Abstract
OBJECTIVE: This study was conducted to evaluate the amount of medication adsorbed into extracorporeal membrane oxygenation (ECMO) circuits with a polymethylpentane membrane oxygenator and heparin-coated polyvinyl chloride tubing.
METHODS: An ECMO circuit with the aforementioned components was set up ex vivo and primed with expired blood. Midazolam, lorazepam, morphine, and fentanyl were administered to the circuit. Fifteen minutes after medication administration, 60 mL of blood were removed and stored in a 60-mL syringe to serve as a control. Medication levels were drawn from the ECMO circuit (test) and control syringe (control) 15 minutes, 24 hours, and 48 hours after the medications were administered. ECMO circuit medication levels were compared to their corresponding syringe control medication levels. Descriptive statistics were used to determine the percentage of medication remaining in the blood and compare it to the control value.
RESULTS: Except for morphine, there was a large decline in medication levels over the 48-hour period. Compared to control values, 17.2% of midazolam, 41.3% of lorazepam, 32.6% of fentanyl, and 102% of morphine remained in the ECMO circuit.
CONCLUSION: Despite the use of newer components in ECMO circuits, a large quantity of medication is adsorbed into the ECMO circuit. Midazolam, lorazepam, and fentanyl all showed reductions in medication levels greater than 50%. Morphine may have advantages for patients on ECMO, as its concentration does not appear to be affected.
INDEX TERMS: extracorporeal, pharmacokinetics, polymethylpentane
INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) has been in use since the 1970s. During this time, the use of ECMO has grown and changed as the equipment has improved. Membrane oxygenators (MO) have been altered to make them smaller and more efficient, and they are now fabricated from products such as polymethylpentane (PMP) instead of silicone. Polyvinyl chloride (PVC) tubing has also been improved by coating it with heparin.
ECMO support has been shown to affect medication pharmacokinetics in patients.1 Numerous studies have shown that patients on ECMO have larger volumes of distribution, in conjunction with lower clearance.2–,7 Therefore, patients on ECMO support may require vastly different drug dosing regimens. These effects in patients on ECMO may be a result of changes associated with their illness or of the ECMO circuit itself.
Several studies have been conducted to analyze the effects of the ECMO circuit on medications.8–,12 Drastic changes have been observed in certain drugs, such as midazolam, lorazepam, morphine, and fentanyl. This suggests that these agents may not be as effective in patients on ECMO. Previous studies were conducted with ECMO circuits utilizing silicone MO and uncoated PVC. As circuit components have improved, our working knowledge of the effects of ECMO on medications has become outdated. It has been suggested that the newer components may have less medication binding. In a recent study of Shekar et al,8 significant decreases were observed in medication concentrations, despite the use of contemporary circuits and components such as PMP MO and albumin/heparin-coated tubing. This particular study reported medication loss of fentanyl, midazolam, and meropenem. In contrast to the studies conducted with silicone MO and uncoated PVC tubing, Shekar and colleagues did not observe significant reductions in vancomycin or morphine levels.
The goal of this study was to analyze the rates of decrease of medication concentrations in an ECMO circuit utilizing a PMP MO and heparin-coated tubing to determine circuit adsorption of the medication.
MATERIALS AND METHODS
This study was conducted ex vivo and received expedited approval from the local IRB. A Sorin SIII (Milan, Italy) ECMO pump was utilized. The circuit components included a PMP MO (Quadrox D, Maquet, Rastatt, Germany), PVC heparin-coated tubing (Carmeda, Medtronic Inc, Minneapolis, MN), centrifugal pump (Sorin SIII), and a heater (Cincinnati Sub-Zero, Cincinnati, OH). The priming volume of the MO was 250 mL, and the gas exchange surface area measured 1.8 m2. The circuit was primed with 400 mL of expired blood, 20 mL of 25% albumin, 3 mL of heparin 100 units/mL, 15 mL of sodium bicarbonate 1 mEq/mL, and 1 mL of calcium chloride 100 mg/mL. The circuit loop was closed by attaching the venous and arterial tubing to a reservoir bag containing 400 mL of blood. The reservoir bag was made of heparin-bonded PVC and was used to provide an adequate volume of blood to maintain the patency of the circuit.
