Abstract
Cancer continues to be the major cause of morbidity and death of more than 500,000 people in the US annually. Alternatively activated macrophages (M2 macrophages or TAMs) can facilitate tumor invasiveness and metastasis. As an invasive species in the tumor microenvironment, they provide an ideal therapeutic target.
Keywords: epithelial to mesenchymal transition, metastasis, prostate cancer, tumor associated macrophages
Introduction
Cancer is a leading cause of death in the US, and in the past year, over 500,000 people died from this disease.1 While localized cancer is highly treatable by surgical resection and radiation, cancer that has metastasized remains incurable. (Fig. 1)
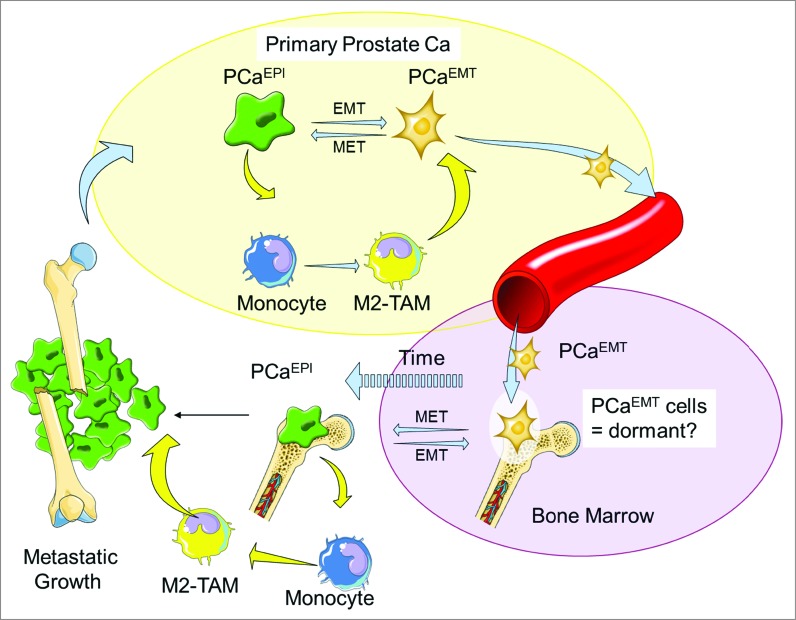
Figure 1.
Prostate Cancer Ecosystem and Diaspora. Prostate cancer epithelial cells (PCaEPI) interact with M2 macrophages and undergo EMT (PCaEMT).10 The M2 macrophages then aide the PCaEMT cells in in situ invasion and intravasation into the lymphatic system. The PCaEMT cells then extravasate, adapt to the bone microenvironment, and remain dormant in the bone marrow before reversing their cellular characteristics by mesenchymal to epithelial transition (MET). As these cells leave dormancy, they increase their proliferative rate, resulting in cancer growth and an osteoblastic reaction in the bone.
Tumor cells of the prostate gland exist as an ecosystem in a complex habitat within their microenvironment, coexisting and exchanging information with the host cells around them.2,3 In the bone, for example, this ecosystem includes haematopoietic stem cell precursors, stromal cells, osteoblasts, osteoclasts, nerve cells, adipocytes, endothelial cells, and multiple cell types of the immune system. Describing tumors as ecological systems defines new opportunities for novel cancer therapies based on disrupting cancer cell–host cell–microenvironment networks.2,3
Tumor Associated Macrophages (TAMs) as part of the cancer ecosystem
Many malignant tumors are associated with an inflammatory infiltrate as part of the reactive stroma that consists mainly of macrophages (often called tumor-associated macrophages), which in some instances, comprise up to 50% of the cell tumor mass.4-7 These cells are an essential cellular component of the innate immune system and are derived from myeloid progenitor cells in the bone marrow compartment. These progenitor cells develop into pro-monocytes and are released into the circulation where they undergo differentiation into monocytes. These monocytes then migrate into tissues where they differentiate into resident tissue macrophages and help to protect these sites from infection and injury as activated (“M1”) or alternatively activated macrophages (“M2”).4-7 In addition to this role in innate immunity, recent evidence suggests that macrophages also play an important role in the regulation of angiogenesis in both normal and diseased tissues, including malignant tumors.4-7 It is not clear whether TAMs are derived from peripheral blood monocytes recruited into the tumor from the circulation or from resident macrophages already in the healthy tissue before tumor develops and metastasizes. Elevated expression of a number of monocyte chemoattractants, including chemokine (C-C motif) ligand 2 (CCL2), CCL3, CCL4, CCL8 and CCL5 (RANTES) by both tumor and stromal cells within tumors has been shown to positively correlate with increased number of TAMs in many human tumors.4-9
When associated with tumors, macrophages demonstrate functional “polarization” that is heavily dependent on specific cytokine exposure. These cytokines include IFN-γ and lipopolysaccharide (LPS) or IL-4/IL-13 or IL-10. This polarization drives one of two phenotypically different subsets (TH1 or TH2) of macrophages. TH1 (also known as M1 macrophages) are activated by IFN-γ, LPS. This type of activation is known as classical. TH2 (also known as M2 macrophages) are activated by IL-4/IL-13 or IL-10.4-9 M1 macrophages are known to produce NO, pro-inflammatory cytokines (IL-1, IL-6, TNF-α and IL-12), and are tumoricidal while M2 macrophages scavenge debris and promote angiogenesis and wound repair. The M2 macrophage population is thought to be phenotypically similar to the tumor-associated macrophage population that promotes tumor growth development, epithelial to mesenchyme transitions, and subsequent metastasis.10 CCL2 as well as CCL7 and CCL8, is an important chemokine that is known to regulate monoctye/macrophage trafficking and has been reported to be present in several solid tumor beds.
