Abstract
Activation of β-catenin, the intracellular mediator of canonical Wnt signaling, has a critical role in mediating podocyte injury and proteinuria. However, the underlying mechanisms remain poorly understood. Here, we show that β-catenin triggers ubiquitin-mediated protein degradation of Wilms’ tumor 1 (WT1) and functionally antagonizes its action. In mice injected with adriamycin, WT1 protein was progressively lost in glomerular podocytes at 1, 3, and 5 weeks after injection. Notably, loss of WT1 apparently did not result from podocyte depletion but was closely associated with upregulation of β-catenin. This change in WT1/β-catenin ratio was accompanied by loss of podocyte-specific nephrin, podocalyxin, and synaptopodin and acquisition of mesenchymal markers Snail1, α-smooth muscle actin, and fibroblast-specific protein 1. In vitro, overexpression of β-catenin induced WT1 protein degradation through the ubiquitin proteasomal pathway, which was blocked by MG-132. WT1 and β-catenin also competed for binding to common transcriptional coactivator CREB-binding protein and mutually repressed the expression of their respective target genes. In glomerular miniorgan culture, activation of β-catenin by Wnt3a repressed WT1 and its target gene expression. In vivo, blockade of Wnt/β-catenin signaling by endogenous antagonist Klotho induced WT1 and restored podocyte integrity in adriamycin nephropathy. These results show that β-catenin specifically targets WT1 for ubiquitin-mediated degradation, leading to podocyte dedifferentiation and mesenchymal transition. Our data also suggest that WT1 and β-catenin have opposing roles in podocyte biology, and that the ratio of their expression levels dictates the state of podocyte health and disease in vivo.
Keywords: podocyte, proteinuria, glomerular disease, WT1, β-catenin
Podocyte injury is a major pathologic feature in a wide variety of proteinuric kidney diseases, such as FSGS and diabetic nephropathy.1,2 As a crucial element of the glomerular filtration apparatus, the state of podocyte health is essential for establishing and maintaining a functional size and charge barrier to prevent proteinuria.3,4 Extensive experimental and clinical evidences indicate that podocyte injury not only plays a fundamental role in the initiation and progression of proteinuria but also is a key factor that drives glomerulosclerosis in the pathogenesis of CKD.5,6 In this context, deciphering the signaling pathways underlying podocyte injury would be paramount for understanding the pathomechanisms of proteinuria as well as for designing therapeutic strategies to combat proteinuric CKD.
We and others previously reported that activation of β-catenin, the intracellular mediator of canonical Wnt signaling, plays a critical role in mediating podocyte injury/dysfunction in proteinuric CKD.7–10 As a transcriptional regulator, β-catenin is translocated into the nucleus on stimulation, wherein it binds to members of the T cell factor (TCF)/lymphoid enhancer-binding factor family of transcription factors. Nuclear β-catenin/TCF then assembles a transcriptionally active complex by recruiting transcriptional coactivators, such as the cAMP response element-binding protein-binding protein (CBP), to stimulate the transcription of its target genes.11–13 Extensive studies have shown aberrant activation of β-catenin in glomerular podocytes in a range of animal models of proteinuric CKD and human biopsies from patients with various kidney disorders, such as FSGS, diabetic nephropathy, and IgA nephropathy.7,14,15 Consistently, either genetic ablation of β-catenin in a podocyte-specific fashion or pharmacologic inhibition of β-catenin signaling protects mice against podocyte dysfunction and proteinuria after injury.7,10,16 These studies suggest that dysregulated activation of β-catenin signaling could be a common mechanism leading to podocyte dysfunction and proteinuria. However, the mechanism by which β-catenin induces podocyte injury remains poorly understood.
Wilms’ tumor 1 (WT1) protein is a pivotal transcription factor that is exclusively expressed in glomerular podocytes in adult kidneys. As a zinc finger transcription factor, WT1 is also able to bind to the transcriptional coactivator CBP and drives the expression of a host of podocyte-specific genes, such as nephrin and podocalyxin.17–21 WT1 has been shown to be essential and required for normal embryonic kidney development as well as the maintenance of the differentiated state of podocytes in adult kidneys.22 Genetic deletion and mutation cause severe kidney disorders characterized by glomerular lesions and proteinuria.23–25 As a podocyte-specific nuclear protein, WT1 is often used as a molecular marker for assessing podocyte density in the kidneys, and loss of WT1 in pathologic conditions is commonly interpreted as a result of podocyte depletion.26 However, exactly how WT1 is regulated in glomerular podocytes in vivo remains largely unknown.
In this study, we show that β-catenin signaling specifically targets WT1 for ubiquitin-mediated protein degradation, and loss of WT1, in turn, amplifies β-catenin signaling. Furthermore, we show that WT1 and β-catenin mutually antagonize each other through competition for binding to the common transcriptional coactivator CBP. Our results suggest that the balance between WT1 and β-catenin plays a decisive role in dictating the state of podocyte health and sickness in vivo.
Results
Loss of WT1 in Adriamycin Nephropathy Is Not Caused by Podocyte Depletion
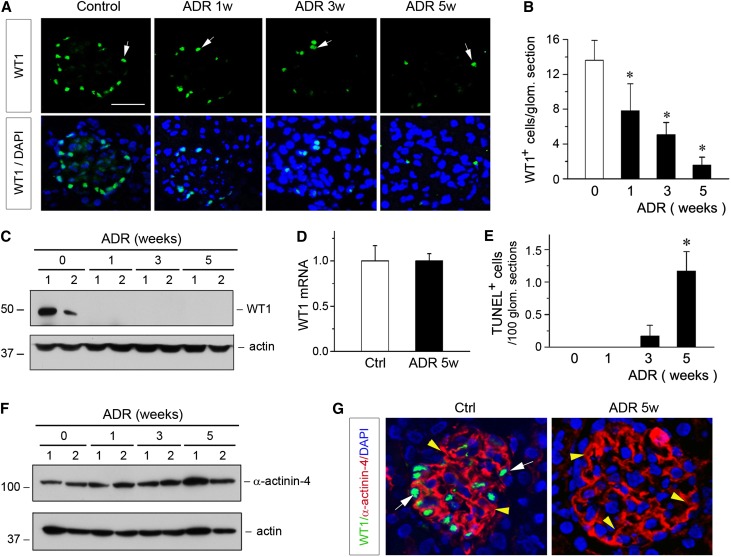
We first examined the expression of WT1 in adrimycin (ADR) nephropathy, a model of human FSGS.27 As shown in Figure 1A, immunofluorescence staining showed that WT1-positive cells were dramatically reduced in a time-dependent manner after ADR injection. Quantitative determination of the WT1-positive podocytes in mouse kidneys at 1, 3, and 5 weeks after ADR injection is presented in Figure 1B. Both the numbers of the WT1-positive cells per glomerular section and the intensity of WT1 protein staining progressively decreased according to the duration of the injury. Loss of WT1 in ADR nephropathy was also confirmed by Western blot analyses of whole-kidney lysates. As illustrated in Figure 1C, WT1 protein almost completely vanished as early as 1 week after ADR injection. Therefore, loss of WT1 is an early and robust pathologic finding in ADR nephropathy.
Figure 1.
Loss of WT1 in ADR nephropathy is not caused by podocyte depletion. (A) Representative micrographs show glomerular WT1 staining at different time points after ADR injection. BALB/c mice were injected with ADR, and kidney tissues were collected at 1, 3 and 5 weeks postinjection, and stained for WT1. Arrows indicate WT1-positive staining. DAPI, 4′,6-diamidino-2-phenylindole. (B) Quantitative determination of the WT1-positive cells in different groups. WT1-positive cells were counted in 10 randomly chosen glomerular sections, and the averages of the WT1-positive cells per glomerular section were calculated. *P<0.05 versus normal controls (n=5–6). (C) Representative Western blotting shows renal WT1 protein levels in various groups as indicated. Numbers (1 and 2) indicate the individual animal in a given group. (D) qRT-PCR analyses show no difference in renal WT1 mRNA expression at 5 weeks after ADR injection. Ctrl, control. (E) Quantitative determination of the apoptotic glomerular cells. Kidney paraffin sections were subjected to TUNEL staining. TUNEL-positive glomerular cells were counted in different groups. Data are presented as numbers of apoptotic cells per 100 glomerular sections. *P<0.05 versus normal controls (n=5–6). (F) Western blotting shows α-actinin-4 protein abundance at different time points after ADR injection. Numbers (1 and 2) indicate the individual animal in a given group. (G) Representative micrographs show the double immunostaining for WT1 (green) and α-actinin-4 (red) in the kidneys of control (Ctrl) and ADR mice at 5 weeks after injection (ADR 5w). White arrows indicate WT-1–positive staining, whereas yellow arrowheads show α-actinin-4–positive staining.
