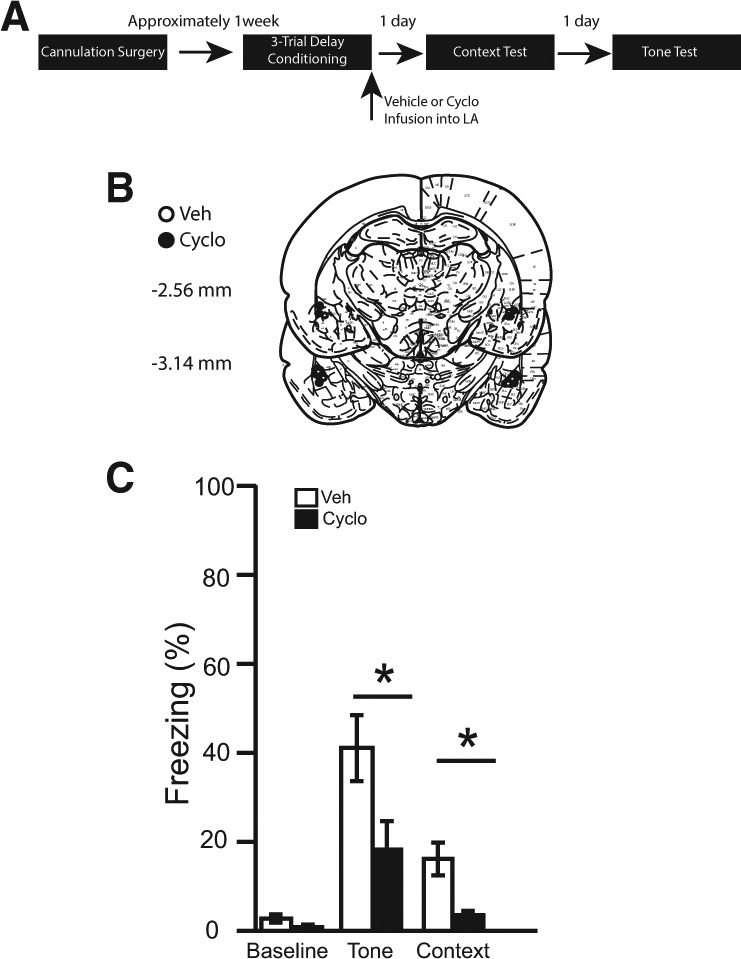
(A) Timeline for Experiment 5. (B) Cannula placement for all animals included in Experiment 5 (atlas images taken and modified from Paxinos and Watson 1998 with permission from Elsevier 1998). The number of animals in each group was as follows: delay veh, n = 10; delay cyclo, n = 10; N = 20. (C) The percentage of time spent freezing during the baseline period (first 3 min), tone, and context.
Verification of infusion location
Brains were sliced and stained with cresyl violet to verify cannulae placements. Four rats were excluded from statistical analysis due to misplaced cannulae. The cannulae placements in the remaining 20 rats were deemed acceptable and included in all statistical analyses (see Fig. 5B).
Tone test
During the 180-sec baseline period of the tone test, no differences were observed among infusion groups [t(18) = 1.843, P = 0.08]. It was revealed that post-training infusions of cycloheximide significantly attenuated freezing relative to controls during the tone, [t(18) = 2.35, P < 0.05] (see Fig. 5C).
Context test
The average percentage of time spent freezing over the entire 8 min of the context test was calculated (Fig. 5C). It was revealed that post-training infusions of cycloheximide significantly attenuated freezing relative to controls [t(18) = 3.32, P < 0.01].
Discussion
The present data provide strong support for the involvement of the basolateral amygdala in trace fear conditioning (see also Kwapis et al. 2011). Pretraining lesions of the BLA disrupt freezing to tone and context in both trace and delay conditioned animals. Post-training infusions of the protein synthesis inhibitor, cycloheximide, into the BA attenuate freezing during the tone, trace interval, and context test in trace conditioned rats. However, similar infusions into the BA had no significant effect on three- or 10-trial delay fear conditioning. By contrast, post-training infusions of cycloheximide into the LA disrupt three-trial delay and context freezing, but have no significant effect on trace or 10-trial delay fear memory consolidation. These data suggest that trace and delay fear conditioning may be differentially distributed in the BA and LA, respectively.