Targeting tumor associated macrophages for cancer therapy
In the field of cancer biology, it is imperative to fully investigate the regulation of infiltrating TAMs and to define their function in primary and metastatic disease. Essentially, tumors are “wounds that do not heal” and M2-TAMs are constantly being attracted to cancer lesions where they try to scavenge debris and end up facilitating tumor growth and dissemination. Our analysis of metastases by flow cytometry revealed increased numbers of CD206-positive M2-TAMs in metastatic tissues of PCa patients at rapid autopsy (approximately 40% of cells in bone metastases).8 These findings are indicative of the importance of M2-TAMs in not only primary but also in PCa metastasis. As outlined by Tang and colleagues, TAM targeted therapy strategies can be binned into 4 categories: (i) inhibiting macrophage recruitment; (ii) suppressing TAM survival; (iii) enhancing M1-like tumoricidal activity of TAMs; (iv) blocking M2-like tumor-promoting activity of TAMs.6 Blocking chemoattraction and/or developing macrophage cytotoxic specific therapeutics are attractive ideas that have been reported to help inhibit tumorigenesis and attenuate cancer metastasis.5,8,9 Inducing death of TAMs using bisphosphonates (e.g. zoledronic acid and clodronate) have been reported to decrease differentiation and survival of TAMs. These bisphosphonates have been shown to lead to the accumulation of cytotoxic ATP analogs which can induce apoptosis. However, bisphosphonates typically possess very poor cellular uptake. Another plausible strategy would be to increase the activity and the amount of M1 macrophages to promote tumor cell death by using CpG-ODN or TLR7/8 agonists which aide in up-regulating pro-inflammatory cytokines by positive regulation of the NF-κB pathway.5 Lastly, strategies that target specific antigens on the cell surface of TAMs, such as the mannose receptor, the macrophage scavenging receptor, IL4 receptors, and macrophage colony stimulating factor receptor have been reported and are all currently under further development.5 Each of these formidable strategies would ultimately ablate the interaction of M2 macrophages with the prostate tumor, aide in reprogramming the reactive tumor stroma, leading to tumor shrinkage and a better patient prognosis.
Funding Statement
This work is supported by the NCI grant nos. U54CA143803, CA163124, CA093900 and CA143055.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014 Jan-Feb; 64(1):9-29; PMID: ; http://dx.doi.org/10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- 2.Yang KR, Mooney S, Zarif JC, Coffey DS, Taichman RS, Pienta KJ. Niche inheritance: a cooperative pathway to enhance cancer cell fitness through ecosystem engineering. J Cell Biochem 2014 Sep; 115(9):1478-85; http://dx.doi.org/10.1002/jcb.24813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camacho DF, Pienta KJ. Disrupting the networks of cancer. Clin Cancer Res 2012 May 15; 18(10):2801-8; PMID: ; http://dx.doi.org/10.1158/1078-0432.CCR-12-0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vinogradov S, Warren G, Wei X. Macrophages associated with tumors as potential targets and therapeutic intermediates. Nanomedicine (Lond) 2014 Apr; 9(5):695-707; PMID: ; http://dx.doi.org/10.2217/nnm.14.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cook J, Hagemann T. Tumour-associated macrophages and cancer. Curr Opin Pharmacol 2013 Aug; 13(4):595-601; PMID: ; http://dx.doi.org/10.1016/j.coph.2013.05.017 [DOI] [PubMed] [Google Scholar]
- 6. Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumour-associated macrophages. Immunology. 2013 Feb; 138(2):93-104; PMID: ; http://dx.doi.org/10.1111/imm.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Palma M, Lewis CE.. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013 Mar 18; 23(3):277-86; PMID: ; http://dx.doi.org/10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 8. Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem 2009 Dec 4; 284(49):34342-54; PMID: ; http://dx.doi.org/10.1074/jbc.M109.042671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res 2007 Oct 1; 67(19):9417-24; PMID: ; http://dx.doi.org/10.1158/0008-5472.CAN-07-1286 [DOI] [PubMed] [Google Scholar]
- 10. Roca H, Hernandez J, Weidner S, McEachin RC, Fuller D, Sud S, Schumann T, Wilkinson JE, Zaslavsky A, Li H, et al. Transcription factors OVOL1 and OVOL2 induce the mesenchymal to epithelial transition in human cancer. PLoS One 2013 Oct 4; 8(10):e76773; PMID: ; http://dx.doi.org/10.1371/journal.pone.0076773 [DOI] [PMC free article] [PubMed] [Google Scholar]