To our surprise, despite a dramatic loss of WT1 protein, WT1 mRNA levels were not changed during the course of ADR nephropathy. As shown in Figure 1D, no difference in WT1 mRNA level was found, even at 5 weeks after ADR injection, compared with the controls. These results suggest that loss of WT1 protein is unlikely to be a result of reduced WT1 mRNA expression or loss of the WT1-expressing podocytes. To further address this issue, we investigated podocyte apoptosis by terminal deoxynucleotidyl transferase-mediated deoxyuridine nick-end labeling (TUNEL) staining. As shown in Figure 1E, no apoptotic cells were detected in the glomeruli at 1 week after ADR injection, a time point when WT1 protein was largely lost (Figure 1C). Even at 5 weeks after ADR injection, glomerular cell apoptosis was extremely rare, at approximately one apoptotic cell per 100 glomerular sections (Figure 1E). Consistent with this notion, we found that α-actinin-4, a podocyte-specific cytoskeleton protein, was not changed during the entire course of ADR nephropathy, which was assessed by Western blot analyses (Figure 1F) and immunostaining (data not shown), indicating the physical presence of podocytes. Costaining for WT1 and α-actinin-4 revealed that, even at 5 weeks after ADR injection when WT1 was lost, glomerular α-actinin-4 protein remained completely preserved (Figure 1G). Taken together, these data suggest that loss of WT1 is not a result of podocyte depletion in this model.
Loss of WT1 Is Associated with β-Catenin Activation and Podocyte Dedifferentiation
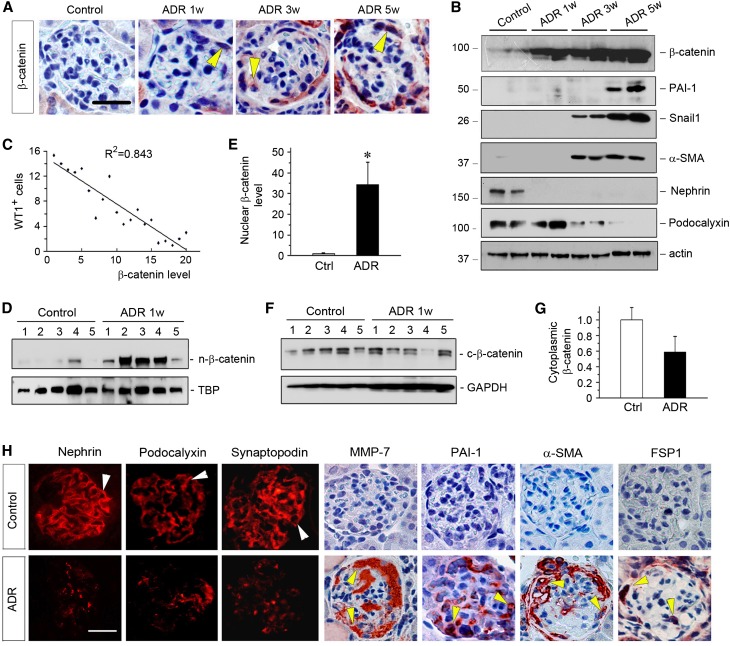
We found that loss of WT1 was closely associated with glomerular activation of β-catenin. As shown in Figure 2A, β-catenin was upregulated predominantly in glomerular podocytes in a time-dependent fashion after ADR injection. Induction of β-catenin protein was also confirmed by Western blot analyses (Figure 2B), although the absolute levels of renal β-catenin in different groups remained unknown. Additional linear regression analysis revealed an inverse correlation between the numbers of the WT1-positive podocytes and the abundances of β-catenin in ADR nephropathy (Figure 2C). Nuclear translocation of β-catenin was evident in the kidneys as early as 1 week after ADR injection (Figure 1, D–G), suggesting an early activation of β-catenin signaling in this model.7 These results suggest that loss of WT1 might be linked to β-catenin activation in podocytes after ADR injury.
Figure 2.
Loss of WT1 is associated with β-catenin activation and phenotypic transition of podocytes in ADR nephropathy. (A) Representative micrographs show glomerular β-catenin staining in different groups as indicated. Arrowheads indicate β-catenin–positive podocytes. Scale bar, 35 µm. (B) Representative Western blotting shows dramatic changes in podocyte-specific proteins as well as β-catenin and its target genes. Kidney lysates were immunoblotted with specific antibodies against β-catenin, PAI-1, Snail1, α-SMA, nephrin, podocalyxin, and actin. (C) Linear regression shows an inverse correlation between WT1-positive cells per glomerular section and β-catenin protein abundance (arbitrary units) at different time points after ADR injection. The correlation coefficient (R2) is shown. (D–G) Activation and nuclear translocation of β-catenin occurs at 1 week after ADR injection. Nuclear and cytoplasmic proteins were fractionated from the kidneys of control and ADR mice at 1 week after injection and subjected to Western blot analyses. Nuclear β-catenin was normalized to TATA-binding protein (TBP), whereas cytoplasmic β-catenin was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (D and F) Western blots and (E and G) quantitative data are presented. Numbers (1–5) represent individual animals in a given group. *P<0.05 versus normal controls (n=5). Ctrl, control. (H) Representative micrographs show phenotypic changes of glomerular podocytes in ADR nephropathy. Kidney sections prepared from mice at 5 weeks after ADR injection or controls were stained with specific antibodies against nephrin, podocalyxin, synaptopodin, MMP-7, PAI-1, α-SMA, and FSP-1. Arrowheads indicate positive staining. Scale bar, 30 µm.
We then investigated the expression of the WT1 and β-catenin downstream target genes. As shown in Figure 2B, podocyte-specific WT1 target genes, such as nephrin and podocalyxin, were downregulated in whole-kidney lysates after ADR injection. Meanwhile, β-catenin target genes, such as plasminogen activator inhibitor-1 (PAI-1),28 Snail1,7 and α-smooth muscle actin (α-SMA), were markedly induced (Figure 2B). Quantitative real-time RT-PCR (qRT-PCR) show that matrix metalloproteinase-7 (MMP-7), another direct downstream target gene of β-catenin,29 was also induced in a time-dependent manner (data not shown). Immunostaining confirmed the downregulation of podocyte-specific proteins, such as nephrin, podocalyxin, and synaptopodin after ADR injury, which was accompanied by de novo expression of MMP-7, PAI-1, fibroblast-specific protein-1 (FSP-1), and α-SMA specifically in glomerular podocytes (Figure 2H). These data suggest that loss of WT1 is also associated with podocyte dedifferentiation and mesenchymal transition in ADR nephropathy.