In the present series of experiments, 10 acquisition trials initially were used for both trace and delay fear conditioning. While 10 trials is typical for studies of trace fear conditioning in order to acquire a robust fear response to the tone, delay conditioning can be acquired using fewer tone–footshock pairings. Thus, 10 trials of delay conditioning yield very strong conditioning with asymptotic responding. It is possible that the lack of a deficit in cycloheximide-infused 10-trial delay conditioned animals (in Experiment 2) is a function of overtraining, rather than evidence of BA-independent delay conditioning. However, previous studies have shown that even animals given 75 overtraining trials using delay conditioning with an intact BLA subsequently display a significant deficit in freezing to the tone following BLA lesion or inactivation (Ponnusamy et al. 2007; Zimmerman et al. 2007). This suggests that in animals overtrained with intact basal and lateral nuclei of the amygdala, delay fear memory remains dependent upon those nuclei. However, due to the extended length of the training session in our 10-trial delay conditioning (45 min, 40 sec), it is possible that protein synthesis following the initial trials may occur prior to the infusion of cycloheximide that occurs following termination of the entire session. For this reason, a much shorter three-trial procedure with a much shorter session duration (6 min, 48 sec) was used in Experiments 4 and 5. In these experiments, post-training cycloheximide infusions targeting the LA, but not BA, attenuated freezing to the tone. This is consistent with numerous previous reports of LA involvement in delay fear conditioning (Schafe and LeDoux 2000; Pape and Pare 2010; Kwapis et al. 2011).
Raybuck and Lattal (2011) demonstrated that muscimol inactivation of the amygdala impaired delay, but not trace, fear conditioning in mice. The discrepancy between their findings and ours (as well as those of Kwapis et al. 2011) might be explained by a number of differences in our approaches. The present study and Kwapis et al. (2011) used rats rather than mice. Additionally, these rat studies used more conditioning trials than did Raybuck and Lattal (2011), who used one, two, or four trials. However, this does not seem an entirely sufficient explanation as animals in all studies froze to the CS at reasonable levels during testing. The specific pharmacological manipulation may provide a better explanation. Raybuck and Lattal (2011) inactivated the amygdala using muscimol, while the present experiments and Kwapis et al. (2011) inhibited protein synthesis. As noted by Kwapis et al. (2011), protein synthesis and reconsolidation can take place in an inactivated amygdala under some conditions (e.g., Ben Mamou et al. 2006). As such, inactivation via muscimol may fail to prevent the consolidation of trace fear memory where protein synthesis inhibitors are effective. Additionally, it is possible that alternative mechanisms are able to compensate for the amygdala in trace fear conditioning that occurs when the amygdala is inactivated, as trace conditioning critically depends upon a number of other structures such as the hippocampus (e.g., Quinn et al. 2005) and medial prefrontal cortex (Gilmartin and Helmstetter 2010). Under some conditions, learning that is normally hippocampus-dependent can be acquired via alternative mechanisms if the hippocampus has been inactivated (e.g., Rudy and O'Reilly 1999; Wiltgen et al. 2006). It is possible that the inactivation procedure used by Raybuck and Lattal (2011) facilitated the use of extra-amygdalar compensatory mechanisms, while protein synthesis inhibition used in the present experiment and by Kwapis et al. (2011) did not. Thus, amygdalar protein synthesis inhibition results in deficits in trace fear conditioning, while muscimol inactivation may not.