The circuit ran for 15 minutes at 1 L/min. The flow rate continued at 1 L/min to prevent potential clotting with slower flow rates. Doses of midazolam, lorazepam, morphine, and fentanyl were administered via the venous line access port. The doses administered were calculated for a patient with a body weight of 10 kg. After each medication administration, a 3-mL saline flush was given to avoid pooling of the drug at the administration site. The administration regimen was: 1 mg/1 mL of midazolam, 3 mL of saline, 1 mg/0.5 mL of lorazepam, 3 mL of saline, 1 mg/0.5 mL of morphine, 3 mL of saline, 10 mcg/0.2 mL of fentanyl, 3 mL of saline. No activity was conducted for 15 minutes after the medications were administered to allow for equilibration of the drugs throughout the circuit. After 15 minutes had passed, a control sample was drawn and stored in a 60-mL plastic syringe. Both the ECMO circuit and the control syringe were stored at 37°C. Medication levels were drawn from the ECMO circuit and the control syringe at 15 minutes, 24 hours, and 48 hours after drug administration. After each set of samples was drawn, they were immediately transported to the laboratory department for preparation and storage. Morphine and fentanyl samples were frozen (< −20°C), whereas midazolam and lorazepam samples were refrigerated (2°C to 8°C) during transport to their respective locations, per third-party laboratory specifications. The laboratory then sent the samples to outside facilities for processing. Morphine and fentanyl samples were analyzed at the Mayo Clinic Laboratories, Rochester, MN, while midazolam and lorazepam samples were analyzed at Medtox Laboratories, Saint Paul, MN. Fentanyl sample analysis utilized liquid chromatography and tandem mass spectrometry. Morphine sample analysis utilized gas chromatography/mass spectrometry using selected ion monitoring. Midazolam and lorazepam sample analysis utilized gas chromatography/electron capture detection. The assay coefficients of daily variation for morphine and fentanyl were 6% and 4.6%, respectively. Assay coefficients were not available for midazolam and lorazepam samples.
Descriptive statistics (specifically, percentages) were calculated to compare the test values to those of the control samples. The following equations were used report the percentage of medication remaining in the blood in the ECMO circuit.
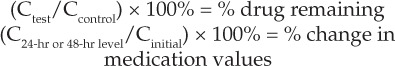 |
RESULTS
The measured values for control and test samples are shown in Table 1. Control levels for morphine at 24 hours and fentanyl at 48 hours could not be determined. The concentrations were cancelled by the third-party laboratory after 4 days without a result. There were no additional complications, such as clotting or mechanical failures. Figures 1 through 4 show the changes in control and test values from baseline for mid-azolam, lorazepam, fentanyl, and morphine, respectively.
Table 1.
Medication Levels in PMP MO/Heparin-Coated PVC ECMO Circuits (Test) and in a Control Syringe
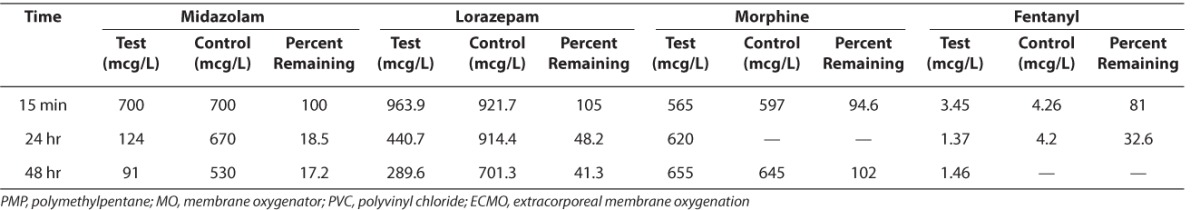
Figure 1.
The percent change of midazolam concentrations when compared to the initial values in control and test samples.
 Midazolam, control;
Midazolam, control;  Midazolam, test
Midazolam, test
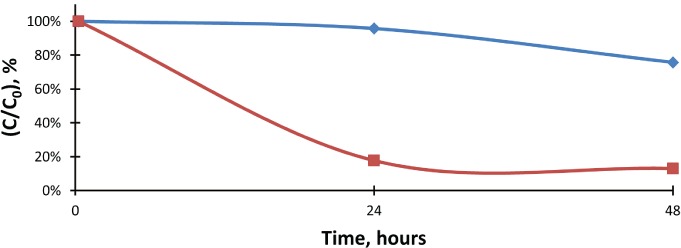
Figure 2.
The percent change of lorazepam concentrations when compared to the initial values in lorazepam control and test samples.
