Abstract
OBJECTIVES: In 2011, the Food and Drug Administration (FDA) approved intravenous esomeprazole 0.5 mg/day for children aged >1 month and oral esomeprazole for infants aged 1 month to <1 year at doses of 2.5, 5, and 10 mg based on weight. Prior to 2011, proton pump inhibitors (PPIs) were not approved for use in infants aged <1 year. This study determined PPI usage rates prior to the FDA approval among newborns and infants in both the inpatient and outpatient settings and compared PPI and histamine-2 receptor antagonist (H2RA) usage in the inpatient setting.
METHODS: We conducted a retrospective analysis of PPI prescribing patterns for newborns and infants from 2003 to 2008 using data from the Premier Perspective Inpatient Hospital Database and the PharMetrics Patient-Centric Database for inpatient and outpatient data, respectively. PPI use and diagnoses were determined from clinical and charge records from more than 500 hospitals. Descriptive statistics were used to summarize the findings.
RESULTS: Our analysis showed that PPIs were prescribed for approximately 5000 newborns (0.13%) and 15,000 infants (2.65%) each year in the hospital setting and 1.6% of newborns and infants, as a group, in the outpatient setting. Newborns and infants receiving PPIs most often had diagnoses of gastroesophageal reflux disease (GERD) and were generally prescribed an adult PPI dose, although the actual dose administered could not be substantiated.
CONCLUSIONS: Although no PPI was approved by the FDA for patients aged <1 year at the time of this study, results of this analysis indicate that PPIs were commonly prescribed for newborns and infants, mostly in hospital, but also in outpatient settings. Most PPIs were prescribed for infants with a diagnosis of GERD.
INDEX TERMS: esomeprazole, gastroesophageal reflux disease, GERD, pediatric use
INTRODUCTION
Gastroesophageal reflux is common in preterm and healthy infants and children.1 Reflux of gastric contents, if it causes troublesome symptoms and/or complications, may predispose infants to development of gastroesophageal reflux disease (GERD).2 Symptoms of GERD include recurrent vomiting, regurgitation, back arching, crying, irritability, and food refusal.1,3 More serious signs and conditions traditionally associated with GERD include failure to thrive, esophagitis, apnea, and recurrent pneumonia.1,3,4
Treatment of GERD in infants generally includes modifications to nutrition, feeding frequency/volume, and positioning, as well as pharmacologic therapies such as gastric acid-buffering agents, mucosal surface barriers, and gastric antisecretory agents.3 Findings from a recent study showed that the proton pump inhibitor (PPI) esomeprazole reduces esophageal acidity and acidic reflux events in infants with GERD.5 Since the time of the present analysis, the US Food and Drug Administration (FDA) has approved only one PPI for oral and intravenous use in infants <1 year of age (esomeprazole, Nexium [AstraZeneca LP, Wilmington, DE]); esomeprazole was approved in April of 2011 for intravenous use in patients aged 1 month to 17 years and approved in December of 2011 for oral use in patients 1 month to younger than 12 months of age.6,7
The prevalence of PPI use in infants <1 year of age increased fourfold from 2000 to 2003 to approximately 400 infants per 100,000, according to findings from a retrospective observational study.8 Because PPIs are reportedly prescribed in various clinical settings in patients aged <1 year,8 a real-world data analysis was performed to evaluate the nature of off-label PPI use in this age group in the United States and to understand the reported use of PPIs and histamine-2 receptor antagonists (H2RAs) in the inpatient setting and PPIs in the outpatient setting during a 5-year period.
MATERIALS AND METHODS
Inpatients
Newborns (aged <1 month at discharge) and infants (aged ≥1 month to <1 year at discharge) who were discharged from January 1, 2004, through December 31, 2008, were identified from the Premier Perspective Inpatient Hospital Database, which contains clinical and charge data from approximately 500 hospitals in the United States. Estimates of patient numbers were projected to the total national hospital discharge population. GERD diagnosis was based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes given at discharge.