Some studies suggest that the BA is important in conditioned fear (e.g., Sananes and Davis 1992) and, more specifically, delay fear conditioning (Goosens and Maren 2001; Amano et al. 2011). However, other sources suggest that the BA does not play a role in delay fear conditioning (e.g., Killcross et al. 1997; Amorapanth et al. 2000; Nader et al. 2001). Similarly, we observe no deficit in delay fear conditioning as a result of post-training administration of cycloheximide into the BA using either a 10- or three-trial conditioning procedure. Differences in procedure may account for discrepant results. Goosens and Maren (2001) utilized a pretraining lesion procedure in which rats received a large electrolytic lesion of the amygdala on one side, and a nucleus-specific neurotoxic lesion on the contralateral side. Lesions targeting the BA resulted in deficits to delay fear conditioning, but had no effect if the anterior portion of the BA was spared. It is possible that our cycloheximide infusions similarly spared the most anterior portion of the BA. Alternatively, it is also possible that while lesions of the BA disrupt delay conditioning, the formation of this association does not depend upon de novo protein synthesis in the BA. Finally, fibers of passage that would be destroyed with an electrolytic lesion of the BA are spared during cycloheximide infusion, which may account for differences in the two manipulations. However, it is important to mention that Amano et al. (2011) found that a substantial portion of BA neurons acquire excitatory responses to the CS during delay fear conditioning. Specifically, basomedial responses persist long after CS-offset, suggesting that they are not merely passive relays of rapidly adapting LA input. Additionally, they demonstrated that pretesting muscimol inactivation of the entire BA (including medial and lateral portions) attenuated freezing to the tone. This result strengthens the possibility that our cycloheximide infusions may have partially spared the BA.
Post-training BA infusions of cycloheximide produced a deficit in contextual fear conditioning in trace, as well as three-trial delay, conditioned animals. This was an expected result, as there is strong evidence that the BA is critical for contextual fear conditioning (Muller et al. 1997; Goosens and Maren 2001; Vlachos et al. 2011). However, no deficits were observed in contextual fear conditioning in 10-trial delay conditioned animals. This is most likely due to a floor effect, as both vehicle- and cycloheximide-infused delay animals froze at relatively low levels during the context test (see Fig. 2D). In a 10-trial delay conditioning procedure, it is reasonable to expect that conditioning to the context would be relatively weak since the associative strength of the tone is very strong.
Protein synthesis inhibitors are sometimes criticized for their nonspecific effects, such as cell death and catecholamine synthesis inhibition (Flexner and Goodman 1975; Radulovic and Tronson 2008; Rudy 2008). However, there is an established history of experiments examining amygdalar contributions to delay fear conditioning using protein synthesis inhibitors as amnesic agents, (e.g., Bailey et al. 1999; Schafe and LeDoux 2000; Maren et al. 2003; Kwapis et al. 2011). As little is known about amygdalar contributions to trace fear conditioning, it is a sound practice to use a broad approach rather than attempting to target a more specific signaling cascade. Cycloheximide is a less commonly used protein synthesis inhibitor than anisomycin, but it is sometimes preferred as it is easier to keep in solution. There is no evidence to suggest that it is less effective than other protein synthesis inhibitors (e.g., Milekic et al. 2006; Lai et al. 2008), and it has been successfully used in the amygdala as an amnesic agent in a number of studies (e.g., Berman et al. 1978; Duvarci et al. 2005; Pedroso et al. 2013), including the present study.
Though the diffusion extent of cycloheximide was not measured for the present experiments, evidence suggests diffusion was confined to the targeted subnucleus. A labeling study carried out by Parsons et al. (2006) demonstrated that another protein synthesis inhibitor, anisomycin, remained within the boundaries of the amygdala using a similar infusion size (0.5 µL). Similarly, Amano et al. (2011) administered 0.3 µL of 0.5 mM fluorescent muscimol dissolved in aCSF targeting the lateral or medial portion of the BA. Imaging revealed that infusions targeting the individual basal subnuclei were reasonably well-contained 10 min after infusion time. While inactivation of either subnucleus alone had no effect, combined inactivation of the basal medial and basal lateral nuclei resulted in a deficit in delay fear conditioning learning. Finally, the present pattern of behavioral results reveals differential involvement of LA and BA as a function of training condition (delay vs. trace). This suggests that our cycloheximide infusions were relatively well contained within the targeted amygdala nucleus. Protein synthesis in the lateral amygdala has been shown to be critical for the consolidation of delay fear conditioning (e.g., Schafe and LeDoux 2000; Kwapis et al. 2011), and LA, but not BA, infusions of cycloheximide disrupted three-trial delay conditioning in the present experiments.
In conclusion, the present data support a role for the BLA in trace, delay, and contextual fear conditioning. Trace fear conditioning appears to be more dependent upon BA processing, since infusions of cycloheximide into this region, but not into the LA, disrupt consolidation of trace fear memories. However, it is worth noting the trend toward a deficit following cycloheximide infusions into LA. Consistent with previous findings, delay fear conditioning appears to be more dependent upon LA processing, since infusions of cycloheximide into this region, but not into the BA, disrupt consolidation of delay fear memories (at least when using a three-trial delay procedure). This dissociation is strengthened by a recent finding showing that expression of the immediate early gene, activity-regulated cytoskeleton-associated protein (Arc/Arg3.1), is elevated in BA, but not LA, following trace fear conditioning (Chau et al. 2013).