 Lorazepam, control;
Lorazepam, control;  Lorazepam, test
Lorazepam, test
Figure 3.
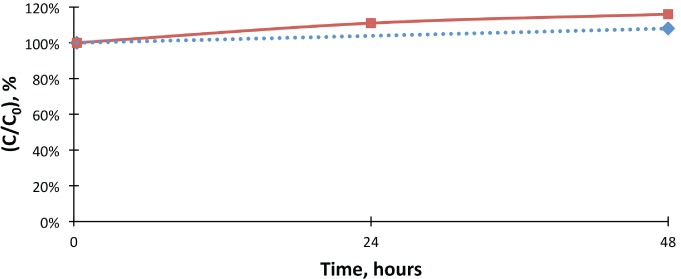
The percent change of morphine concentrations when compared to the initial values in morphine control and test samples. Control morphine levels were extrapolated, as the 24-hour control value was not available.
 Morphine, control;
Morphine, control;  Morphine, test;
Morphine, test;  Expon. (Morphine, control)
Expon. (Morphine, control)
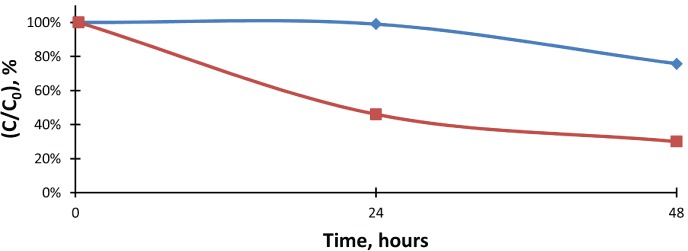
Figure 4.
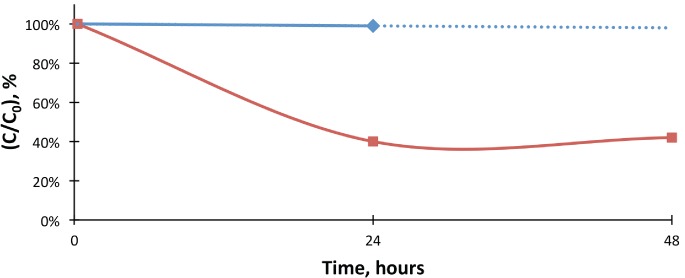
The percent change of lorazepam concentrations when compared to the initial values in fentanyl control and test samples. Control fentanyl levels were extrapolated, as the 48-hour control value was not available.
 Lorazepam, control;
Lorazepam, control;  Lorazepam, test;
Lorazepam, test;  Expon. (Lorazepam, control)
Expon. (Lorazepam, control)
DISCUSSION
Several studies have shown significant adsorption of medications into ECMO circuits that used silicone MO and PVC tubing. In the last few years, new ECMO products have become available and increasingly common. PMP MO and heparin-coated PVC tubing have replaced older products. With this transition, the literature regarding medication adsorption into ECMO circuits has lost much of its utility in the clinical setting. The common consensus has been that newer ECMO circuit components have less drug adsorption than the older circuits. Although one other study has been conducted, the circuit components were slightly different.8
The results of this study show that, despite improvements in MO and tubing, there is still a loss of medication in ECMO circuits. The study was not designed to compare the PMP MO and heparin-coated PVC tubing to silicone MO and uncoated PVC tubing; the cost of such an analysis and the inability to secure older circuit components prevented this comparison. However, the medications used in this study have been analyzed in several previous articles with silicone MO/PVC tubing.
Table 2 provides a comparison of this study to the results of previous studies8–,13 with these medications and various MOs. When comparing the current ECMO setup to silicone MO/uncoated PVC tubing, it does appear that there is an identifiable difference in medication adsorption into the current circuits. Medications with a high lipophilicity, such as fentanyl, maintained higher concentrations in the PMP/heparin-coated circuit. In previous circuits, the extremely low concentrations of available fentanyl rendered the drug ineffective. This study found that approximately 33% of the fentanyl dose remained. Thus, fentanyl may now be more effective than with previous components. Morphine remains the opioid of choice, as its concentration appears to not be affected by the current components. Both midazolam and lorazepam values declined by over 50%, with resulting concentrations of 18.5% and 48.2%, respectively. Although there was a greater loss in midazolam concentration, it may remain the anxiolytic medication of choice to avoid the possible intravenous lorazepam toxicities associated with propylene glycol. Therefore, with only 20% of the midazolam dose remaining, ECMO patients may require higher dosages to attain desired sedation parameters.