β-Catenin Triggers WT1 Protein Degradation through the Ubiquitin–Proteasome Pathway
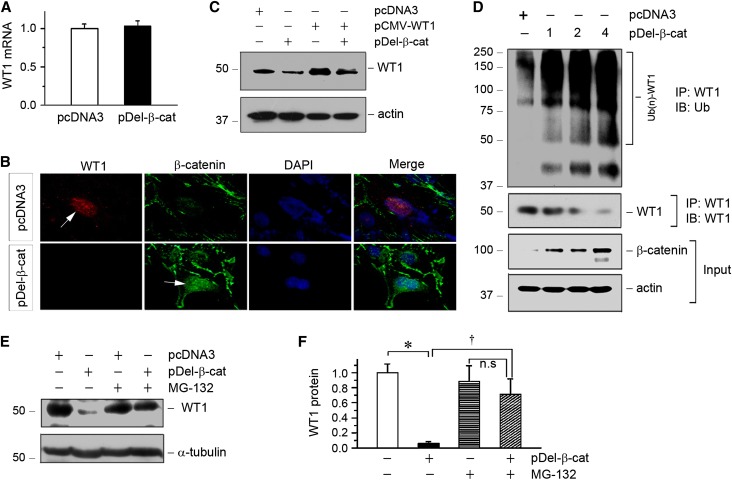
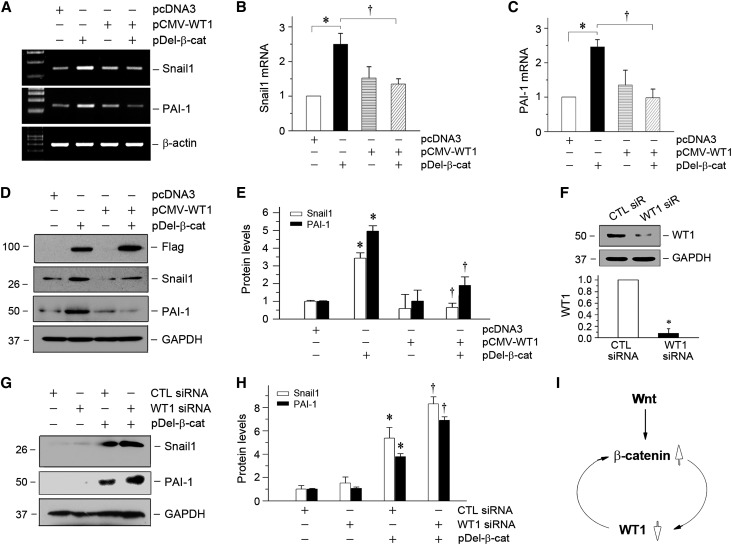
To explore whether the loss of WT1 is mechanistically coupled with β-catenin activation, we examined the possibility that β-catenin regulates WT1 expression. To this end, mouse podocytes were transfected with the N-terminally truncated, constitutively activated β-catenin expression vector (pDel-β-cat) in vitro. As shown in Figure 3A, overexpression of β-catenin did not affect WT1 mRNA expression, which was assessed by qRT-PCR, suggesting that β-catenin has no role in regulating WT1 gene transcription. However, immunostaining revealed that overexpression of β-catenin reduced endogenous WT1 protein in cultured podocytes (Figure 3B), because nuclear β-catenin and WT1 often existed in a mutually exclusive fashion. To further address this issue, we transfected mouse podocytes with pDel-β-cat and/or WT1 expression vector with exogenous WT1 under the control of cytomegalovirus (CMV) promoter (pCMV-WT1). As shown in Figure 3C, β-catenin also markedly reduced exogenous WT1 protein expression in podocytes, but it did not affect WT1 mRNA level (data not shown). These results imply a crucial role for β-catenin in controlling WT1 protein stability and degradation.
Figure 3.
β-Catenin promotes WT1 protein degradation through the ubiquitin–proteasome pathway. (A) qRT-PCR analysis shows that overexpression of constitutively activated β-catenin did not affect WT1 mRNA expression in mouse podocytes. (B) Ectopic expression of β-catenin inhibited endogenous WT1 protein expression. Mouse podocytes were transfected with pDel-β-cat or empty vector (pcDNA3) followed by immunofluorescence staining for WT1 and β-catenin. Arrows indicate positive staining. DAPI, 4′,6-diamidino-2-phenylindole. (C) Representative Western blotting shows that β-catenin reduced WT1 protein. Mouse podocytes were cotransfected with various plasmids as indicated. Cell lysates were immunoblotted with antibodies against WT1 and actin. (D) β-Catenin dose-dependently induced WT1 ubiquitination and its subsequent proteasome-dependant degradation. Mouse podocytes were transfected with increasing amounts of pDel-β-cat as indicated. Cell lysates were then immunoprecipitated with anti-WT1 antibody followed by immunoblotting (IB) with antiubiquitin (anti-Ub) or anti-WT1. Whole-cell lysates were also immunoblotted with antibodies against β-catenin and actin. (E) MG-132 preserved WT1 protein expression in podocytes. Mouse podocytes were pretreated with MG-132 (5 μM) at 37°C for 30 minutes and then transfected with β-catenin expression vector. Cell lysates were immunoblotted with antibodies against WT1 and α-tubulin. (F) Quantitative data show that MG-132 inhibited the β-catenin–mediated WT1 degradation. *P<0.05 versus pcDNA3 controls; †P<0.05 versus pDel-β-cat alone (n=3).
We further investigated the potential role of the ubiquitin-mediated protein degradation in the WT1 downregulation, because this system plays a major role in mediating intracellular protein degradation in eukaryotic cells.30,31 As shown in Figure 3D, coimmunoprecipitation (co-IP) showed that overexpression of β-catenin dose-dependently promoted WT1 ubiquitination and its proteasome-dependent degradation. To further confirm the involvement of the ubiquitin–proteasome pathway in mediating β-catenin–triggered WT1 degradation, we examined the effect of MG-132, a specific proteasome inhibitor.32,33 As shown in Figure 3, E and F, WT1 degradation was efficiently blocked by pretreatment with MG132. Therefore, these results indicate that β-catenin targets WT1 protein for degradation through the ubiquitin–proteasome pathway.
β-Catenin Represses WT1-Mediated Gene Expression and Induces Podocyte Dedifferentiation
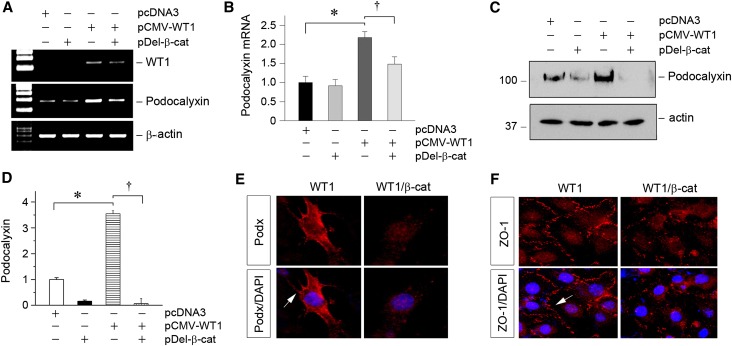
Given that β-catenin induces WT1 protein degradation, we next tested whether β-catenin signaling suppresses WT1-mediated gene expression in podocytes. As shown in Figure 4, A and B, podocalyxin, a direct WT1 downstream gene,20,21 was induced in glomerular podocytes by overexpression of WT1. However, podocalyxin mRNA expression was substantially repressed by cotransfection of pDel-β-cat. Similar results were obtained when podocalyxin protein was assessed by Western blotting and immunofluorescence staining (Figure 4, C–E). In addition, β-catenin also inhibited the expression of zona occludens 1 (ZO-1), an epithelial marker that is abundantly expressed in podocytes. As shown in Figure 4F, immunofluorescence staining showed that ZO-1 was present at the sites of cell-to-cell contact with a characteristic zipper-like pattern between the interdigitating cell processes of podocytes after WT1 transfection.34 However, cotransfection of β-catenin significantly reduced the overall intensity of ZO-1 staining, and its zipper-like staining pattern was changed to a noncontinuous belt along the cell borders (Figure 4F), consistent with the notion that β-catenin antagonizes WT1 and induces podocyte dedifferentiation.
Figure 4.
β-Catenin induces podocyte dedifferentiation by antagonizing WT1-mediated gene expression. (A and B) RT-PCR analyses show that overexpression of β-catenin abolished WT1-mediated podocalyxin gene expression. Mouse podocytes were transfected with WT1 expression vector (pCMV-WT1) or/and pDel-β-cat. Representative (A) RT-PCR and (B) quantitative data of podocalyxin mRNA levels are presented. *P<0.05 versus pcDNA3 controls; †P<0.05 versus pCMV-WT1 alone (n=3). (C and D) Western blotting analyses show that β-catenin abolished WT1-mediated podocalyxin protein expression in mouse podocytes. Representative (C) Western blot and (D) quantitative data are presented. *P<0.05 versus pcDNA3 controls; †P<0.05 versus pCMV-WT1 alone (n=3). (E) Representative micrographs show that β-catenin blocked the WT1-mediated podocalyxin expression. Arrow indicates positive staining for podocalyxin. (F) Immunofluorescence staining shows that ectopic expression of β-catenin blocked the WT1-mediated ZO-1 expression. Arrow indicates positive staining for ZO-1. DAPI, 4′,6-diamidino-2-phenylindole.