PPI and H2RA use was based on billing charges; usage was summarized and compared in the inpatient setting. Premier Perspective Standard Charge Master and Standard Quantity for charged items were used to calculate the daily dose for each PPI agent. The most frequently prescribed PPI in 2008 was determined based on the number of patients exposed. The most frequently prescribed PPI daily dose was determined based on the number of patient-days of exposure. Both oral and parenteral formulations were analyzed for inpatients, and the data were grouped together for each drug.
Outpatients
Data from the PharMetrics Patient-Centric Database were used to identify patients who were aged <1 year at some point during the analysis period (January 1, 2003 through December 31, 2007), had a diagnosis of GERD, received ≥1 prescription(s) for a PPI, and had not received a prescription for a H2RA before receiving a PPI. Drug utilization was evaluated using the National Drug Code (NDC), drug dose, and days-of-supply information in the PharMetrics database. The mean duration of PPI therapy was based on “days of pharmacy therapy” (duration of therapy = last refill date-first fill date + last refill days supply). GERD diagnosis was based on ICD-9-CM codes (530.81 and 787.1).
Statistical Analysis
The intent of this study was to report real-world treatment patterns in infants and newborns with GERD-related conditions prior to the approval of PPIs in this population. Descriptive statistics alone were used to report the findings of this study analysis. Comparisons between treatment groups were not conducted.
RESULTS
Inpatients
Projection estimates suggested that the percentage of inpatients who received a PPI increased during a 5-year period (2004–2008) from 0.07% to 0.17% in newborns aged <1 month and from 1.64% to 3.78% in infants aged 1 month to 1 year. On average, 0.13% of newborns and 2.65% of infants, corresponding to approximately 5000 newborns and 15,000 infants, received a PPI in US hospitals each year from 2004 to 2008. Among newborns and infants who received PPIs, the percentage of patients who also received an H2RA during their hospital stay decreased during the 5-year period (2004–2008), from 58.5% to 40.2% in hospitalized newborns and from 37.5% to 30.8% in hospitalized infants. Newborns and infants administered PPIs and H2RAs were more likely to receive H2RAs initially than PPIs (Table). Infants were more likely than newborns to start PPIs and H2RAs at the same time (Table).
Table.
Hospitalized Newborns and Infants Given PPIs and H2RAs by Drug Administered First
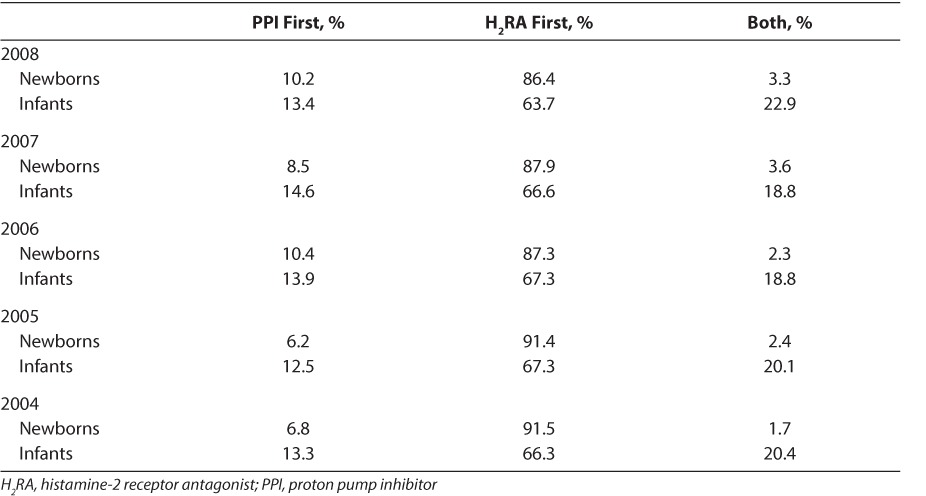
In the inpatient setting, most newborns (70.7%) and infants (75.4%) who received PPIs in 2008 received lansoprazole. Percentages of patients receiving omeprazole, pantoprazole, and esomeprazole were 25.6%, 5.1%, and 1.6% for newborns and 17.9%, 9.2%, and 1.5% for infants, respectively. This prescribing pattern was similar for the 2004 to 2007 period. In 2008, lansoprazole was prescribed orally in 61.8% of newborns and 71.2% of infants, and claims data suggest that these children were frequently prescribed doses of 60 and 30 mg daily, respectively. In all other years included in the study, the oral lansoprazole dose most frequently prescribed for inpatient newborns was 30 mg daily regardless of weight. The route of administration for omeprazole was unknown in ≥78% of newborns and infants; when administered orally, claims data suggest it was most frequently prescribed at <20 mg (12 mg) and 40 mg daily doses, respectively. Pantoprazole was prescribed parenterally for 45.3% of newborns and 56.5% of infants; claims data suggest that daily administration of 80 mg pantoprazole (regardless of weight) was the most common dosage in both age groups.