Table 2.
Percent Remaining of Midazolam, Lorazepam, Morphine, and Fentanyl in 7 Different Studies
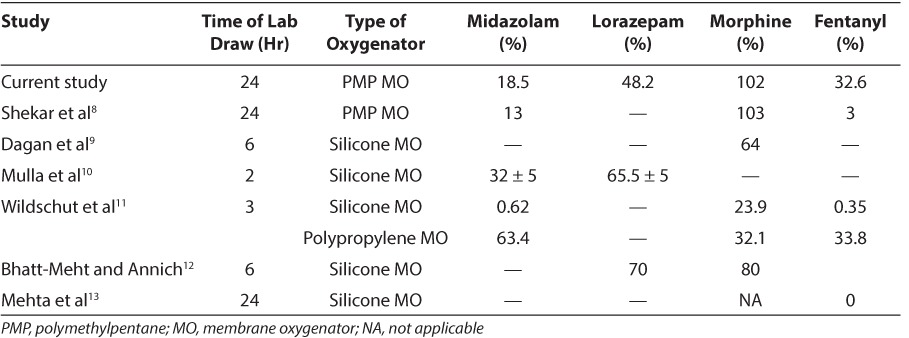
Other studies have shown that available drug concentrations are higher with polypropylene MO than with silicone MO.11,14 As with silicone MO, the authors are unaware of any data comparing PMP MO to polypropylene. Polypropylene is a microporous polymer, whereas PMP is a microporous polymer with a thin non-porous matrix on the blood side requiring diffusion and pressure gradients for molecules to cross.15 The non-porous matrix may minimize the amount of plasma leakage across the membrane and has resulted in decreased adsorption of inhaled anesthetics.16,17 The results of this study differ from those of a previous study.11 Wildschut et al11 had a rate of fentanyl loss of around 33%, similar to our experiment. Morphine levels were lower in that study (32.1% vs. 100%) but midazolam levels were higher (63% vs. 18%) than in the present study. In a study of Rosen and colleagues,14 fentanyl concentrations decreased by less than 1%. The differences between these studies may be multifactorial, including laboratory error, sampling differences, differences in ECMO circuit configuration, and overall study conduct. It is difficult to compare PMP to polypropylene utilizing the data from this study alone.
The study that was most similar to this study was that conducted by Shekar and colleagues.8 They also utilized a PMP MO, but the tubing was coated with albumin and heparin instead of heparin alone. Both studies noted that morphine levels did not decrease during the course of the study. The similarities between the studies suggest that morphine concentrations are not affected by contemporary circuits and that the usual dosing regimens can be utilized. The concentrations of fentanyl and midazolam were lower in that study than in the present study. This variation could be related to the albumin-coated tubing or, more likely, the limited sample size of this study. This may also be related to changes in variables such as pH and albumin levels, which are known to affect adsorption rates and free drug levels.18
A comparison of the results of this study with those of the previously listed studies (Table 2) may have limitations. There were dissimilarities in the methods used in each study. Priming volumes and the surface area of the ECMO components may have been different across the studies. This study was not designed to assess the site of medication loss. Other circuit components, such as the heater or centrifugal pump, could play a role in the resultant drug levels. Further studies are needed to ascertain the differences in medication adsorption between the varying ECMO components.
Several of the studies included in Table 2 showed rapid adsorption of medications into ECMO circuits. As many as 7 samples were taken within the first hour. However, in other studies, only 1 sample was drawn. The baseline sample was drawn at 15 minutes in this study to allow time for the medications to reach equilibrium. Because rapid adsorption has already been observed in other studies, additional samples would have added significant cost with minimal change in results and were therefore not obtained. Although the figures suggest a continuous change in drug concentration over time, it is more likely that there is a rapid shift in medication from the blood to the circuit, as shown by other studies.
In all the studies cited in Table 2, a bolus dose was administered to the circuit. This differs from standard practice, as many of the medications studied are utilized as a continuous infusion. Because the circuit in the current study was not connected to a patient, medication levels would be expected to increase if continuous infusions were utilized. Metabolism and excretion functions performed by the patient were not available, and bolus dosing was done so that a more accurate representation of circuit adsorption could be observed. Therefore, the medication concentration values in this study are not representative of values in an actual patient. The intention of the medication values was to monitor the percentage changes over time to show how much medication could potentially be adsorbed by the circuit materials themselves.