Materials and Methods
Animals
All rats were experimentally naïve Long-Evans rats. Thirty-six female rats were bred in-house for use in Experiment 1. One hundred two male rats were purchased from Harlan Laboratories (Indianapolis, IN) for use in Experiments 2, 4, and 5. Fifty male rats were bred in-house for use in Experiment 3. All rats were pair-housed in standard colony caging on a 12:12-h light:dark cycle and given ad libitum access to food and water. The rats were handled for 1 min per day for five consecutive days prior to surgery. All procedures were performed during the light cycle and were approved by the Miami University Institutional Animal Care and Use Committee in accordance with the NIH Guidelines for the Care and Use of Experimental Animals.
Lesion surgery
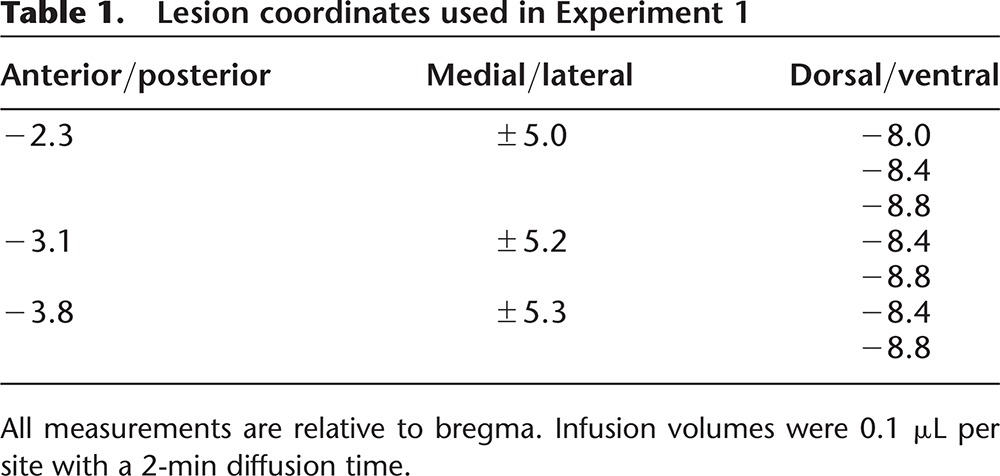
Rats were anesthetized with 5% isoflurane (Vedco) in an induction chamber. They were placed in a standard stereotaxic instrument and maintained on 2%–3% isoflurane at 1 L/min. Body temperature was maintained on a heating pad located under the rat throughout surgery. The scalp was shaved, incised, and retracted. The head was leveled by equating bregma and λ in the horizontal plane. Stainless steel tubing (28 gauge; Plastics One) connected to 10 μL Hamilton syringes using clear polyethylene tubing (PE20) were lowered into the brain bilaterally targeting the basolateral amygdala. For coordinates, see Table 1. N-Methyl-D-aspartate (NMDA; 20 µg/µL; Sigma-Aldrich) was infused into each site (0.1 µL/site), followed by a 2-min diffusion time. Following the last infusion, the skull was dried and the scalp was closed using stainless steel wound clips. Sham surgery consisted of the incision, retraction, and closing of the scalp; no infusions of any kind were administered. At the end of surgery, the rats were given two subcutaneous injections: 3 mL of 0.9% saline for rehydration and 5 mg/kg/mL of Rimadyl to reduce pain and inflammation. Following surgery, the rats were placed into a recovery cage on a heating pad until they fully awoke from anesthesia. Post-operative care was performed for five consecutive days after surgery. Rimadyl (5 mg/kg/mL; s.c.) was administered at 24 and 48 h post-surgery. Saline (0.9%; 3 mL; s.c.) was given as needed for signs of dehydration.
Table 1.