Neonatal jaundice associated with preterm delivery was the most frequent discharge diagnosis among newborns who received PPIs (with or without H2RAs) in each year included in the study, and it is not known how many of these newborns had additional diagnoses of GERD. Either GERD or respiratory distress syndrome was the second most frequent discharge diagnosis for newborns. GERD was the most frequent discharge diagnosis among infants who received PPIs (with or without H2RAs). The second most frequent discharge diagnosis in infants who received PPIs was acute bronchiolitis because of respiratory syncytial virus or failure to thrive, depending on the year. In each year included in the study, >50% of newborns and >64% of infants who received PPIs (with or without H2RAs) had discharge diagnoses of GERD. PPI prescriptions among newborns and infants with GERD increased during the 5-year period (2004–2008), but patients with GERD were most commonly prescribed H2RAs only (Figure).
Figure.
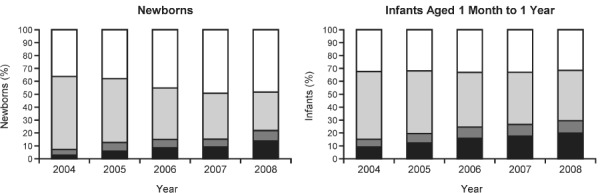
Percentage of hospitalized newborns and infants with gastroesophageal reflux disease who were given PPIs, H2RAs, both, or neither.
H2RA, histamine-2 receptor antagonist; PPI, proton pump inhibitor.
 PPI Only;
PPI Only;  PPI and H2RA;
PPI and H2RA;  H2RA only; □ None
H2RA only; □ None
Outpatients
Of the newborns and infants in the outpatient analysis group (n = 246,236), 48,849 (19.8%) were diagnosed with GERD, of which 4018 (8.2%) received PPIs, which was 1.6% of the overall out-patient group. In the outpatient setting, the most common PPIs used in newborns and infants with GERD were lansoprazole (86.2%), omeprazole (13.2%), esomeprazole (0.4%), and pantoprazole (0.1%). Newborns and infants with GERD were most frequently prescribed daily doses of 15 mg lansoprazole, 20 mg omeprazole, 20 or 40 mg esomeprazole, or 40 mg pantoprazole. Of the newborns and infants with GERD who were initially prescribed PPIs (n = 4018), 77.5% were on monotherapy, 11.6% switched to H2RAs, and 7.5% were titrated to a higher dose at a later time within the 1-year period. The median duration of PPI therapy for newborns and infants with GERD was 86 days (range = 14–364 days).
DISCUSSION
The results of this study show that although PPIs were not approved in the United States for patients younger than 1 year of age at the time of the study, they often were prescribed for infants in both inpatient and outpatient settings. Hospital charge data suggest that infants aged <1 year may have been prescribed PPIs at adult doses, although the actual dose administered may have varied and could not be validated due to limitations in study design. PPI prescriptions for newborns and infants in the hospital setting more than doubled from 2004 to 2008; whether this is due to an increase in incidence of GERD diagnosis in newborns and infants or increase in preference for prescribing PPIs over H2RAs is unknown. There have been data reported that the incidence of GERD diagnosis in infants ≤1 year of age is increasing.9 Nelson et al9 found a greater than threefold increase in diagnosis of GERD and acid-related conditions from 2000 to 2005 in this age group, based on US claims data.
Most patients aged <1 year in US hospitals who received prescriptions for PPIs first had received prescriptions for H2RAs. If an H2RA was not effective, adding or switching to a PPI would be consistent with the practice of stepping up therapy.
