Abstract
Propylene glycol (PG) is a commonly used solvent for oral, intravenous, and topical pharmaceutical agents. Although PG is generally considered safe, when used in high doses or for prolonged periods, PG toxicity can occur. Reported adverse effects from PG include central nervous system (CNS) toxicity, hyperosmolarity, hemolysis, cardiac arrhythmia, seizures, agitation, and lactic acidosis. Patients at risk for toxicity include infants, those with renal or hepatic insuficiency, epilepsy, and burn patients receiving extensive dermal applications of PG containing products. Laboratory monitoring of PG levels, osmolarity, lactate, pyruvate, bicarbonate, creatinine, and anion gap can assist practitioners in making the diagnosis of PG toxicity. Numerous studies and case reports have been published on PG toxicity in adults. However, very few have been reported in pediatric patient populations. A review of the literature is presented.
INDEX TERMS: adverse effects, pediatric, propylene glycol, toxicity
INTRODUCTION
Propylene glycol (1, 2-propanediol) is an organic compound with multiple uses including: drug solvent, humectant food additive, and as a moisturizer in medicines, cosmetics, and tobacco products. Propylene glycol (PG) has been used as a carrier in fragrance oils; non-toxic antifreeze for winterizing drinking water systems; as a coolant in liquid cooling systems; and is the main ingredient in deodorant sticks. In 1982, the US Food and Drug Administration classified PG as a compound that is “generally regarded as safe.” Although considered safe for use as a vehicle for intravenous medications, PG toxicity has been reported.1
Adverse effects of PG have occurred following topical, oral, and intravenous administration.2–,5 The adverse effects associated with PG include CNS toxicity, hyperosmolarity, hemolysis, cardiac arrhythmia, and lactic acidosis.6–,8 Oral and intravenous multivitamin preparations containing PG have been associated with adverse effects. A 15-month-old boy who received large doses of vitamin C suspended in PG had episodes of unresponsiveness, tachypnea, tachycardia, diaphoresis, and hypoglycemia.3 Seizures have been reported following ingestion of PG used as a vehicle to administer vitamin D.4 Hyperosmolarity in small infants has also been reported following the intravenous administration of a multivitamin preparation containing PG.5
In 1974, the World Health Organization recommended a maximum dose of 25 mg/kg/day of PG when ingested as a food additive. This limit does not address its use as a drug solvent. A safe maximum intravenous PG dose has not been reported in the literature. However, one study reported that serum levels of PG greater than 18 mg/dL can be toxic.9 Other investigators have shown that toxicity is most likely to occur when the serum PG concentrations exceed 25 mg/dL.10,11
This review article is intended to aid prescribers in recognizing PG toxicity and provide prescribing guidelines for pediatric patients.
METHODS
A systematic literature search was performed using keywords: propylene glycol, PG, PG toxicity, pediatrics, and neonates. The search was limited to studies between 1970 and 2010, published in English and those involving human participants. The medical literature included case reports, retrospective and prospective studies, prospective controlled observational studies, and review articles. The search was further limited to adult and pediatric abstracts and full text articles relevant to the topic. Reference lists for selected retrieved articles were also reviewed.