Lesion coordinates used in Experiment 1
Cannulation surgery
Rats were anesthetized and skulls were leveled as described previously. Guide cannulae (22 gauge; Plastics One) were lowered into the brain bilaterally targeting the BA or LA using the following coordinates: BA (AP −3.0 mm, ML ± 5.3 mm, DV −7.9 mm); LA (AP −2.9 mm, ML ± 5.0 mm, DV −6.8 mm) relative to bregma (Paxinos and Watson 1998). Four skull screws and dental acrylic were used to secure the guide cannulae within the skull. Obturators were placed into the guide cannulae to prevent debris from entering. Following surgery, post-operative care was administered as described above.
Behavioral apparatus
Animals were fear conditioned and context tested in four identical Context A chambers (32.4 × 25.4 × 21.6 cm; MED-Associates, Inc.). The ceiling and front door of each chamber were made of clear Plexiglas, the back wall was white Plexiglas and the two side walls were aluminum. The floor consisted of 19 equally spaced stainless steel rods. The grid floor in each chamber was wired to a shock generator and scrambler (MED-Associates, Inc.). The conditioning chambers were wiped down with an odorless 5% sodium hydroxide solution and scented with 50% vanilla flavor (Meijer) solution. The chamber was brightly lit (125 lux) by a light box located above the conditioning chamber.
Animals were tested for freezing to tone in Context B. These chambers (32.4 × 25.4 × 21.6 cm; MED-Associates, Inc.) were located in a different experimental room and were distinct from Context A. They consisted of a Plexiglas floor and a Plexiglas equilateral triangular insert. The context was cleaned and scented with a 1% glacial acetic acid solution. The light box above the chamber provided near-infrared lighting (0 lux).
The rats were continuously monitored by a progressive scan video camera with a visible light filter (VID-CAM-MONO-2A; MED-Associates, Inc.) connected to a computer in the experimental room running Video-Freeze software (MED-Associates, Inc.) designed for automated assessment of defensive freezing (see Anagnostaras et al. 2010).
Infusions
Injectors (28 gauge) were connected to 10 μL Hamilton syringes using clear polyethylene tubing (PE20). The injectors were inserted into the cannulae so that they extended 1 mm below the guide. All infusions were delivered via an infusion pump (KD Scientific, Inc.) at a rate of 0.1 μL/min for 5 min. Rats were placed in plastic bins with ∼3 cm standard bedding during infusions, and were left for 4 min following infusion to allow for diffusion. In Experiments 2–5, the protein synthesis inhibitor, cycloheximide (50 mg/mL; Sigma-Aldrich, Inc.), was dissolved in 50%DMSO/50%aCSF and infused bilaterally into the BA or LA. In control rats, the vehicle was infused into the same location at the same rate and duration.
Procedure Experiment 1
Rats were randomly assigned to one of four conditions: (1) trace conditioned rats that received pretraining sham surgeries; (2) trace conditioned rats that received pretraining BLA lesions; (3) delay conditioned rats that received pretraining sham surgeries; and (4) delay conditioned rats that received pretraining BLA lesions. Trace conditioned rats were given a 120-sec acclimation period, followed by 10 trials consisting of a 16-sec tone (2 kHz), followed by a 28-sec trace interval and then a 2-sec footshock (0.9 mA). Delay conditioned rats were given a 120-sec acclimation period, followed by 10 trials consisting of a 16-sec tone (2 kHz), coterminating with a 2-sec footshock (0.9 mA). The intertrial interval (ITI) was 256 sec (tone onset to tone onset). The session durations for trace and delay conditioning were equal. On day 2, all rats were tested for context freezing in Context A during an 8-min session. Freezing is defined as the absence of all movement except that necessary for respiration (e.g., Fanselow 1980), with significant muscle tone. On day 3, rats underwent tone testing in a novel context (Context B), which consisted of a 180-sec baseline period, followed by three discrete tone presentations separated by 256 sec.
Procedure Experiment 2
Rats were randomly assigned to one of five conditions: (1) unpaired controls that received post-training vehicle infusions; (2) trace conditioned rats that received post-training vehicle infusions; (3) trace conditioned rats that received post-training cycloheximide infusions; (4) delay conditioned rats that received post-training vehicle infusions; and (5) delay conditioned rats that received post-training cycloheximide infusions. The procedure was identical to Experiment 1 except that an unpaired training condition was included. The unpaired conditioned rats were given a 120-sec acclimation period, followed by 10 tones and then 10 footshocks, or vice versa. The interstimulus interval (ISI) was 130 sec (stimulus onset to stimulus onset). Session duration was equal to that of trace and delay conditioned animals. Additionally, rats underwent pretraining cannulation surgery targeting the BA, and received immediate post-training infusions of vehicle or cycloheximide.
