Abstract
On the basis of previous observations that deletion of the von Hippel–Lindau protein (pVHL) in juxtaglomerular (JG) cells of the kidney suppresses renin and induces erythropoietin expression, this study aimed to characterize the events underlying this striking change of hormone expression. We found that renin cell-specific deletion of pVHL in mice leads to a phenotype switch in JG cells, from a cuboid and multiple vesicle-containing form into a flat and elongated form without vesicles. This shift of cell phenotype was accompanied by the disappearance of marker proteins for renin cells (e.g., aldo-keto reductase family 1, member 7 and connexin 40) and by the appearance of markers of fibroblast-like cells (e.g., collagen I, ecto-5′-nucleotidase, and PDGF receptor-β). Furthermore, hypoxia-inducible transcription factor-2α (HIF-2α) protein constitutively accumulated in these transformed cells. Codeletion of pVHL and HIF-2α in JG cells completely prevented the phenotypic changes. Similar to renin expression in normal JG cells, angiotensin II negatively regulated erythropoietin expression in the transformed cells. In summary, chronic activation of HIF-2 in renal JG cells leads to a reprogramming of the cells into fibroblast-like cells resembling native erythropoietin-producing cells located in the tubulointerstitium.
Keywords: erythropoietin, fibroblast, renin-angiotensin system
Renin-expressing cells of the kidney are the key regulators of the renin-angiotensin system (RAS). The origin and development of renal renin-expressing cells are not well understood. During kidney development, renin-expressing cells in the kidney first appear within the renal mesenchyme1 and then form mural cells of the developing preglomerular arteriolar tree.2–4 With ongoing kidney maturation, mural renin-expressing cells transform into vascular smooth muscle–like cells.5 This transformation process is reversible and can be reverted in the adult kidney in states in which sodium or BP homeostasis of the body is threatened.6,7 Because the signaling pathways triggering the developmental differentiation of renin-expressing cells are widely unknown, we recently examined the role of hypoxia-inducible transcription factors (HIFs) in this context.8 As an experimental approach, we generated Ren1+/Cre Vhlfl/fl mice with stabilized HIFs by cell-specific deletion of the von Hippel–Lindau gene (Vhl)9 in the renin cell lineage.10
Unexpectedly, we found that Vhl deletion inhibited renin expression and induced erythropoietin (EPO) expression8 in juxtaglomerular (JG) cells. Normally, EPO is expressed by interstitial mesenchymal cells in the renal cortex.11–13 The origin and phenotype of these cells are still poorly characterized. Originally, the cells were considered as fibroblast-like cells. After demonstration of certain neuronal proteins in EPO-expressing cells, they were hypothesized to be of neuronal origin.14 The majority of EPO-producing cells express CD7312–17 and the β-receptor for PDGF (PDGFR-β).15 Because reversible phenotype shifts of renin-expressing cells, particularly into smooth muscle cells, are well known,10 we addressed the hypothesis that deletion of Vhl from JG cells could induce a switch to a cellular phenotype resembling typical renal EPO-producing cells. To this aim, we determined the expression of typical marker proteins for renin cells (e.g., aldo-keto reductase family 1, member 7 [Akr1b7]5,18 or connexin 40 [Cx40]19) on the one hand and for EPO-producing cells on the other. Because transcription factor HIF-2 is the essential molecular regulator of EPO gene expression and because deletion of von Hippel–Lindau protein (pVHL) in JG cells leads to a clear upregulation of HIF-2α but not HIF-1α protein,8 we further addressed the hypothesis that the switch of JG cells from renin to EPO expression is essentially triggered by HIF-2.
To consider this issue, we aimed to generate mice deficient for both pVHL and for HIF-2α in renin-expressing cells (Ren1+/Cre Vhlfl/fl HIF-2αfl/fl mice). Because pVHL is known as a tumor suppressor protein,20 we were interested in determining whether deletion of the Vhl gene might induce temporally dynamic changes of the functions in JG cells or if enhanced EPO production and suppression of renin production remain stable throughout the adult life of mice.