PHARMACOKINETICS, PATHOPHYSIOLOGY, AND CLINICAL PRESENTATION
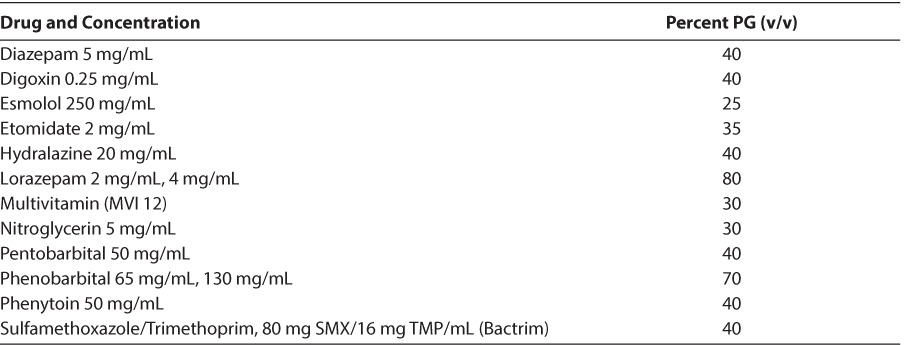
PG is primarily eliminated via the kidneys (12%–45%), with the remainder being metabolized in the liver to form lactate, acetate, and pyruvate.12,13 In adults with normal liver and kidney function, the terminal half-life of PG ranges from 1.4 to 3.3 hours.14 In contrast, the mean half-life is significantly longer in infants 19.3 hours (range, 10.8–30.5 hours) due to decreased renal elimination.2,5 Renal elimination decreases as the dose administered increases (390 mL/min/1.73 m2 at a dose of 5 g/day) versus 144 mL/min/1.73 m2 at a dose of 21 g/day.13,14 A list of intravenous medications containing PG along with their percent PG volume/volume contents is noted in Table 1.
Table 1.
Parenteral Pharmaceutical Preparations Containing Propylene Glycol15
Studies suggest that serum PG concentrations correlate positively with the duration12 or rate13,16 of lorazepam infusion. However, the level beyond which PG accumulation is detrimental is still unknown. Wilson et al17 reported clinical deterioration in patients with PG levels ranging from 104 to 144 mg/dL. In contrast, in subjects with only metabolic abnormalities, the same authors found serum PG levels ranging from 58 to 126 mg/dL. The authors suggest that higher concentrations of PG are more likely to be associated with clinical deterioration.17
Laboratory values can assist a practitioner in making the diagnosis of PG intoxication. The most common include osmolality, lactate and pyruvate concentrations, in addition to bicarbonate level, serum creatinine, and anion gap. A definitive diagnosis is determined by serum PG concentrations. However, most institutions do not have the capability of running PG levels and therefore must send them to a specialty laboratory outside of the institution, which can delay confirmation of the diagnosis.4
Byproducts of alcohol metabolism, i.e., elevated lactate and/or pyruvate levels, are increased in patients receiving PG. The risk of metabolic acidosis increases as levels increase. If the correct diagnosis is not made, a patient can progress to hypotension, acidemia, and multisystem organ dysfunction. PG-induced intoxication can also mimic sepsis and systemic inflammatory response syndrome.17,18 Measurement of serum osmolality and/or a PG level can help distinguish the 2 entities; therefore, sepsis must be ruled out first.
Determination of excess serum organic acids, including lactate and pyruvate, is possible through the calculation of the anion gap. The anion gap is the difference between unmeasured anions and cations. Although many forms of metabolic acidosis are characterized by an abnormal anion gap, the accumulation of lactate, pyruvate, or exogenous toxins such as PG often produces an anion gap greater than 12 mmol/L. In the same way, the osmolal gap is the difference between the measured and the calculated serum osmolalities. This gap is often used as a mechanism for determining the presence of low molecular weight compounds in the serum. The osmolal gap will exceed 10 mOsm/L H2O in 2 clinical conditions: (1) decreased serum water content, in hyperlipidemia or hyperproteinemia, where the calculated osmolality is underestimated because of a falsely low serum sodium concentration, and (2) increased serum concentration of unmeasured, osmotically active, low-molecular weight compounds (<150 daltons), such as mannitol, ethanol, PG, and other alcohols.2,8
Glascow and colleagues5 found a direct relationship between the osmolal gap and serum PG concentrations in infants who received intravenous medications containing PG (PG = 47.5 times osmol gap + 9.2; r = 0.96). Similarly, Fligner et al2 determined that the relationship between PG concentration and osmolal gap after topical administration of silver sulfadiazine was as follows:
This calculation was based on a case report of an 8-month-old infant.