A limitation of the current study is that no efficacy evaluation is available. Therefore, no conclusions can be drawn regarding the reasons for adding or switching to a PPI in patients first treated with an H2RA. The outpatient data do suggest that, for GERD patients prescribed PPIs (and who had had no previous prescriptions for H2RAs), most remained on PPI monotherapy with very little switching or adding H2RAs. Another limitation of the study was the use of charge data to identify dose in the inpatient population. The charged dose per day may not be the same as the dose administered on that day.
In conclusion, although no PPI was approved by the FDA for patients aged <1 year at the time of this analysis, the results of a real-world data analysis indicate that PPIs were commonly prescribed for newborns and infants in the hospital setting and, to a lesser extent, in the outpatient setting. In this study, newborns and infants receiving PPIs most often had diagnoses of GERD and generally were prescribed PPIs at doses equal to or greater than those indicated for adults and children aged >1 year. As stated earlier, the actual dose administered may not be the same as the dose prescribed and it cannot be validated or substantiated using the database for this study.
ACKNOWLEDGMENT
This study was funded by AstraZeneca LP. We acknowledge Yan Liu, formerly of AstraZeneca LP, for data analysis and interpretation of results. Medical writing and editorial services were provided by Kristen Quinn, PhD, and Stella Chow, PhD, of Scientific Connexions, East Norriton, Pennsylvania (funded by AstraZeneca LP).
ABBREVIATIONS
- FDA
Food and Drug Administration
- GERD
gastroesophageal reflux disease
- H2RA,
histamine-2 receptor antagonist
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- PPIs
proton pump inhibitors
Footnotes
Disclosures Berhanu Alemayehu and Nze Shoetan are employees of AstraZeneca LP, Wilmington, Delaware. Marta Illueca and Huiying Yang are former employees of Astra-Zeneca LP, Wilmington, Delaware. All authors, with the exception of Nze Shoetan, own stock in AstraZeneca LP.
REFERENCES
- 1.Rudolph CD, Mazur LJ, Liptak GS et al. North American Society for Pediatric Gastroenterology and Nutrition. Guidelines for evaluation and treatment of gastroesophageal reflux in infants and children: recommendations of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 2001;32(suppl 2):S1–S31. doi: 10.1097/00005176-200100002-00001. [DOI] [PubMed] [Google Scholar]
- 2.Sherman PM, Hassall E, Fagundes-Neto U et al. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. Am J Gastroenterol. 2009;104(5):1278–1295. doi: 10.1038/ajg.2009.129. [DOI] [PubMed] [Google Scholar]
- 3.Vandenplas Y, Rudolph CD, Di Lorenzo C. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J Pediatr Gastroenterol Nutr. 2009;49(4):498–547. doi: 10.1097/MPG.0b013e3181b7f563. et al; North American Society for Pediatric Gastroenterology Hepatology and Nutrition, European Society for Pediatric Gastroenterology Hepatology and Nutrition. [DOI] [PubMed] [Google Scholar]
- 4.Novak DA. Gastroesophageal reflux in the preterm infant. Clin Perinatol. 1996;23(2):305–320. [PubMed] [Google Scholar]
- 5.Illueca M, Thomson M, Davidson G et al. Esomeprazole pharmacodynamics assessed with pH/impedance monitoring in neonates with gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2010;51(suppl 2):102. [Google Scholar]
- 6.Wilmington, DE: AstraZeneca LP; 2012. NEXIUM I.V. (esomeprazole sodium) for Injection [prescribing information] [Google Scholar]
- 7.Wilmington, DE: AstraZeneca LP; 2012. NEXIUM (esomeprazole magnesium) [prescribing information] [Google Scholar]
- 8.Barron JJ, Tan H, Spalding J et al. Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45(4):421–427. doi: 10.1097/MPG.0b013e31812e0149. [DOI] [PubMed] [Google Scholar]
- 9.Nelson SP, Kothari S, Wu EQ et al. Pediatric gastroesophageal reflux disease and acid-related conditions: trends in incidence of diagnosis and acid suppression therapy. J Med Econ. 2009;12(4):348–355. doi: 10.3111/13696990903378680. [DOI] [PubMed] [Google Scholar]