In this context, we also wondered whether EPO expression in pVHL-deleted JG cells is still regulatable. The master regulator of EPO gene expression is HIF-2,17,21–24 which is elevated in pVHL-deleted JG cells. A central and negative regulator of normal renin cell function is angiotensin II (AngII), which inhibits renin synthesis and secretion.25 Conversely, renin production and secretion are enhanced by AngII antagonists.26 This raised the question of whether AngII antagonists might also regulate EPO production in pVHL-deleted JG cells.
Results
pVHL-Deficient JG Cells Have an Elongated Cell Shape without Prominent Organelles
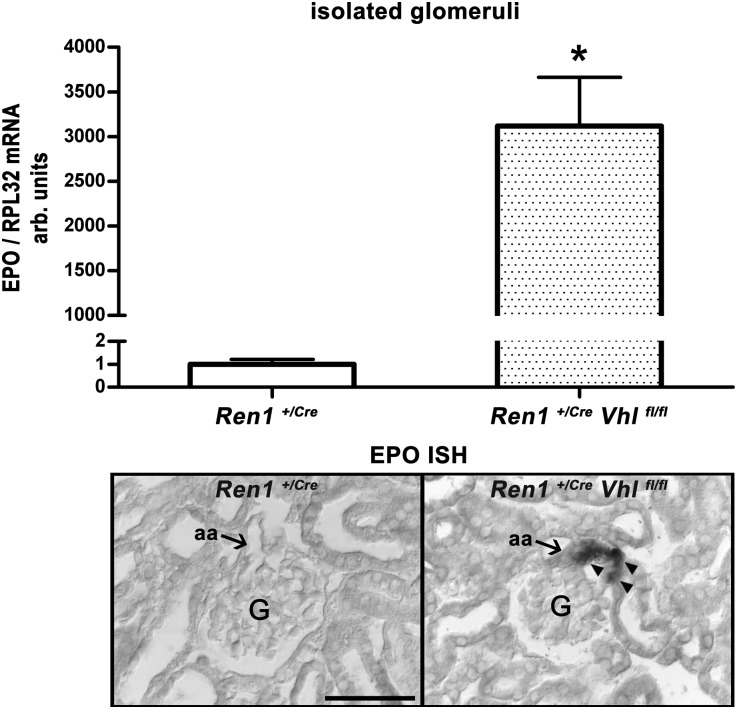
Glomeruli isolated from mouse kidneys with renin cell-specific deletion of pVHL (Ren1+/Cre Vhlfl/fl) contained a high abundance of EPO gene transcripts (Figure 1, upper panel) that were localized to the JG portion of afferent arterioles by in situ hybridization (Figure 1, lower panel). In parallel with the induction of EPO expression, renin expression in the JG areas disappeared (Figure 2). In Ren1+/Cre control kidneys, every glomerulus contains renin-immunoreactive cells at its vascular pole (Figure 2A). In Ren1+/Cre Vhlfl/fl kidneys, the JG areas are free of renin expression. Few residual renin cells are found in the proximal parts of afferent arterioles (Figure 2B).
Figure 1.
EPO gene expression in isolated glomeruli and EPO in situ hybridization on kidney sections of Ren1+/Cre and Ren1+/Cre Vhlfl/fl mice. EPO mRNA abundance of isolated glomeruli is given as ratio over RPL32 mRNA (upper panel), which is considered as a standard. The ratio of control values is set to 1. Data are the mean±SEM of six mice in each group. *P<0.05 by the t test. Localization of EPO mRNA expression by performing in situ hybridization on kidney sections of Ren1+/Cre and Ren1+/Cre Vhlfl/fl mice (lower panel). Arrows indicate JG cells/vascular poles. Arrowheads show JG EPO-expressing cells. aa, afferent arteriole; G, glomeruli; ISH, in situ hybridization; RPL32, ribosomal protein L32. Scale bar, 50 µm.