Arroliga and colleagues13 published a theoretical formula for predicting serum PG concentrations from the osmol gap (−82.1 + [osmol gap × 6.5]) in critically ill adults receiving high dose lorazepam. However, the authors noted that this formula needs further validation and may help identify as early as 48 hours those patients at risk for PG toxicity.
In a study by Yahwak et al19 in 2008, the authors found that an osmol gap of 10 or greater was predictive of elevated PG concentrations and values of 12 or greater were predictive of clinical changes suggestive of PG toxicity. The authors also concluded that screening for PG toxicity with the osmol gap may be helpful for patients receiving intravenous lorazepam in doses greater than 1 mg/kg/day or higher.
ADVERSE DRUG REACTIONS AND PREVENTION OF PG TOXICITY
When PG is administered intravenously, adverse effects may present in different ways. With too rapid an infusion, hypotension and cardiac arrhythmias can occur. The combination of PG and its metabolites have been implicated in various clinical scenarios such as hyperosmolarity and metabolic acidosis. These conditions can lead to hemolysis, renal failure, CNS depression with seizures, and various presentations of cardiovascular decompensation.8
Patients with underlying kidney disease, or impaired alcohol dehydrogenase enzyme systems (e.g. children younger than the age of 4, pregnant women, patients with hepatic disease; and patients treated with disulfiram or metronidazole), are all prone to PG accumulation.16 Based on the significant renal clearance of PG, patients with underlying chronic kidney disease or newly developed acute kidney injury would be at greater risk for toxicity.18 Providers should consider a 50% reduction in the maximum daily dose in patients with underlying risk factors (liver disease, renal failure, pregnancy, concurrent treatment with metronidazole or disulfiram, age <4 years).18 Yorgin et al20 reported results from a renal biopsy suggesting that renal impairment caused by PG is attributable to proximal tubular cell injury.
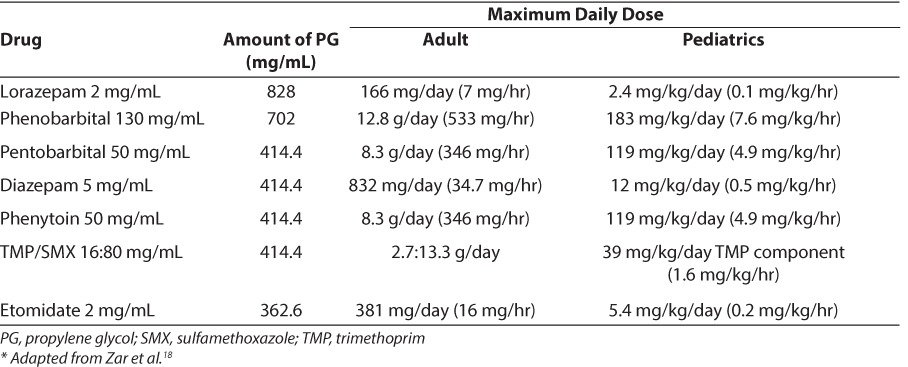
Table 2 provides dose limits of commonly used intravenous drugs to avoid PG toxicity based on a cumulative maximum dose of 69 g/day.18 The maximum daily dose of drug for a pediatric patient can be extrapolated from the adult data (based on a 70-kg patient).
Table 2.