Because only 1 sample was drawn for each level at each time point, there is a risk of sampling bias in the current study. This can be seen in the results of the morphine concentration, as it increased over time. During the 48-hour span of the study, morphine levels rose without further medication administration. An additional source for this bias occurred when a control morphine and fentanyl sample could not be analyzed. The third-party laboratory stated that the labs were cancelled after several days but did not give a reason for this. This may have been due to interference from other molecules in the blood that prevented the values from being assessed. Cross-reactivity between morphine and fentanyl was not considered to be a potential source of error with the types of mass spectrometry performed. A potential means to minimize sampling bias would include the collection of multiple samples for analysis. This could have been done by drawing multiple test and control values at the same time or by performing multiple circuit runs. Although these options were considered, additional samples were not obtained due to cost restraints.
The use of expired blood products in this experiment may constitute an additional limitation. The degraded hemoglobin would not have provided as many potential binding sites for medication adherence. This may have led to falsely elevated drug levels. However, this should not have affected the ability of the ECMO circuit to adsorb the medication. The entirety of the study utilized expired blood products, and any effects on drug levels would have been consistent over the 48-hour course. Because the study measured percentages of medication remaining in the blood, overall increases in medication levels would not have affected these results. A similar limitation exists with possible cross-reactivity between medication levels. Data regarding cross-reactivity was not available from the outside laboratories. Because agents from the same medication class were analyzed, there is a possibility of falsely elevated levels. However, since this cross-reactivity should occur in both control and test specimens, any falsely elevated levels would have remained consistently elevated. The reported percentages should not be affected because of the consistently higher levels, and the results remain valid.
This study assumes that the difference between the test and control variables is associated with medication adsorption into the circuit. Additional factors may have influenced the loss of medication. Medication degradation over time could have resulted in lower drug levels. This was accounted for by collecting a control value with each test value to monitor for the rate of drug loss caused by spontaneous degradation. Another possible influence on medication levels is the destruction of medication via the ECMO components, specifically the centrifugal pump. In addition, pH values and albumin can affect medication concentrations. These parameters were not monitored in this study due to cost constraints. These values may have resulted in lower levels in the ECMO circuit. Whether a medication is adsorbed or destroyed, the medication is still unavailable to the patient. Unless a significant amount of medication leaches out of the circuit over time, this difference may not be of clinical relevance.
This study was conducted in an ex vivo fashion. Patient-related variables, especially in the pharmacodynamically altered environment associated with ECMO patients, may affect the amount of medication the patient receives or needs. This study was designed to assess the effect of the ECMO circuitry on medication levels. Because patient metabolism and excretion were not included in the ex vivo analyses, only a single dose of each medication was necessary to maintain drug levels. Renal or hepatic insufficiency is a common occurrence in this patient population.19–,21 Medication dosages need to be altered to reflect patient changes in metabolism and excretion, as well as the effects of the ECMO components.
CONCLUSION
Despite improvements in ECMO circuit components, medication adsorption remains a significant problem. Midazolam, lorazepam, and fentanyl levels all declined by at least 50%, suggesting that larger doses or alternative agents may be needed to achieve desired sedation and analgesic levels. Further analysis is necessary to evaluate the true significance of this effect.
ACKNOWLEDGMENT
The authors would like to thank Elizabeth Harthan, PharmD, BCPS, and Melinda Davis, MS, MT(ASCP), for their assistance and expertise in the preparation of this study.
ABBREVIATIONS
- C0
initial concentration
- ECMO
extracorporeal membrane
- MO
membrane oxygenator
- PMP
polymethylpentane
- PVC
polyvinyl chloride
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. Dr Harthan had full access to all the data in the study and takes responsibility for the integrity of the paper and accuracy of the data analysis. All funding for this study was provided by the Department of Pharmacy at the Children's Hospital of Illinois.