Loss of WT1 Amplifies β-Catenin Signaling in Podocytes
We next tested the hypothesis that loss of WT1 might affect β-catenin signaling and its downstream gene expression. To this end, we examined the effects of manipulating WT1 levels on β-catenin target gene expression in podocytes. As illustrated in Figure 5, A–C, overexpression of WT1 completely abolished Snail1 and PAI-1 mRNA induction by β-catenin. Consistently, WT1 also inhibited β-catenin–mediated Snail1 and PAI-1 protein expression (Figure 5, D and E). To further assess the functional interplay between WT1 and β-catenin, we next knocked down the endogenous WT1 protein in podocytes by using small interfering RNA (siRNA) (Figure 5F). As shown in Figure 5, G and H, knockdown of WT1 significantly augmented the expression of Snail1 and PAI-1 induced by β-catenin. These results suggest that loss of WT1 in pathologic conditions would further amplify β-catenin signaling in podocytes. Therefore, as illustrated in Figure 5I, β-catenin activation and loss of WT1 effectively form a vicious cycle, leading to podocyte dedifferentiation and mesenchymal transition.
Figure 5.
Loss of WT1 amplifies β-catenin signaling and augments its downstream gene expression in vitro. (A–C) RT-PCR analyses show that overexpression of WT1 abolished β-catenin–mediated gene expression. Mouse podocytes were transfected with WT1 expression vector (pCMV-WT1) or/and pDel-β-cat as indicated. The mRNA expressions of Snail1 and PAI-1 were assessed by RT-PCR. Representative (A) RT-PCR and quantitative data for (B) Snail1 or (C) PAI-1 are presented. *P<0.05 versus pcDNA3 controls; †P<0.05 versus pDel-β-cat alone (n=3). (D and E) Western blotting shows that overexpression of WT1 blocked the induction of β-catenin target genes in moue podocytes. (D) Representative Western blot was performed to analyze the expression of Snail1 and PAI-1. Expression of exogenous β-catenin was verified by assessing Flag. Quantitative data are presented in E. *P<0.05 versus pcDNA3 controls; †P<0.05 versus pDel-β-cat alone (n=3). (F) Knockdown of endogenous WT1 protein in podocytes. Mouse podocytes were transfected with control or WT1-specific siRNA, and WT1 protein expression was analyzed by Western blotting. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to confirm equivalent loading. *P<0.05 versus siRNA (n=3). CTL, control; siR, siRNA. (G–I) Knockdown of endogenous WT1 augments β-catenin–mediated Snail1 and PAI-1 expression. Representative (G) Western blot and (H) quantitative data for Snail1 and PAI-1 are presented. *P<0.05 versus CTL siRNA; †P<0.05 versus pDel-β-cat plus CTL siRNA (n=3). (I) Diagram indicates that β-catenin activation and loss of WT1 constitute a vicious cycle, leading to podocyte dedifferentiation.
WT1 and β-Catenin Mutually Antagonize through Competition for Binding to CBP
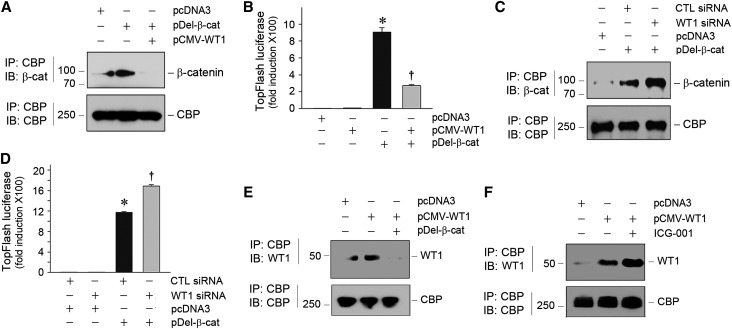
Both WT1 and β-catenin activate the transcription of their target genes by recruiting and interacting with common transcriptional coactivator CBP. In this context, we hypothesized that the mutual antagonism between WT1 and β-catenin might also be mediated through competition for binding to CBP. To test this hypothesis, we examined the interactions among WT1, β-catenin, and CBP in podocytes by co-IP. As shown in Figure 6A, overexpression of β-catenin induced a significant increase in β-catenin/CBP complex formation, which was inhibited by cotransfection of WT1 expression vector, suggesting that WT1 blocks β-catenin interaction with CBP. Such an inhibition of β-catenin/CBP interaction is functionally significant, because WT1 abolished β-catenin–mediated gene transcription in a TOPFlash reporter luciferase assay (Figure 6B). In contrast, knockdown of WT1 by siRNA enhanced β-catenin/CBP interaction (Figure 6C) and augmented β-catenin–mediated gene transcription (Figure 6D).
Figure 6.
WT1 and β-catenin mutually antagonize each other through competing for binding to coactivator CBP. (A) Co-IP shows that WT1 inhibited β-catenin/CBP interaction. Mouse podocytes were transfected with different plasmid vectors as indicated. Cell lysates were immunoprecipitated with anti-CBP followed by immunoblotting (IB) with anti–β-catenin or anti-CBP. (B) WT1 inhibited β-catenin–mediated gene transcription. Mouse podocytes were cotransfected with TOPFlash luciferase reporter plasmid as well as pCMV-WT1 or pDel-β-cat as indicated. *P<0.05 versus pcDNA3 controls; †P<0.05 versus pDel-β-cat alone (n=3). (C) Knockdown of WT1 augmented β-catenin/CBP interaction. Mouse podocytes were transfected with control or WT1-specific siRNA and then subjected to co-IP as indicated. CTL, control. (D) Knockdown of WT1 amplified β-catenin–mediated gene transcription. Mouse podocytes were cotransfected with TOPFlash luciferase reporter and control or WT1-specific siRNA in the absence or presence of pDel-β-cat. *P<0.05 versus pcDNA3 controls; †P<0.05 versus pDel-β-cat alone (n=3). (E) β-Catenin reciprocally inhibited WT1/CBP interaction. Mouse podocytes were transfected with various expression vectors as indicated. WT1/CBP interaction was assessed by co-IP. (F) Blockade of β-catenin/CBP interaction by small molecule ICG-001 enhanced WT1/CBP interaction. Mouse podocytes were treated with ICG-001 (5 µM) and transfected with pCMV-WT1 as indicated. WT1/CBP interaction was assessed by co-IP.
In the reciprocal experiments, we found that β-catenin also blocked WT1/CBP interaction, because overexpression of β-catenin abolished WT1/CBP complex formation in podocytes (Figure 6E). In addition, incubation of podocytes with ICG-001, a small molecule that specifically disrupts β-catenin/CBP interaction,35,36 was able to enhance WT1/CBP interaction (Figure 6F). These results suggest that WT1 and β-catenin could mutually antagonize each other through competing for binding to CBP.