Dose Limits of Commonly Used Intravenous Drugs to Avoid Propylene Glycol Intoxication Based on a Maximum Amount of PG Equal to 69 g/day18
REVIEW OF THE MEDICAL LITERATURE
Pediatric Case Reports of CNS and Cardiotoxicity Associated With PG Toxicity
In a case report by O'Donnell et al,8 a 32-day-old term female developed meconium aspiration pneumonitis at birth and was discharged on day 7 of life but later developed severe respiratory distress. The patient was admitted to the neonatal intensive care unit (NICU), intubated, and started on a lorazepam drip. A pump programming error resulted in the patient receiving 24 mg of lorazepam over a 1.5- to 2-hour period. The drip was discontinued and there was no evidence of PG toxicity. The next morning, the patient was restless and a lorazepam drip was restarted at 2 mg/hr and subsequently increased to 4 mg/hr. The patient continued to receive the lorazepam drip for 8 days with the rate ranging from 2 mg/hr to 7 mg/hr. On day 8, the patient began seizing and the drip was increased to 6 mg/hr, and phenytoin and phenobarbital were added. The patient developed cardiovascular decompensation; dopamine and dobutamine were started, and the serum sodium, calcium, and carnitine levels were monitored. Physicians considered the hyperosmolarity (serum = 672 mOsm/kg) secondary to the PG found in the lorazepam preparation. However, other clinical features not associated with these events were the absence of an increased anion gap, absence of lactic acidosis, and absence of cardiac arrhythmias. The physicians stopped the lorazepam infusion and obtained a serum PG level. The peak anion gap occurred 9.5 hours later. The patient had an elevated serum osmolality that correlated with a wide osmolal gap. In contrast to the anion gap, the osmolal gap peaked during the lorazepam infusion and dropped steadily following its discontinuation. These values correlated with the presence of the PG parent compound.8
In another case report, an 8-month-old developed cardiorespiratory arrest associated with a peak serum PG concentration of 1059 mg/dL.2 The infant was treated with topical silver sulfadiazine for a burn and toxic necrolysis involving 78% of his total body surface area. The day after the cardiac arrest, the osmolal gap (discrepancy between the measured and calculated osmolalities) was 74 mOsm/kg H2O. During the course of the day, the measured serum osmolality increased to a peak of 420 mOsm/kg H2O with a peak osmolal gap of 130 mOsm/kg H2O. The following day, the serum glycol screen showed a large amount of PG, later quantitated at 771 mg/dL. Silver sulfadiazine was discontinued after all other medications were ruled out as a contributing source of PG. The authors reported that during the preceding 70 hours of hospitalization, the patient had received a topical PG dose of 9 g/kg/24 hr. Four days after discontinuation of the silver sulfadiazine, the patient's serum osmolality gradually decreased to 298 mOsm/kg H2O, the osmolal gap normalized to less than 9 mOsm/H2O, and the lactic acidosis resolved.2
SUMMARY OF THE LITERATURE
Pediatric Studies
Shehab et al21 conducted a retrospective, observational study in neonatal and pediatric intensive care unit patients to document neonatal exposures to the potentially harmful pharmaceutical excipients benzyl alcohol (BA) and PG. Lorazepam, phenobarbital, and digoxin were the only medications involved in the PG exposures. The authors observed a wide range in cumulative excipient doses received by neonates. The median range for PG was 204.9 (17.3–9472.7 mg/kg/day). Patients who received continuous infusions versus intermittent dosing received higher excipient doses. The median cumulative excipient doses (PG, 4554.5 mg/kg/day) were 180 times the acceptable oral daily intakes of PG (25 mg/kg/day).
Chicella et al22 conducted a prospective study in 11 intubated patients in a tertiary care pediatric intensive care unit (PICU). The author's objective was to determine if PG accumulates in children receiving continuous lorazepam infusion and, if accumulation occurs, to determine if it is associated with significant laboratory abnormalities. The authors concluded that PG accumulated significantly in PICU patients receiving continuous lorazepam infusion and PG concentration correlated with the cumulative lorazepam dose the patient received. However, significant lab abnormalities due to PG accumulation were not observed. MacDonald et al23 reported an increased incidence of seizures (33% vs 14%, p = 0.21) in low birth weight infants who received MVI-12 (PG 3 g/day), compared to infants receiving MVI concentrate (PG 300 mg/day).
TREATMENT OF PG TOXICITY
PG is a small molecular weight alcohol, 76.1 daltons, that is nonionic, highly water soluble (DVD 0.7–0.9 L/kg), and lacks significant serum protein binding, making it readily cleared by hemodialysis. Intermittent dialysis is the preferred method of treatment as it rapidly lowers PG levels (without a rebound in its level) and corrects associated metabolic abnormalities.15 In a study by Parker et al,15 the authors report that hemodialysis significantly reduced serum concentrations of PG.