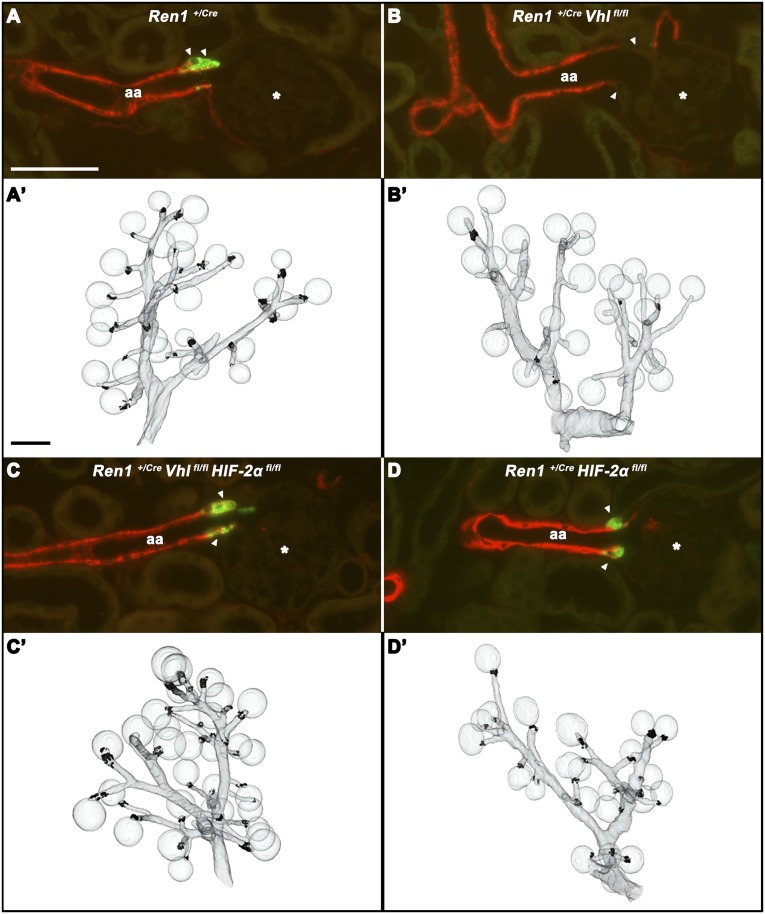
Figure 2.
Number and distribution of renin-expressing cells of the different genotypes. Immunohistochemistry for renin (green) and α-SMA (red) in kidney sections of a wild-type mouse (A), a mouse with deletion of pVHL in the renin cell lineage (B), a mouse with codeletion of pVHL and HIF-2α in the renin cell lineage (C), and a mouse with deletion of HIF-2α in the renin cell lineage (D). From each genotype, a total of 100 glomeruli from two kidneys are analyzed with regard to renin expression. (A) In wild-type kidneys, 100% of the glomeruli show renin expression at their vascular poles. (B) In kidneys of mice with deletion of pVHL in the renin cell lineage, 3% of the glomeruli show weak renin immunoreactivity at their vascular poles. In these kidneys, 19% of afferent arterioles contain single renin cells in their proximal parts. (C) In kidneys of mice with codeletion of pVHL and HIF-2α, 92% of glomeruli show renin expression at their vascular poles. (D) In kidneys of mice with HIF-2α deletion, 99% of glomeruli show renin expression at their vascular poles. Asterisks mark glomeruli, whereas the arrowheads indicate JG areas/vascular poles of afferent arterioles. (A'–D') Three-dimensional reconstructions of α-SMA– (gray) and renin-immunoreactive (black) areas of the respective kidneys. aa, afferent arteriole. Bar, 50 µm in A–D; 100 µm in A’–D’.