REFERENCES
- 1.Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet. 2003;42(5):403–417. doi: 10.2165/00003088-200342050-00001. [DOI] [PubMed] [Google Scholar]
- 2.Cohen P, Collart L, Prober CG et al. Gentamicin pharmacokinetics in neonates undergoing extracorporeal membrane oxygenation. Pediatr Infect Dis J. 1990;9(8):562–566. doi: 10.1097/00006454-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Dodge WF, Jelliffe RW, Zwischenberger JB et al. Population pharmacokinetic models: effect of explicit versus assumed constant serum concentration assay error patterns upon parameter values of gentamicin in infants on and off extracorporeal membrane oxygenation. Ther Drug Monit. 1994;16(6):552–559. [PubMed] [Google Scholar]
- 4.Amaker RD, DiPiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1996;40(5):1139–1142. doi: 10.1128/aac.40.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buck ML. Vancomycin pharmacokinetics in neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1998;18(5):1082–1086. [PubMed] [Google Scholar]
- 6.Ahsman MJ, Hanekamp M, Wildschut ED et al. Population pharmacokinetics of midazolam and its metabolites during venoarterial extracorporeal membrane oxygenation in neonates. Clin Pharmacokinet. 2010;49(6):407–419. doi: 10.2165/11319970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Fontana M, Massironi E, Rossi A et al. Ranitidine pharmacokinetics in newborn infants. Arch Dis Child. 1993;68(5):602–603. doi: 10.1136/adc.68.5_spec_no.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shekar K, Roberts JA, McDonald Cl et al. Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care. 2012;16(5):R194. doi: 10.1186/cc11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dagan O, Klein J, Gruenwald C et al. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit. 1993;15(4):263–266. doi: 10.1097/00007691-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Mulla H, Lawson G, von Anrep C et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion. 2000;15(1):21–26. doi: 10.1177/026765910001500104. [DOI] [PubMed] [Google Scholar]
- 11.Wildschut ED, Ahsman MJ, Allegaert K et al. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36(12):2109–2116. doi: 10.1007/s00134-010-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt-Meht V, Annich G. Sedative clearance during extracorporeal membrane oxygenation. Perfusion. 2005;20(6):309–315. doi: 10.1191/0267659105pf827oa. [DOI] [PubMed] [Google Scholar]
- 13.Mehta NM, Halwick DR, Dodson BL et al. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med. 2007;33(6):1018–1024. doi: 10.1007/s00134-007-0606-2. [DOI] [PubMed] [Google Scholar]
- 14.Rosen DA, Rosen KR, Silvasi DL. In vitro variability in fentanyl absorption by different membrane oxygenators. J Cardiothorac Anesth. 1990;4(3):332–335. doi: 10.1016/0888-6296(90)90041-d. [DOI] [PubMed] [Google Scholar]
- 15.Hinz J, Molder JM, Hanekop GG et al. Reduced sevoflurane loss during cardiopulmonary bypass when using a polymethylpentane versus a polypropylene oxygenator. Int J Artif Organs. 2013;36(4):233–239. doi: 10.5301/ijao.5000208. [DOI] [PubMed] [Google Scholar]
- 16.Wiesenack C, Wiesner G, Keyl C et al. In vivo uptake and elimination of isoflurane by different membrane oxygenators during cardiopulmonary bypass. Anesthesiology. 2002;97(1):133–138. doi: 10.1097/00000542-200207000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Philipp A, Wiesenack C, Behr R et al. High risk of intraoperative awareness during cardiopulmonary bypass with isoflurane administration via diffusion membrane oxygenators. Perfusion. 2002;17(3):175–178. doi: 10.1191/0267659102pf566oa. [DOI] [PubMed] [Google Scholar]
- 18.Skacel M, Knott C, Reynolds F et al. Extracorporeal circuit sequestration of fentanyl and alfentanil. Br J Anaesth. 1986;58(9):947–949. doi: 10.1093/bja/58.9.947. [DOI] [PubMed] [Google Scholar]
- 19.Burda G, Trittenwein G. Issues of pharmacology in pediatric cardiac extracorporeal membrane oxygenation with special reference to analgesia and sedation. Artif Organs. 1999;23(11):1015–1019. doi: 10.1046/j.1525-1594.1999.06458.x. [DOI] [PubMed] [Google Scholar]
- 20.Sell LL, Cullen ML, Whittlesey GC et al. Experience with renal failure during extracorporeal membrane oxygenation: treatment with continuous hemofiltration. J Pediatr Surg. 1987;22(7):600–602. doi: 10.1016/s0022-3468(87)80107-0. [DOI] [PubMed] [Google Scholar]
- 21.Roy BJ, Cornish JD, Clark RH. Venovenous extracorporeal membrane oxygenation affects renal function. Pediatrics. 1995;95(4):573–578. [PubMed] [Google Scholar]