Wnt/β-Catenin Suppresses WT1 and Its Target Genes in Glomerular Miniorgan Culture
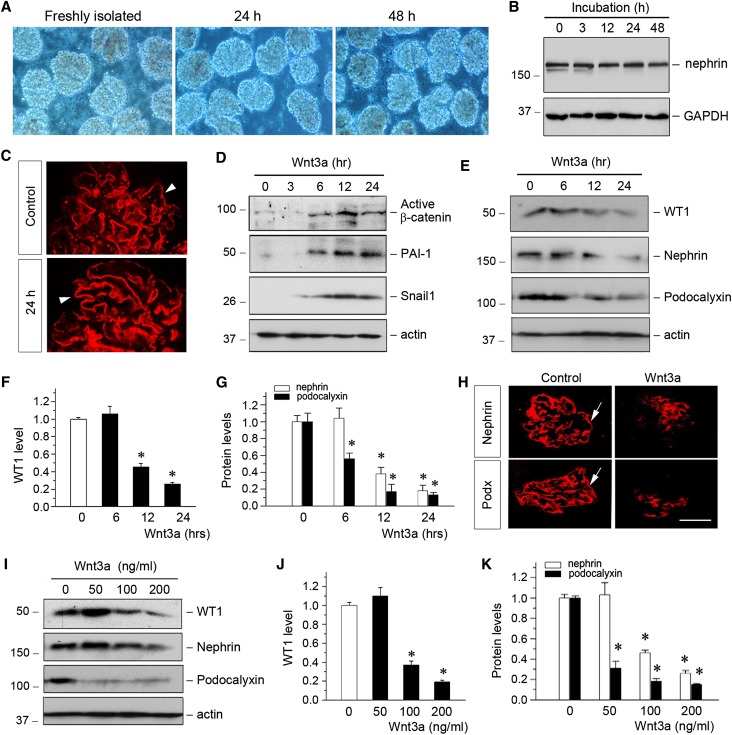
To ascertain the role of β-catenin in controlling WT1 protein and its target genes, we used glomerular miniorgan culture, a model system that largely preserves the sophisticated three-dimensional morphology of podocytes in vivo. Rat glomeruli were chosen, because it was relatively easy to obtain a large amount of glomeruli with high purity from this species. As shown in Figure 7A, these glomeruli could be cultivated for 2 days in suspension, with relatively intact glomerular structure. Major slit diaphragm-associated proteins, such as nephrin, were well preserved during the period of cultivation (Figure 7B). Immuostaining revealed a fine staining pattern of nephrin in these cultured glomeruli (Figure 7C), suggesting the well preserved glomerular integrity.
Figure 7.
Wnt/β-catenin signaling suppresses WT1 and its downstream gene expression in glomerular miniorgan culture. (A) Representative micrograph shows glomerular miniorgan culture for different periods of time as indicated in vitro. Glomeruli were isolated from rat kidneys and cultivated in suspension for 24 and 48 hours. (B) Western blot analyses show nephrin expression in cultured glomeruli at different time points as indicated. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (C) Representative micrographs show nephrin expression and distribution in freshly isolated rat glomerulus (control) and glomerulus cultured for 24 hours. Arrowheads indicate positive staining. (D) Representative Western blotting shows the activation of β-catenin and its downstream PAI-1 and Snail1 after Wnt3a stimulation. Isolated glomeruli were incubated with Wnt3a (100 ng/ml) for different periods of time as indicated. Glomerular lysates were immunoblotted with specific antibodies against active β-catenin, PAI-1, Snail1, and actin. (E–G) Wnt3a inhibited WT1 and its downstream nephrin and podocalyxin in glomerular miniorgan culture. Glomeruli were incubated with Wnt3a for various periods of time as indicated. Representative (D) Western blots and quantitative data for (F) WT1 or (G) nephrin and podocalyxin are presented. *P<0.05 versus controls (n=3). (H) Immunofluorescence staining shows the downregulation of nephrin and podocalyxin (podx) by Wnt3a in glomerular culture. Arrows indicate positive staining. (I–K) Wnt3a inhibited WT1 and its downstream target genes in a dose-dependent fashion. Representative (I) Western blotting and quantitative data for (J) WT1 or (K) nephrin and podocalyxin are presented. *P<0.05 versus controls (n=3).
Incubation of rat glomeruli with Wnt3a clearly activated β-catenin and induced its downstream target genes, such as PAI-1 and Snail1 expression, which was shown by Western blot analyses of glomerular miniorgan culture (Figure 7D). Interestingly, activation of β-catenin by Wnt3a also resulted in the loss of WT1 protein and reduced nephrin and podocalyxin in a time-dependent manner (Figure 7, E–G). Similar results were obtained by immunofluorescence staining (Figure 7H). Wnt3a also dose-dependently downregulated WT1, nephrin, and podocalyxin in glomerular miniorgan culture (Figure 7, I–K).
Blockade of Wnt Signaling Restores WT1 and Preserves Podocyte Integrity In Vivo
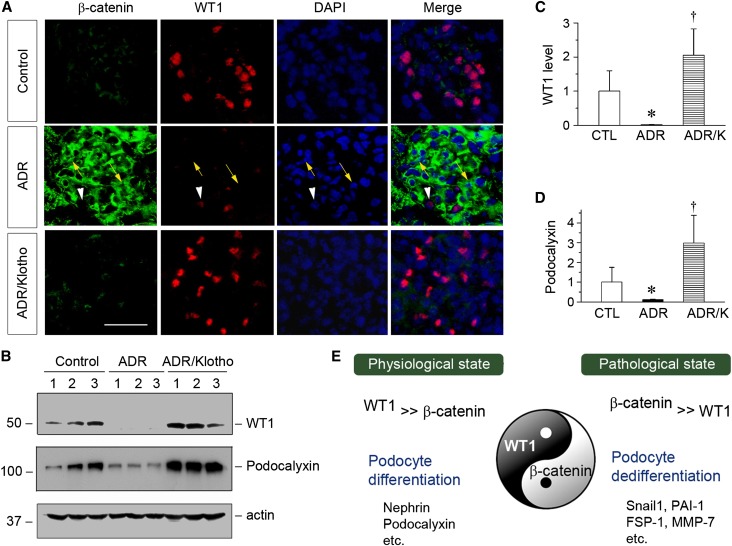
We finally investigated the in vivo relevance of the interplay between WT1 and β-catenin in dictating podocyte health and injury. To this end, we inhibited Wnt/β-catenin signaling by using Klotho, because previous studies have shown that Klotho is an endogenous antagonist of Wnt signaling by physically binding to and sequestering Wnt ligands.16,37 BALB/c mice were injected with ADR, and 1 week later, mice were injected with a soluble secreted form of Klotho expression vector (pV5-sKlotho), which was reported previously.16 ADR induced marked activation of β-catenin (Figure 8A, green) in podocytes and concomitant downregulation of WT1 (Figure 8A, red). However, inhibition of Wnt signaling by soluble Klotho abolished β-catenin activation and restored WT1 expression (Figure 8A). Of note, costaining of WT1 and β-catenin was extremely rare, indicating that they were present in podocytes in a mutually exclusive manner in vivo. We further examined the expression of WT1 and its downstream podocalyxin by Western blotting. As shown in Figure 8, B–D, ADR induced significant downregulation of WT1 and podocalyxin, and Klotho completely preserved both WT1 and podocalyxin expression. As reported previously,16 exogenous Klotho also reduced proteinuria in this model. These data further illustrate an opposing role for WT1 and β-catenin in podocyte biology in vivo.
Figure 8.
Blockade of Wnt signaling by Klotho inhibits β-catenin activation and preserves renal WT1 expression in ADR nephropathy in vivo. (A) Coimmunofluorescence staining shows glomerular β-catenin and WT1 expression in different groups. Kidney cryosections were coimmunostained for β-catenin (green) and WT1 (red). Nuclei were visualized by 4′,6-diamidino-2-phenylindole (DAPI) staining. Yellow arrows indicate β-catenin–positive cells, whereas white arrowheads denote WT1-positive podocytes. Scale bar, 30 µm. (B–D) Western blotting analyses of kidney lysates show that blockade of Wnt signaling by Klotho preserved WT1 and promoted podocalyxin expression. Representative (B) Western blots and quantitative data for (C) WT1 or (D) podocalyxin are presented. CTL, control. *P<0.05 versus controls; †P<0.05 versus ADR (n=5). (E) The diagram shows that WT1 and β-catenin, as Yin and Yang, are two key transcription regulators that play opposing roles in podocyte biology. Their ratio dictates the state of podocyte health (differentiation) and disease (dedifferentiation) in vivo.