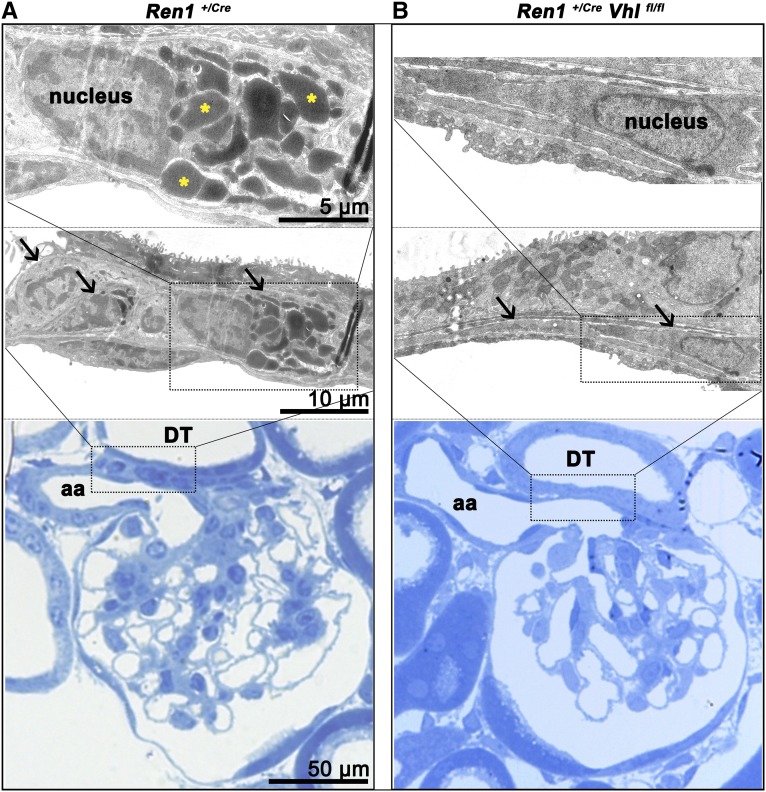
We aimed to characterize the cellular phenotype that results from deletion of pVHL in JG cells. Electron microscopy shows that normal JG cells have a cuboid-like morphology that results from accumulation of prominent electron-dense renin storage vesicles, as shown in Figure 3A. The morphology of pVHL-deficient JG cells is markedly different from that of typical JG cells. pVHL deletion leads to flat and elongated cell bodies of the JG cells, and the characteristic electron-dense granules are also absent (Figure 3B).
Figure 3.
Altered morphology of juxtaglomerular cells due to pVHL deficiency. Electron microscopy analysis of JG cells of a Ren1+/Cre control (A) and a Ren1+/Cre Vhlfl/fl (B) kidney. Lower panels show a regional overview of the JG apparatus in Richardson’s stain, middle panels show electron microscopy, and upper panels illustrate one single JG cell of each genotype at a higher magnification. (A) JG cells in the control kidney have a cuboid-like shape with huge electron-dense intracellular vesicles; for demonstration, three renin-containing vesicles are highlighted by an asterisk. (B) In contrast with the control kidney the afferent arteriole in the Ren1+/Cre Vhlfl/fl kidney appears widened with a thin wall in the terminal (JG) portion. JG cells appear as flat and elongated cells without prominent substructures or cell organelles. The vessel walls are in contact with portions of distal tubules. Arrows indicate JG cells. aa, afferent arteriole; DT, distal tubule.
pVHL-Deficient JG Cells Express the Fibroblast Markers Collagen I, CD73, and PDGFR-β
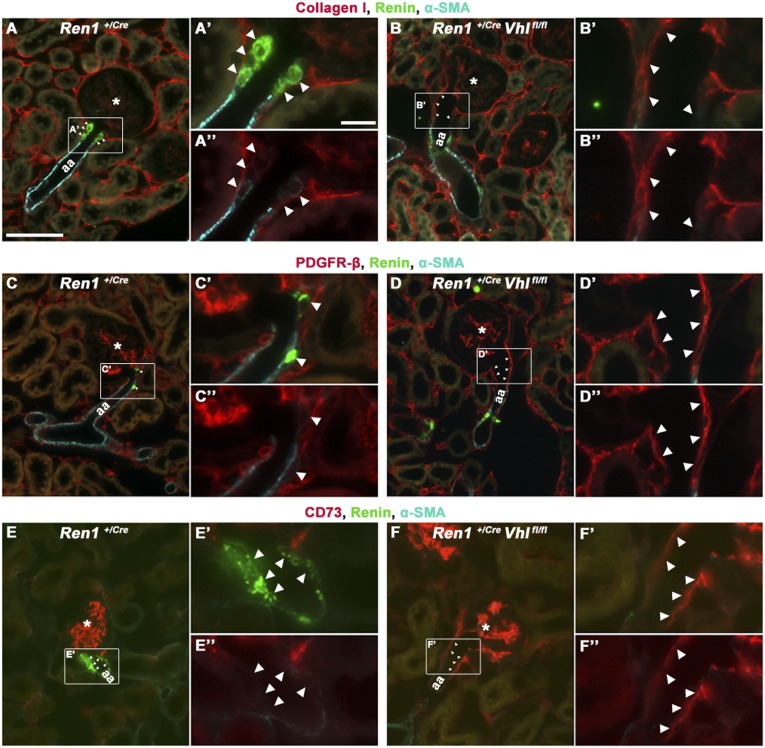
We next performed immunohistochemical studies with marker proteins typical either for renin cells or characteristic for EPO cells to investigate the expression profile of pVHL-deficient JG cells with an abnormal cell morphology. Whereas renin-producing cells typically coexpress Akr1b7 and Cx40, pVHL-deficient JG cells express none of these proteins (not shown). Because downregulation of renin expression is known to occur during metaplastic transformation of renin-expressing cells into vascular smooth muscle cells, we considered the possibility that deletion of pVHL might have shifted the JG cells to the phenotype of vascular smooth muscle cells. It turned out that pVHL-deficient JG cells also do not express smooth muscle cell markers such as α-smooth muscle actin (α-SMA) (Figure 4, B, D, and F), calponin, or transgelin (not shown). Instead, JG cells deficient for pVHL express collagen I, PDGFR-β, and CD73 (Figure 4, B, D, and F, respectively), all of which are typically expressed by fibroblast-like cells resembling native EPO-producing cells.27,28