CONCLUSION
Although PG is considered safe for use as a vehicle for intravenous medications by the US Food and Drug Administration, at high levels, PG can accumulate and cause lactic acidosis, CNS depression, coma, hypoglycemia, seizures, and hemolysis. Patients at risk for toxicity include infants, patients with renal insufficiency, patients with epilepsy, or burn patients who receive extensive dermal applications of PG. PG toxicity can occur at dosing of lorazepam as low as 4 to 6 mg per hour in adults. The clinical diagnosis of PG intoxication may be difficult since many institutions do not measure PG levels. However, the osmolar gap, anion gap, and lactate are commonly elevated in PG intoxication.
Practitioners should exercise caution when treating patients with large doses or prolonged use of medications containing PG especially in infants or patients with renal or hepatic dysfunction. Propylene glycol toxicity should be suspected in any patient with an unexplained anion gap, metabolic acidosis, hyperosmolarity, and/or clinical deterioration who has received drug therapy with PG as a drug solvent. Additional studies in the pediatric population are warranted.
ACKNOWLEDGMENT
The authors thank Dr Shabnam Gaskari, PharmD, for her insight and contributions.
ABBREVIATIONS
- BA
benzyl alcohol
- CNS
central nervous system
- NICU
neonatal intensive care unit
- PG
propylene glycol
- PICU
pediatric intensive care unit
Footnotes
Disclosure The authors declares no conflict or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts and honoraria.
REFERENCES
- 1.Food and Drug Administration. Generally Recognized as Safe (GRAS) status of propylene glycol. http://www.accessdata.fda.gov/scripts/fcn/fcnDetailNavigation.cfm?rpt=scogsListing&id=262. Accessed April 29, 2010.
- 2.Fligner CL, Jack R, Twiggs GA et al. Hyperosmolarity induced by propylene glycol, a complication of silver sulfadiazine therapy. JAMA. 1985;253(11):1606–1609. [PubMed] [Google Scholar]
- 3.Martin G, Finberg L. Propylene glycol: a potentially toxic vehicle in liquid dosage form. J Pediatr. 1970;77(5):877–878. doi: 10.1016/s0022-3476(70)80253-0. [DOI] [PubMed] [Google Scholar]
- 4.Arulanantham K, Genel M. Central nervous system toxicity associated with ingestion of propylene glycol. J Pediatr. 1978;93(3):515–516. doi: 10.1016/s0022-3476(78)81183-4. [DOI] [PubMed] [Google Scholar]
- 5.Glascow AM, Boeckx RL, Miller MK et al. Hyperosmolarity in small infants due to propylene glycol. Pediatrics. 1983;72(3):353–355. [PubMed] [Google Scholar]
- 6.Tuohy KA, Nicholson WJ, Schiffman F. Agitation by sedation. Lancet. 2003;361(9354):308. doi: 10.1016/S0140-6736(03)12326-4. [DOI] [PubMed] [Google Scholar]
- 7.Kelner MJ, Bailey DN. Propylene glycol as a cause of lactic acidosis. J Anal Toxicol. 1985;9(1):40–42. doi: 10.1093/jat/9.1.40. [DOI] [PubMed] [Google Scholar]
- 8.O'Donnell J, Mertl SL, Kelly WN. Propylene glycol toxicity in a pediatric patient: the dangers of diluents. J Pharm Pract. 2000;13(3):214–224. [Google Scholar]
- 9.Arbour R, Esparis B. Osmolar gap metabolic acidosis in a 60-year-old man treated for hypoxemic respiratory failure. Chest. 2000;118(2):545–546. doi: 10.1378/chest.118.2.545. [DOI] [PubMed] [Google Scholar]
- 10.Barnes BJ, Gerst CG, Smith JR et al. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacology. 2006;26(1):23–33. doi: 10.1592/phco.2006.26.1.23. [DOI] [PubMed] [Google Scholar]