Discussion
Podocytes are a unique population of terminally differentiated glomerular visceral epithelial cells that possess distinctive morphologic features exemplified by sophisticated foot processes.3,4 Under pathologic conditions, injurious stimuli often cause podocyte dedifferentiation characterized by loss of podocyte-specific features, thereby leading to podocyte dysfunction and defective glomerular filtration.1,2 In this study, we have identified WT1 and β-catenin as two master transcriptional regulators that play opposing roles in podocyte biology. As depicted in Figure 8E, WT1, a key transcription factor that is exclusively expressed in glomerular podocytes in normal adult kidneys, is the predominant force that drives podocyte differentiation by promoting podocyte-specific gene expression as well as antagonizing β-catenin action. However, β-catenin, as a master transcription regulator that is induced in glomerular podocytes after injury, orchestrates podocyte dedifferentiation by triggering ubiquitin-mediated WT1 protein degradation as well as promoting podocyte epithelial–mesenchymal transition.34 Therefore, WT1 and β-catenin, functioning as Yin and Yang, antagonize each other and control podocyte differentiation and dedifferentiation program, respectively (Figure 8E). On the basis of these findings, we propose that the balance between WT1 and β-catenin is a key determinant that dictates the state of podocyte health and disease in vivo.
One intriguing finding in this study is that loss of WT1 in ADR nephropathy is not caused by a reduced WT1 mRNA expression or a loss of the WT1-expressing podocytes. Rather, it primarily results from increased WT1 protein degradation through an ubiquitin-mediated, proteasome-dependent pathway. WT1, as a podocyte-specific nuclear protein, is often used as a molecular signature of podocytes, and loss of WT1 in diseased kidneys is commonly interpreted as a sign of podocyte depletion.26,38 However, our results underscore that loss of WT1 in pathologic conditions does not necessarily indicate podocyte absence. Therefore, the methodology of using WT1 staining for estimating podocyte density in diseased kidneys, a common practice in the field, seems problematic and inappropriate. For that matter, staining for other podocyte-specific proteins, such as nephrin, podocalyxin, and synaptopodin, does not help either, because loss of these proteins cannot be interpreted as a result of podocyte deficiency as well (Figure 2). In this context, the earlier observations of podocyte depletion, as assessed after WT1 staining, in the pathogenesis of proteinuira in common CKD, such as diabetic nephropathy, should be interpreted with caution.26,38 We believe that podocyte dedifferentiation rather than depletion is the primary mechanism that causes proteinuria in the vast majority of CKD. Consistent with this view, robust proteinuria already occurred at 1 week after ADR injection, a time point when glomerular cell apoptosis was undetectable (Figure 1).
The novel finding that β-catenin targets WT1 for ubiquitin-mediated degradation provides a mechanistic insight into the pathomechanism of β-catenin action in podocytes. Earlier studies have established that Wnt/β-catenin signaling is implicated in mediating podocyte injury and proteinuria in a variety of CKD,7,10 but the mechanisms underlying β-catenin action remain elusive. This study links β-catenin activation to the ubiquitin-mediated protein degradation of WT1, a crucial transcription factor that safeguards podocyte integrity and differentiated status.20,25,39 It should be stressed that the molecular details on how β-catenin leads to WT1 ubiquitination and subsequent degradation remain enigmatic. We found that there was no physical interaction between WT1 and β-catenin by co-IP (data not shown), suggesting that β-catenin may not directly induce WT1 degradation. It is most likely that β-catenin mediates expression of a ubiquitin ligase or some other target genes, which in turn, leads to WT1 degradation. Exactly which ubiquitin ligase is induced by β-catenin and responsible for WT1 degradation is unknown at this stage. This question warrants additional investigation.
Downregulation of WT1 by β-catenin only takes place in protein level and not WT1 mRNA. We have previously shown that blockade of Wnt/β-catenin by DKK1 does not affect renal WT1 mRNA expression in the TGF-β1–induced podocyte injury, suggesting that WT1 gene transcription is not controlled by Wnt/β-catenin.15 This finding is consistent with the observations that β-catenin did not affect WT1 mRNA expression in vitro (Figure 3A), and there was no change in WT1 mRNA in ADR nephropathy in vivo (Figure 1D), despite a marked β-catenin activation in this model (Figure 2B). Taken together, these studies indicate that Wnt/β-catenin does not affect WT1 mRNA expression, but exclusively induces its protein degradation. The notion that β-catenin induces WT1 protein degradation is supported by multiple lines of evidence. It not only occurs in cultured podocytes (Figure 3) but also, glomerular miniorgan culture (Figure 7) and in vivo (Figure 8), because WT1 and β-catenin rarely coexist in the nuclei of podocytes. Given the critical role of WT1 in maintaining podocyte differentiation, it is conceivable that loss of WT1 after β-catenin activation, per se, would result in podocyte dedifferentiation. Of interest, loss of WT1, in turn, is able to amplify β-catenin signaling, because WT1 is a negative inhibitor of Wnt/β-catenin signaling.40,41 Therefore, β-catenin activation and loss of WT1 effectively form a feed-forward vicious cycle, leading to exaggerated podocyte injury (Figure 5I).
WT1 and β-catenin are able to mutually antagonize each other and repress the expression of their respective target genes (Figures 4 and 5). It should be noted that overexpression of β-catenin in podocytes resulted in almost complete inhibition of WT1-mediated podocalyxin expression (Figure 4), although the transfection efficiency was estimated to be 50% (data not shown). This discrepancy is probably caused by the underestimation of the transfection efficiency using GFP expression; some transfected cells might not be identifiable, because the GFP levels did not reach the detection threshold by microscopy. The mutual antagonism of WT1 and β-catenin is most likely to be mediated by their competition for binding to common transcriptional coactivator CBP (Figure 6). Because both WT1 and β-catenin require CBP for efficiently stimulating the expression of their target genes, it is predictable that activation of either WT1 or β-catenin would compete for a limited pool of CBP. Consistent with this finding, knockdown of endogenous WT1 promotes β-catenin/CBP interaction, whereas specific inhibition of β-catenin/CBP interaction by small molecule ICG-001 enhances WT1/CBP binding (Figure 6). It is plausible to speculate that, in normal podocytes, nuclear WT1 abundance overwhelms β-catenin, thereby favoring WT1/CBP interaction, whereas in damaged podocytes, β-catenin overpowers WT1, leading to β-catenin/CBP complex formation (Figure 8E). Therefore, through this mechanism, WT1 and β-catenin can mutually antagonize each other and dictate distinct transcriptional programs.
Activation of β-catenin occurs in an assortment of proteinuric CKD and represents a common pathway leading to podocyte injury. This finding is in harmony with earlier observations that many pathogenic cues, such as Wnt, TGF-β, integrin-linked kinase, angiotensin II, and high glucose, induce, either directly or indirectly, β-catenin activation.7,15,42,43 This study shows that β-catenin could trigger podocyte dedifferentiation/injury by multiple mechanisms. It targets WT1 for ubiquitin-mediated degradation. In addition, it competes with WT1 for binding to CBP. Furthermore, β-catenin, as a transcriptional regulator, induces de novo expression of a battery of its target genes, such as Snail1, PAI-1, FSP-1, α-SMA, and MMP-7, as well as the renin-angiotensin system44 in podocytes. Therefore, β-catenin activation not only induces podocyte dedifferentiation characterized by loss of podocyte-specific markers but also, promotes their mesenchymal transition. Consistently, therapeutic intervention of β-catenin or its upstream signaling is quite effective in restoring WT1 expression and podocyte integrity in an animal model of proteinuric CKD (Figure 8).
In summary, we have shown that loss of WT1 is associated with β-catenin activation in ADR nephropathy and that β-catenin targets WT1 for ubiquitin-mediated degradation. In addition, we have identified WT1 and β-catenin as two master transcription factors that play opposing roles in regulating podocyte differentiation and dedifferentiation, respectively. Our studies suggest that the ratio of nuclear WT1/β-catenin is a determining factor that dictates the state of podocyte health and disease in vivo.