Figure 4.
pVHL-deficient juxtaglomerular cells express fibroblast-related proteins. Coimmunostainings for renin (green), α-SMA (cyan), and collagen I (red) (A and B), PDGFR-β (red) (C and D), and CD73 (red) (E and F) in JG areas of Ren1+/Cre (A, C, and E) and Ren1+/Cre Vhlfl/fl (B, D, and F) kidneys. (A, C, E) In Ren1+/Cre control kidneys, JG cells express renin (indicated by arrowheads). (B, D, and F) In the Ren1+/Cre Vhlfl/fl kidneys, renin- or α-SMA–expressing cells are lacking in JG position. Instead, cells expressing collagen I (B), PDGFR-β (D), and CD73 (F) form the arteriolar vessel wall in the JG area (indicated by arrowheads) of Ren1+/Cre Vhlfl/fl kidneys. Furthermore, the afferent arterioles appear widened at the glomerular entrance in Ren1+/Cre Vhlfl/fl kidneys. The JG area of each illustration is shown in a higher magnification with renin staining (A’–F’) and without renin staining (A’’–F’’). Asterisks indicate glomeruli. aa, afferent arteriole. Bar, 50 µm in A–F; 10 µm in A’–F’ and A’’–F’’.
Codeletion of pVHL and HIF-2α in JG Cells Prevents the Changes Caused by pVHL Deficiency
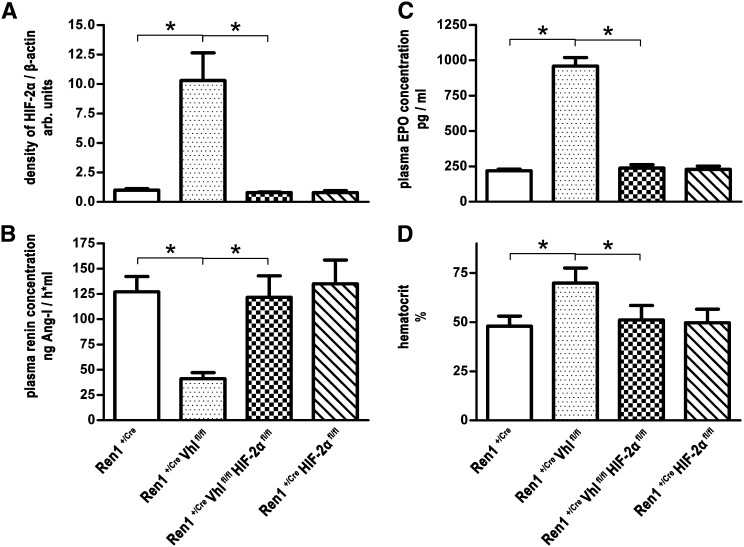
In Ren1+/Cre Vhlfl/fl kidneys, cells in the JG position show clear HIF-2α (Figure 5B) but not HIF-1α (not shown) accumulation, which is paralleled by an approximately 10-fold enrichment of HIF-2α protein abundance in glomerulus preparations (Figure 6A). To determine a possible causal role of HIF-2 for the striking switches of cellular morphology and endocrine function induced by pVHL deletion in JG cells, we generated and characterized pVHL-deficient mice with an additional knockout of HIF-2α in the renin cell lineage (Ren1+/Cre Vhlfl/fl HIF-2αfl/fl mice). As expected, the increases of JG HIF-2α immunoreactivity and glomerular HIF-2α protein abundance observed in Ren1+/Cre Vhlfl/fl kidneys were reversed in Ren1+/Cre Vhlfl/fl HIF-2αfl/fl mice (Figures 5C and 6A). Apparently, codeletion of pVHL and HIF-2α also prevented the functional changes caused by pVHL deficiency alone. Thus, the numbers and distribution of renin cells (Figure 2C), plasma renin concentrations (Figure 6B), plasma EPO