- 11.Nelsen JL, Haas CE, Habtemariam B et al. A prospective evaluation of propylene glycol clearance and accumulation during continuous-infusion lorazepam in critically ill patients. J Intensive Care Med. 2008;23(3):184–194. doi: 10.1177/0885066608315808. [DOI] [PubMed] [Google Scholar]
- 12.Yaucher NE, Fish JT, Smith HW et al. Propylene glycol-associated renal toxicity from lorazepam infusion. Pharmacotherapy. 2003;23(9):1094–1099. doi: 10.1592/phco.23.10.1094.32762. [DOI] [PubMed] [Google Scholar]
- 13.Arroliga AC, Shehab N, McCarthy K et al. Relationship of continuous infusion lorazepam to serum propylene glycol concentration in critically ill adults. Crit Care Med. 2004;32(8):1709–1714. doi: 10.1097/01.ccm.0000134831.40466.39. [DOI] [PubMed] [Google Scholar]
- 14.Speth PA, Vree TB, Neilen NF et al. Propylene glycol pharmacokinetics and effects after intravenous infusion in humans. Ther Drug Monit. 1987;9(3):255–258. doi: 10.1097/00007691-198709000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Parker MG, Fraser GL, Watson DM et al. Removal of propylene glycol and correction of increased osmolar gap by hemodialysis in a patient on high dose lorazepam infusion therapy. Intensive Care Med. 2002;28(1):81–84. doi: 10.1007/s00134-001-1125-1. [DOI] [PubMed] [Google Scholar]
- 16.Horinek El, Kiser TH, Fish DN et al. Propylene glycol accumulation in critically ill patients receiving continuous intravenous lorazepam infusions. Ann Pharmacother. 2009;43(12):1964–1071. doi: 10.1345/aph.1M313. [DOI] [PubMed] [Google Scholar]
- 17.Wilson KC, Reardon C, Theodore AC et al. Propylene glycol toxicity: a severe iatrogenic illness in ICU patients receiving IV benzodiazepines. A case series and prospective, observational pilot study. Chest. 2005;128(3):1674–1681. doi: 10.1378/chest.128.3.1674. [DOI] [PubMed] [Google Scholar]
- 18.Zar T, Graeber C, Perazella MA. Recognition, treatment, and prevention of propylene glycol toxicity. Semin Dial. 2007;20(3):217–219. doi: 10.1111/j.1525-139X.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 19.Yahwak JA, Riker RR, Fraser GL et al. Pharmacotherapy determination of a lorazepam dose threshold for using the osmol gap to monitor for propylene glycol toxicity. Pharmacotherapy. 2008;28(8):984–991. doi: 10.1592/phco.28.8.984. [DOI] [PubMed] [Google Scholar]
- 20.Yorgin PD, Theodorou AA, Al-Uzri A et al. Propylene glycol-induced proximal renal tubular cell injury. Am J Kidney Dis. 1997;30(1):134–139. doi: 10.1016/s0272-6386(97)90577-1. [DOI] [PubMed] [Google Scholar]
- 21.Shehab N, Lewis CL, Streetman DD et al. Exposure to the pharmaceutical excipients benzyl alcohol and propylene glycol among critically ill neonates. Pediatr Crit Care Med. 2009;10(2):256–259. doi: 10.1097/PCC.0b013e31819a383c. [DOI] [PubMed] [Google Scholar]
- 22.Chicella M, Jansen P, Parthiban A et al. Propylene glycol accumulation associated with continuous infusion of lorazepam in pediatric intensive care patients. Crit Care Med. 2002;30(12):2752–2756. doi: 10.1097/00003246-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald MG, Getson PR, Glasgow AM et al. Propylene glycol: increased incidence of seizures in low birth weight infants. Pediatrics. 1987;79(4):622–625. [PubMed] [Google Scholar]