Concise Methods
Animal Models
Male BALB/c mice, weighing approximately 20–22 g, were purchased from Harlan Sprague–Dawley (Indianapolis, IN). For the ADR nephropathy model, BALB/c mice were administered by a single intravenous injection of ADR (doxorubicin hydrochloride; Sigma-Aldrich, St. Louis, MO) at 10 mg/kg body wt. Groups of mice (n=5) were euthanized at 1, 3, and 5 weeks after ADR injection, and kidney tissues were collected for various analyses. In the second set of experiments, mice were administered with pV5-sKlotho (Addgene, Cambridge, MA) or empty vector (pcDNA3) by rapid injection of a large volume of DNA solution through the tail vein as described previously.16,45 Three groups of BALB/c mice were used: (1) normal control (n=6), (2) ADR mice injected with pcDNA3 vector (n=7), and (3) ADR mice injected with pV5-sKlotho plasmid (n=7). Plasmids were administered at weeks 1 and 2 after ADR injection, and mice were euthanized at 3 weeks post-ADR. All animal studies were performed by use of the procedures approved by the Animal Ethics Committee at the Southern Medical University and the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Cell Culture and Treatment
The conditionally immortalized mouse podocyte cell line was provided by Peter Mundel (Massachusetts General Hospital, Boston, MA) and maintained as described previously.7,20 To propagate podocytes, cells were cultured at 33°C in RPMI1640 medium supplemented with 10% FBS and 10 units/ml mouse recombinant IFN-γ (R&D Systems, Minneapolis, MN) to enhance the expression of a thermosensitive T antigen. To induce differentiation, podocytes were grown under nonpermissive conditions at 37°C in the absence of IFN-γ. For some studies, podocytes were transiently transfected with WT1 expression vector (pCMV-WT1) and/or N-terminally truncated pDel-β-cat by using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) as described previously.20
TUNEL Staining
Apoptotic cell death was determined by using TUNEL staining with the DeadEnd Fluorometric Apoptosis Detection System according to the procedures specified by the manufacturer (Promega, Madison, WI).
Histology and Immunohistochemical Staining
Paraffin-embedded mouse kidney sections (3-µm thickness) were prepared by a routine procedure. Immunohistochemical staining was performed using routine protocols. The following antibodies were used: rabbit polyclonal anti–β-catenin (ab15180; Abcam, Inc., Cambridge, MA), rabbit polyclonal anti–MMP-7 (GTX11603; Genetex, Irvine, CA), mouse polyclonal anti–PAI-1 (sc-5297; Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti–α-SMA (A2547; Sigma-Aldrich), and rabbit polyclonal anti–FSP-1 (S100A4; A5114; Dako, Carpinteria, CA). After incubation with primary antibodies at 4°C overnight, the slides were then stained with horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Nonimmune normal IgG was used to replace primary antibodies as a negative control, and no staining occurred. Slides were viewed under a Nikon Eclipse E600 microscope equipped with a digital camera (Melville, NY).
Immunofluorescence Staining and Confocal Microscopy
Kidney cryosections were fixed with 3.7% paraformaldehyde for 15 minutes at room temperature. Mouse podocytes cultured on coverslips were fixed with cold methanol/acetone (1:1) for 10 minutes at −20°C. After blocking with 10% donkey serum for 30 minutes, the slides were immunostained with primary antibodies against nephrin (20R-NP002; Fitzgerald Industries International, Concord, MA), WT1 (sc-192; Santa Cruz Biotechnology), Synaptopodin (sc-21536; Santa Cruz Biotechnology), α-actinin-4 (ALX-210–356-C050; Enzo Life Sciences, Farmingdale, NY), podocalyxin (AF1556; R&D Systems), ZO-1 (61–7300; Invitrogen), and β-catenin (610154; BD Biosciences, San Jose, CA). For costaining of WT1 and α-actinin-4, kidney cryosections were immunostained with mouse mAb against WT1 (sc-7385; Santa Cruz Biotechnology) and rabbit polyclonal Ab against α-actinin-4 (ALX-210–356-C050; Enzo Life Sciences), respectively. The slides were then stained with Cy3- or Cy2-conjugated secondary antibody (Jackson ImmunoResearch Laboratories). Slides were viewed under a Nikon Eclipse E600 microscope equipped with a digital camera or a Leica TCS-SL confocal microscope.
Western Blot Analysis
Protein expression was analyzed by Western blot analysis as described previously.46 The primary antibodies used were as follows: antiactive β-catenin (05–665; EMD Millipore, Billerica, MA), anti–β-catenin (BD Biosciences), anti–PAI-1 (Santa Cruz Biotechnology), anti-Snail1 (ab17732; Abcam, Inc.), anti–α-SMA (Sigma-Aldrich), anti-WT1 (ab15251; Abcam, Inc.), anti–α-actinin-4 (ALX-210–356-c050; Enzo Life Sciences), antinephrin (20R-NP002; Fitzgerald Industries International), antipodocalyxin (R&D Systems), anti-Flag (F4042; Sigma-Aldrich), anti–α-tubulin (T9026; Sigma-Aldrich), antiglyceraldehyde-3-phosphate dehydrogenase (5174; Cell Signaling Technology, Boston, MA), and mouse monoclonal anti-pan–specific actin (MAB1501; EMD Millipore).
Nuclear and Cytoplasmic Fractionation
Whole kidneys from the BALB/c mice injected with ADR for 1 week were collected and homogenized in cold PBS. Nuclear and cytoplasmic protein preparations were made by using the Nuclear and Cytoplasmic Extraction Kit according to the protocols specified by the manufacturer (BB-3102; Bestbio, Shanghai, China). Nuclear and cytoplasmic proteins were then subjected to Western blot analyses using specific antibodies.
Glomerular Miniorgan Culture
Glomeruli were isolated by differential sieving technique from male Sprague–Dawley rats (Harlan Sprague–Dawley). Briefly, kidneys were excised and pressed with a spatula through stainless steel screens through differential sieves (60, 100, and 200 meshes) and washed with cold PBS/1% BSA. The glomeruli were suspended in PBS/1% BSA. After centrifugation at 200×g for 10 minutes, glomeruli were collected for cultivation. The purity of glomeruli was about 95% using this approach. Isolated glomeruli were cultured in RPMI1640/10% FBS medium at 37°C on noncoated six-well plates in the absence or presence of recombinant Wnt3a protein (1324-WN-010; R&D Systems) for various periods of time as indicated.
IP
The interaction of β-catenin or WT1 with CBP and WT1 ubiquitination in mouse podocytes was determined by co-IP as previously described.20 Cell lysates were immunoprecipitated overnight at 4°C with anti-WT1 antibody (Abcam, Inc.), anti-CBP (sc-7300; Santa Cruz Biotechnology), and protein A/G plus agarose (sc-2003; Santa Cruz Biotechnology). The precipitated complexes were washed with lysis buffer and boiled for 5 minutes in SDS sample buffer followed by immunoblotting with rabbit polyclonal anti-Ubiquitin (sc-8017; Santa Cruz Biotechnology), anti-WT1 antibody (Abcam, Inc.), and anti–β-catenin (BD Biosciences).
Real-Time RT-PCR
Total RNA isolation and qRT-PCR were carried out by procedures described previously.16 Briefly, first-strand cDNA synthesis was carried out by using a Reverse Transcription System Kit according to the instructions of the manufacturer (Promega). Real-time RT-PCR was performed on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) as described previously.16,46 The PCR reaction mixture in a 25-µl volume contained 12.5 µl 2× SYBR Green PCR Master Mix (Applied Biosystems), 5 µl diluted RT product (1:10), and 0.5 µM sense and antisense primer sets. PCR reaction was run by using standard conditions. After sequential incubations at 50°C for 2 minutes and 95°C for 10 minutes, the amplification protocol consisted of 50 cycles of denaturing at 95°C for 15 seconds and annealing and extension at 60°C for 60 seconds. The mRNA levels of various genes were calculated after normalizing with β-actin. Primer sequences used for amplifications are presented in Supplemental Table 1.