Procedure Experiment 3
Rats were randomly assigned to one of four conditions: (1) trace conditioned rats that received post-training vehicle infusions; (2) trace conditioned rats that received post-training cycloheximide infusions; (3) delay conditioned rats that received post-training vehicle infusions; and (4) delay conditioned rats that received post-training cycloheximide infusions. The procedure was identical to Experiment 2 except that an unpaired training condition was not included, and post-training infusions targeted the LA.
Procedure Experiment 4
Rats were randomly assigned to one of two conditions: (1) delay conditioned rats that received post-training vehicle infusions and (2) delay conditioned rats that received post-training cycloheximide infusions. Delay conditioning consisted of three tone–footshock trials using a 16-sec tone coterminating with a 2-sec footshock. The ITI was 60 sec and the session duration was 6 min, 48 sec. Infusions targeted the BA.
Procedure Experiment 5
Rats were randomly assigned to one of two conditions: (1) delay conditioned rats that received post-training vehicle infusions and (2) delay conditioned rats that received post-training cycloheximide infusions. The procedure was identical to that of Experiment 4, except that infusions targeted the LA.
Histology
GFAP and NeuN immunofluorescence staining
At the end of behavioral testing in Experiment 1, rats were anesthetized with 0.2 mL Euthasol i.p. (Virbac Animal Health, Inc.; 390 mg pentobarbital sodium + 50 mg phenytoin sodium per mL). The rats were perfused intracardially with a phosphate buffered saline solution followed by 0.4% paraformaldehyde. Brains were removed and placed into 0.4% paraformaldehyde. One day later, each brain was transferred into a 30% glycerol in phosphate buffered saline solution. Brains were frozen and sliced on a cryostat in 40 μm coronal sections. Sections were stored in 0.1% sodium azide in well plates until immunohistochemical staining. Antibodies were directed against: (1) the astrocyte marker glial fibrillary acidic protein (GFAP) and (2) the neuronal nuclei marker NeuN.
Following a series of washes in 0.1 M PBS, sections were incubated overnight in 0.1 M PBS-0.2% Triton-X solution, blocked with normal donkey serum, and then incubated for 48 h at 4°C in primary antibody: Mouse anti-NeuN (Millipore MAB377) and chicken anti-GFAP (Abcam AB64674) diluted in 0.1 M PBS. Following a series of rinses, sections were incubated for 2 h in AlexaFluor conjugated antibodies directed toward the primary host antibody (Alexa Fluor 555 Donkey Antimouse, Life Technologies A-31570; Alexa Fluor 488 Donkey AntiChicken, Jackson Immuno 703-545-155). Sections then were rinsed, mounted on slides, and coverslipped using fluorescent mounting medium with DAPI (Vectashield, Vector Labs H-1200). Images were captured using an Olympus AX-70 Research System microscope.
Cresyl violet staining
At the end of behavioral testing in Experiments 2–5, the rats were anesthetized with 0.2 mL Euthasol i.p. (Virbac Animal Health, Inc.; 390 mg pentobarbital sodium + 50 mg phenytoin sodium per mL). To visualize infusion locations, rats were administered 0.5 μL of Cresyl violet acetate (10% in distilled water; Sigma-Aldrich, Inc.) into each site using the same rate and duration of drug infusions. The rats were perfused intracardially with 0.9% saline followed by 10% formalin. Brains were removed and placed into 10% formalin. One day later, each brain was transferred into a 10% formalin/30% sucrose solution. Brains were frozen and sliced on a cryostat in 50 μm coronal sections. Every fourth slice through the amygdala was collected and mounted onto microscope slides. The brain slices were stained with 0.5% thionin (Sigma-Aldrich, Inc.) and coverslipped. Infusion locations were verified using a light microscope by an observer who was blind to the condition and behavior of each animal.