concentrations (Figure 6C), and hematocrit values (Figure 6D) in Ren1+/Cre Vhlfl/fl HIF-2αfl/fl mice were rather similar to the respective values of control mice. Deletion of HIF-2α alone in the renin cell lineage (Ren1+/Cre HIF-2αfl/fl mice) had no influence on the number and localization of renin cells (Figure 2D), plasma renin concentrations, or plasma EPO concentrations or hematocrit values (Figure 6). Supplemental Figure 1 confirms the efficacy of Cre/flox recombination of all investigated genotypes on the genomic level resulting in stabilization of HIF-2α protein only in glomerulus preparations of Ren1+/Cre Vhlfl/fl mice.
Figure 5.
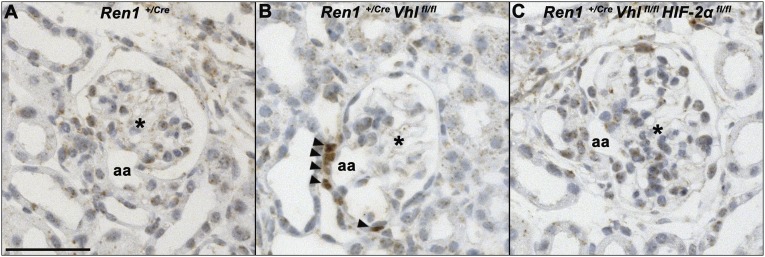
Accumulation of HIF-2α protein in juxtaglomerular cells due to pVHL deficiency. Immunostaining (brown color) for HIF-2α in JG apparatuses of a wild-type mouse (A), a mouse with deletion of pVHL in the renin cell lineage (B), and a mouse with codeletion of pVHL and HIF-2α in the renin cell lineage (C). Arrowheads highlight HIF-2α immunoreactivity. Asterisks mark glomeruli. aa, afferent arteriole. Bar, 50 μm.
Figure 6.
The endocrine switch of juxtaglomerular cells in the Ren1+/Cre Vhlfl/fl mice depends on HIF-2α accumulation. Abundance of HIF-2α protein in isolated glomeruli (A), plasma renin concentration (B), plasma EPO concentration (C), and hematocrit values (D) of Ren1+/Cre, Ren1+/Cre Vhlfl/fl, Ren1+/Cre Vhlfl/fl HIF-2αfl/fl, and Ren1+/Cre HIF-2αfl/fl mice. Renin cell-specific Vhl knockout mice have higher glomerular HIF-2α levels, increased plasma EPO and hematocrit values, but lowered plasma renin concentrations compared with the Ren1+/Cre control mice. These changes in the Ren1+/Cre Vhlfl/fl mice are almost completely reversed by an additional knockout of HIF-2α. Knockout of HIF-2α alone in the renin cell lineage has no influence on renin or EPO expression. Hematocrit values are in line with the EPO data. Data are the mean±SEM of six mice in each group. For determination of HIF-2α abundance, glomerulus preparations from two animals are pooled (see the Concise Methods). After cell lysis and immunoblotting, membranes are incubated with antibodies against HIF-2α or β-actin as a standard (see Supplemental Figure 1). Band intensities are quantified measuring OD on a Gel Doc 2000 (Bio-Rad) and analyzed by Multi-Analyst Software (Bio-Rad). The ratio of control values (glomerulus preparations of Ren1+/Cre mice) is set to 1. *P<0.05.