Transfection and Luciferase Assay
The effect of WT1 on β-catenin–mediated gene transcription was assessed using the TOPFlash reporter plasmid containing two sets of three copies of the TCF-binding site at the upstream of the thymidine kinase minimal promoter and luciferase cDNA (EMD Millipore). Mouse podocytes were cotransfected using Lipofectamine 2000 reagent with TOPFlash plasmid and pDel-β-cat in the absence or presence of WT1 expression vector (pCMV-WT1) or control and WT1-specific siRNA as indicated. An internal control reporter plasmid (0.1 µg) Renilla reniformis luciferase driven under thymidine kinase promoter (Promega) was also cotransfected for normalizing the transfection efficiency. Luciferase assay was performed using a dual luciferase assay system kit according to the manufacturer’s protocols (Promega). Relative luciferase activity (arbitrary units) was reported as fold induction over the controls after normalizing for transfection efficiency.
Statistical Analyses
All data examined were expressed as means±SEMs. Statistical analyses of the data were carried out using SigmaStat software (Jandel Scientific, San Rafael, CA). Comparison between groups was made using one-way ANOVA followed by Newman–Keuls test. P<0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by National Basic Research Program of China Grant 2012CB517700, National Science Foundation of China Grants 81130011 and 81370014, and National Institutes of Health Grants DK064005 and DK091239. L.Z. was supported by National Science Foundation of China Grant 81370014. R.J.T. was supported by American Heart Association Fellow-to-Faculty Transition Grant 13FTF16990086.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013101067/-/DCSupplemental.
References
- 1.Shankland SJ: The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Greka A, Mundel P: Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Mathieson PW: The podocyte as a target for therapies—new and old. Nat Rev Nephrol 8: 52–56, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Patrakka J, Tryggvason K: New insights into the role of podocytes in proteinuria. Nat Rev Nephrol 5: 463–468, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y: Wnt/β-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20: 1997–2008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He W, Kang YS, Dai C, Liu Y: Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato H, Gruenwald A, Suh JH, Miner JH, Barisoni-Thomas L, Taketo MM, Faul C, Millar SE, Holzman LB, Susztak K: Wnt/β-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J Biol Chem 286: 26003–26015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heikkilä E, Juhila J, Lassila M, Messing M, Perälä N, Lehtonen E, Lehtonen S, Sjef Verbeek J, Holthofer H: β-Catenin mediates adriamycin-induced albuminuria and podocyte injury in adult mouse kidneys. Nephrol Dial Transplant 25: 2437–2446, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Clevers H, Nusse R: Wnt/β-catenin signaling and disease. Cell 149: 1192–1205, 2012 [DOI] [PubMed] [Google Scholar]
- 12.MacDonald BT, Tamai K, He X: Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell 17: 9–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angers S, Moon RT: Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10: 468–477, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Zhou T, He X, Cheng R, Zhang B, Zhang RR, Chen Y, Takahashi Y, Murray AR, Lee K, Gao G, Ma JX: Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia 55: 255–266, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Dai C, Li Y, Liu Y: Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria. Kidney Int 80: 1159–1169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y: Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 24: 771–785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner N, Wagner KD, Xing Y, Scholz H, Schedl A: The major podocyte protein nephrin is transcriptionally activated by the Wilms’ tumor suppressor WT1. J Am Soc Nephrol 15: 3044–3051, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Guo G, Morrison DJ, Licht JD, Quaggin SE: WT1 activates a glomerular-specific enhancer identified from the human nephrin gene. J Am Soc Nephrol 15: 2851–2856, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Löwik MM, Groenen PJ, Levtchenko EN, Monnens LA, van den Heuvel LP: Molecular genetic analysis of podocyte genes in focal segmental glomerulosclerosis—a review. Eur J Pediatr 168: 1291–1304, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Li Y, Wu C, Liu Y: PINCH1 is transcriptional regulator in podocytes that interacts with WT1 and represses podocalyxin expression. PLoS ONE 6: e17048, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer RE, Kotsianti A, Cadman B, Boyd T, Gerald W, Haber DA: WT1 regulates the expression of the major glomerular podocyte membrane protein Podocalyxin. Curr Biol 11: 1805–1809, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Morrison AA, Viney RL, Saleem MA, Ladomery MR: New insights into the function of the Wilms tumor suppressor gene WT1 in podocytes. Am J Physiol Renal Physiol 295: F12–F17, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Lahiri D, Dutton JR, Duarte A, Moorwood K, Graham CF, Ward A: Nephropathy and defective spermatogenesis in mice transgenic for a single isoform of the Wilms’ tumour suppressor protein, WT1-KTS, together with one disrupted Wt1 allele. Mol Reprod Dev 74: 300–311, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Menke AL, IJpenberg A, Fleming S, Ross A, Medine CN, Patek CE, Spraggon L, Hughes J, Clarke AR, Hastie ND: The wt1-heterozygous mouse; a model to study the development of glomerular sclerosis. J Pathol 200: 667–674, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Guo JK, Menke AL, Gubler MC, Clarke AR, Harrison D, Hammes A, Hastie ND, Schedl A: WT1 is a key regulator of podocyte function: Reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum Mol Genet 11: 651–659, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Macconi D, Bonomelli M, Benigni A, Plati T, Sangalli F, Longaretti L, Conti S, Kawachi H, Hill P, Remuzzi G, Remuzzi A: Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol 168: 42–54, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 28.He W, Tan R, Dai C, Li Y, Wang D, Hao S, Kahn M, Liu Y: Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/β-catenin signaling. J Biol Chem 285: 24665–24675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He W, Tan RJ, Li Y, Wang D, Nie J, Hou FF, Liu Y: Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol 23: 294–304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan R, Zhang J, Tan X, Zhang X, Yang J, Liu Y: Downregulation of SnoN expression in obstructive nephropathy is mediated by an enhanced ubiquitin-dependent degradation. J Am Soc Nephrol 17: 2781–2791, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Lecker SH, Goldberg AL, Mitch WE: Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17: 1807–1819, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Carmignac V, Quéré R, Durbeej M: Proteasome inhibition improves the muscle of laminin α2 chain-deficient mice. Hum Mol Genet 20: 541–552, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Crawford LJ, Walker B, Ovaa H, Chauhan D, Anderson KC, Morris TC, Irvine AE: Comparative selectivity and specificity of the proteasome inhibitors BzLLLCOCHO, PS-341, and MG-132. Cancer Res 66: 6379–6386, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Kang YS, Dai C, Kiss LP, Wen X, Liu Y: Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am J Pathol 172: 299–308, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eguchi M, Nguyen C, Lee SC, Kahn M: ICG-001, a novel small molecule regulator of TCF/β-catenin transcription. Med Chem 1: 467–472, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y: Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol 22: 1642–1653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T: Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317: 803–806, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Su J, Li SJ, Chen ZH, Zeng CH, Zhou H, Li LS, Liu ZH: Evaluation of podocyte lesion in patients with diabetic nephropathy: Wilms’ tumor-1 protein used as a podocyte marker. Diabetes Res Clin Pract 87: 167–175, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Gebeshuber CA, Kornauth C, Dong L, Sierig R, Seibler J, Reiss M, Tauber S, Bilban M, Wang S, Kain R, Böhmig GA, Moeller MJ, Gröne HJ, Englert C, Martinez J, Kerjaschki D: Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat Med 19: 481–487, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Kim MK, McGarry TJ, O Broin P, Flatow JM, Golden AA, Licht JD: An integrated genome screen identifies the Wnt signaling pathway as a major target of WT1. Proc Natl Acad Sci U S A 106: 11154–11159, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, Angers S, Moon RT: Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science 316: 1043–1046, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Kang YS, Li Y, Dai C, Kiss LP, Wu C, Liu Y: Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int 78: 363–373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang L, Xu L, Song Y, Li J, Mao J, Zhao AZ, He W, Yang J, Dai C: Calmodulin-dependent protein kinase II/cAMP response element-binding protein/Wnt/β-catenin signaling cascade regulates angiotensin II-induced podocyte injury and albuminuria. J Biol Chem 288: 23368–23379, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, Hou FF, Kahn M, Liu Y: Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling [published online ahead of print 2014]. J Am Soc Nephrol 10.1681/ASN.2014010085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arking DE, Krebsova A, Macek M, Sr., Macek M, Jr., Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, Dietz HC: Association of human aging with a functional variant of klotho. Proc Natl Acad Sci U S A 99: 856–861, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y: Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int 82: 537–547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.