Data analysis
All statistics were calculated using SPSS version 20.0. In Experiments 1–3, factorial (training and infusion or training and surgery) and repeated-measures (tone number and trace interval number) analyses of variance (ANOVAs) were conducted to analyze the percentage of time spent freezing during the baseline, tone, trace interval, and context periods. In Experiments 4–5, t-tests were conducted to analyze the percentage of time spent freezing during the baseline, tone, and context periods. A priori planned comparisons between groups were performed using Fisher's LSD. A critical value α = 0.05 was used for all analyses.
Acknowledgments
We thank Kevin D. Lash and Samantha L. Hagerty for their contributions to the project. We also thank Matt Duley of the Center for Advanced Microscopy and Imaging at Miami University for his assistance with imaging. This work was supported by R15 MH100689 and a grant from the Miami University College of Arts and Science (J.J.Q.). Additional support was provided by Miami University Undergraduate Research Awards (E.A.F., Kevin D. Lash, and A.F.P), Miami University Undergraduate Summer Scholar Awards (E.A.F. and A.F.P.), Miami University Undergraduate Dean Scholar Awards (E.C.T. and E.A.F.), and a Miami University DUOS Award (E.A.F. and D.E.K.).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.034918.114.
References
- Amano T, Duvarci S, Popa D, Paré D 2011. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci 31: 15481–15489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorapanth P, LeDoux JE, Nader K 2000. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci 3: 74–79. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Wood SC, Shuman T, Cai DJ, Leduc AD, Zurn KR, Zurn JB, Sage JR, Herrera GM 2010. Automated assessment of Pavlovian conditioned freezing and shock reactivity in mice using the video freeze system. Front Behav Neurosci 10.3389/fnbeh.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DJ, Kim JJ, Sun W, Thompson RF, Helmstetter FJ 1999. Acquisition of fear conditioning in rats requires the synthesis of mRNA in the amygdala. Behav Neurosci 113: 276–282. [DOI] [PubMed] [Google Scholar]
- Barot SK, Chung A, Kim JJ, Bernstein IL 2009. Functional imaging of stimulus convergence in amygdalar neurons during Pavlovian fear conditioning. PLoS One 4: e6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Mamou C, Gamache K, Nader K 2006. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci 9: 1237–1239. [DOI] [PubMed] [Google Scholar]
- Berman R, Kesner R, Partlow L 1978. Passive avoidance impairment in rats following cycloheximide injection into the amygdala. Brain Res 158: 171–188. [DOI] [PubMed] [Google Scholar]
- Chau LS, Prakapenka A, Fleming SA, Davis AS, Galvez R 2013. Elevated Arc/Arg 3.1 protein expression in the basolateral amygdala following auditory trace-cued fear conditioning. Neurobiol Learn Mem 106: 127–133. [DOI] [PubMed] [Google Scholar]
- Chowdhury N, Quinn JJ, Fanselow MS 2005. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behav Neurosci 119: 1396–1402. [DOI] [PubMed] [Google Scholar]
- Davis M 1997. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci 9: 382–402. [DOI] [PubMed] [Google Scholar]
- Davis M 2006. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol 61: 741–756. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE 2005. Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci 21: 283–289. [DOI] [PubMed] [Google Scholar]
- Fanselow MS 1980. Conditional and unconditional components of post-shock freezing. Pavlov J Biol Sci 15: 177–182. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE 1999. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23: 229–232. [DOI] [PubMed] [Google Scholar]
- Flexner L, Goodman RH 1975. Studies on memory: inhibitors of protein synthesis also inhibit catecholamine synthesis. Proc Natl Acad Sci 72: 4660–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS 2004. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci 24: 3810–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ 2010. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem 17: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD 2005a. Single neurons in the dentate gyrus and CA1 of the hippocampus exhibit inverse patterns of encoding during trace fear conditioning. Behav Neurosci 119: 164–179. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD 2005b. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci 119: 1496–1510. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ 2012. Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiol Learn Mem 97: 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S 2001. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav Neurosci 117: 738–750. [DOI] [PubMed] [Google Scholar]
- Han CJ, O'Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, Anderson DJ 2003. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc Natl Acad Sci 100: 13087–13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killcross S, Robbins TW, Everitt BJ 1997. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature 388: 377–380. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Schiff JC, Helmstetter FJ 2011. Memory consolidation in both trace and delay fear conditioning is disrupted by intra-amygdala infusion of the protein synthesis inhibitor anisomycin. Learn Mem 18: 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YT, Fan HY, Cherng CG, Chiang CY, Kao GS, Yu L 2008. Activation of amygdaloid PKC pathway is necessary for conditioned cues-provoked cocaine memory performance. Neurobiol Learn Mem 90: 164–170. [DOI] [PubMed] [Google Scholar]