pVHL Deficiency in JG Cells Results in Stable EPO Production
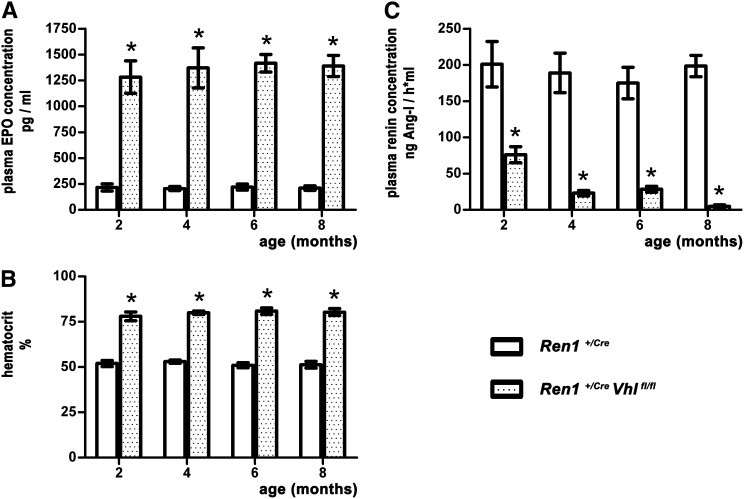
Because pVHL is known as a tumor suppressor protein, we were interested in determining whether the effects induced by pVHL deletion in the renin cell lineage were stable or developed temporal dynamics. To evaluate this issue, we monitored Ren1+/Cre Vhlfl/fl mice and their controls over an 8-month time period and we took blood samples at 2-month intervals. Values for plasma EPO concentrations of the Ren1+/Cre Vhlfl/fl mice were constant between 1283 and 1417 pg/ml, and values for control litters ranged from 206 to 220 pg/ml (Figure 7A). In line with the EPO data, age-related hematocrit levels (between 78.0% and 80.9%) of the renin cell-specific pVHL knockout mice also did not significantly vary (Figure 7B). Instead, plasma renin concentrations of Ren1+/Cre Vhlfl/fl mice declined over time, whereas the values for the control mice were stable (Figure 7C).
Figure 7.
Plasma EPO concentrations, plasma renin concentrations, and hematocrit values in Ren1+/Cre and Ren1+/Cre Vhlfl/fl mice. Blood samples are taken from the mice every 2 months, up to the age of 8 months. Throughout this period, plasma EPO concentrations are in the same range. Similarly, hematocrit values of the Ren1+/Cre Vhlfl/fl mice are constant over the entire observed time. Plasma renin concentrations of the Ren1+/Cre mice have a steady level of approximately 200.0 ng AngI/h⋅ml, whereas PRC of the Ren1+/Cre Vhlfl/fl mice decline from 76.1 to 4.9 ng AngI/h⋅ml. Values are the mean±SEM of six mice in each group. *P<0.05. PRC, plasma renin concentration.