- LeDoux JE 1993. Emotional memory systems in the brain. Behav Brain Res 58: 69–79. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ 1988. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8: 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Choi JS, Brown TH, Kim JJ 2001. Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. J Neurosci 21: 4116–4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S 2008. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci 28: 1661–1666. [DOI] [PubMed] [Google Scholar]
- Maren S, Ferrario CR, Corcoran KA, Desmond TJ, Frek KA 2003. Protein synthesis in the amygdala, but not the auditory thalamus, is required for consolidation of Pavlovian fear conditioning in rats. Eur J Neurosci 18: 3080–3088. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF 1998. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus 8: 638–646. [DOI] [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM 2006. Persistent disruption of an established morphine conditioned place preference. J Neurosci 26: 3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Corodimas KP, Fridel Z, LeDoux JE 1997. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci 111: 683–691. [DOI] [PubMed] [Google Scholar]
- Nader K, Majidishad P, Amorapanth P, LeDoux JE 2001. Damage to the lateral and central, but not other, amygdaloid nuclei prevents the acquisition of auditory fear conditioning. Learn Mem 8: 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape H, Pare D 2010. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Baruch DE, Riedner BA, Helmstetter FJ 2006. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. Eur J Neurosci 23: 1853–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C 1998. The rat brain in stereotaxic coordinates, 4th ed Academic Press, San Diego, CA. [Google Scholar]
- Pedroso TR, Jobim PFC, Carvalho LM, Christoff RR, Maurmann N, Reolon GK, Werenicz A, Roesler R 2013. Inhibition of protein synthesis or mTOR in the basolateral amygdala blocks retrieval-induced memory strengthening. J Neural Transm 120: 1525–1531. [DOI] [PubMed] [Google Scholar]
- Ponnusamy R, Poulos AM, Fanselow MS 2007. Amygdala-dependent and amygdala-independent pathways for contextual fear conditioning. Neuroscience 147: 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JJ, Ommen SS, Morrison GE, Fanselow MS 2002. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus 12: 495–504. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS 2005. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus 15: 665–674. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Wied HM, Ma QD, Tinsley MR, Fanselow MS 2008. Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus 18: 640–654. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Wied HM, Liu D, Fanselow MS 2009. Post-training excitotoxic lesions of the dorsal hippocampus attenuate generalization in auditory delay fear conditioning. Eur J Neurosci 29: 1692–1700. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Pittenger C, Lee AS, Pierson JL, Taylor JR 2013. Striatum-dependent habits are insensitive to both increases and decreases in reinforcer value in mice. Eur J Neurosci 37: 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Tronson NC 2008. Protein synthesis inhibitors, gene superinduction and memory: too little or too much protein? Neurobiol Learn Mem 89: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Lattal KM 2011. Double dissociation of amygdala and hippocampal contributions to trace and delay fear conditioning. PLoS One 6: e15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy J 2008. Is there a baby in the bathwater? Maybe: some methodological issues for the de novo protein synthesis hypothesis. Neurobiol Learn Mem 89: 219–224. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC 1999. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci 113: 867–880. [DOI] [PubMed] [Google Scholar]
- Sananes CB, Davis M 1992. N-methyl-D-aspartate lesions of the lateral and basolateral nuclei of the amygdala block fear-potentiated startle and shock sensitization of startle. Behav Neurosci 106: 72–80. [DOI] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE 2000. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci 20: RC96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R 2011. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333: 104–107. [DOI] [PubMed] [Google Scholar]
- Vlachos I, Herry C, Lüthi A, Aertsen A, Kumar A 2011. Context-dependent encoding of fear and extinction memories in a large-scale network model of the basal amygdala. PLoS Comput Biol 7: e1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE 2006. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 26: 12387–12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS 2006. Context fear learning in the absence of the hippocampus. J Neurosci 26: 5484–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Rabinak CA, McLachlan IG, Maren S 2007. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem 14: 634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]