pVHL Deficiency in JG Cells Results in Regulatable EPO Production
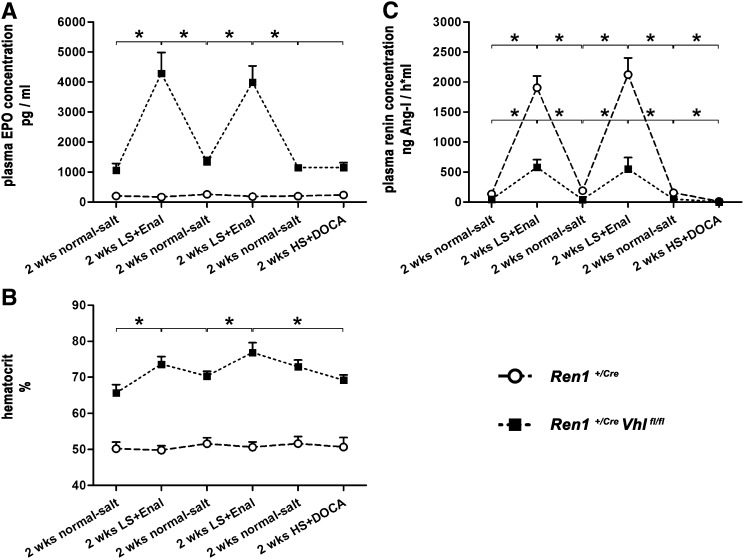
The expression of the renin gene in adult kidneys is controlled by several negative feedback loops involving AngII, BP, or salt balance.6,7,29 Our previous observation of a parallel induction of renin and of EPO expression by angiotensin-converting enzyme inhibition combined with salt depletion in Ren1+/Cre Vhlfl/fl kidneys raised the question of the reversibility of these effects. We thus treated animals intermittently with standard chow containing normal salt content and with a combination of enalapril and a low-salt diet in 2-week intervals each. Plasma renin concentrations, plasma EPO concentrations, and hematocrit values were determined at the end of each treatment period. As shown in Figure 8, combined treatment with enalapril and a low-salt diet led to reproducible and reversible increases of the plasma concentrations of renin and EPO in Ren1+/Cre Vhlfl/fl mice, whereas only plasma concentrations of renin, but not EPO, increased in Ren1+/Cre mice. Treatment with desoxycorticosterone acetate (DOCA) (plus a high-salt diet) applied at the end of the experiment strongly lowered plasma renin concentrations (Figure 8C), but had no effect on plasma EPO concentrations in either genotype (Figure 8A). Hematocrit values changed in parallel with plasma EPO values in Ren1+/Cre Vhlfl/fl mice (Figure 8 B), indicating the biologic efficacy of regulated EPO production in Ren1+/Cre Vhlfl/fl mice.
Figure 8.
EPO expression in pVHL-deficient juxtaglomerular cells is regulatable by parameters that also modulate renin expression in native JG cells. Plasma concentrations of renin (A) and EPO (B) and hematocrit values (C) in Ren1+/Cre controls and Ren1+/Cre Vhlfl/fl mice. Animals are treated intermittently in 2-week periods with a normal-salt diet, a low-salt diet in combination with enalapril (LS+Enal) and a high-salt diet in combination with DOCA (HS+DOCA). Data are the mean±SEM of six mice in each group. *P<0.05 between treatment periods by the t test.
Discussion
This study confirms and extends our previous report8 by showing that deletion of pVHL from renin-expressing cells reliably downregulates renin expression and upregulates EPO expression in the cells and that this reciprocal switch of endocrine function is stable throughout life. This study addressed the hypothesis that the endocrine switch of JG cells is associated with a phenotype switch of the cells. Our results are in line with this hypothesis by showing not only morphologic changes but also changes of the gene expression profile. Proteins typically coexpressed with renin (e.g., Cx4019 or Akr1b75,18) disappear together with renin, whereas other more fibroblast-related proteins (e.g., PDGFR-β, CD73, or collagen I27,28) appear together with EPO. Regarding the protein expression pattern, the cell type induced by deletion of pVHL resembles renocortical interstitial cells,12–17 which are considered as the main physiologic production sites of EPO in the body. PDGFR-β and CD73 are also considered as markers of renal pericytes.29 Because renin cells and pericytes are considered to derive from the same cell lineage,30 our findings could support the concept that EPO-expressing cells are related to pericytes. It is already known that renin cells of the kidney can reversibly transform into another cell type, namely vascular smooth muscle cells. It is thought that several transcription factors and signaling pathways such as myocardin or Nkx2.5 play a major role in triggering the transformation process to smooth muscle cells.5 However, the transformation of JG renin cells into fibroblast-like cells has not yet been reported. We did not obtain signs for activation of signaling pathways relevant for transformation of renin cells into smooth muscle cells when pVHL was deleted from the renin cell lineage. Instead, according to our second hypothesis about a mediator role of hypoxia transcription factors, we obtained strong evidence that the endocrine and phenotype shift of JG cells induced by pVHL deletion was exclusively mediated by HIF-2. Deletion of pVHL led to an accumulation of HIF-2 protein in JG cells. Preventing the accumulation of HIF-2 protein by interruption of the HIF-2α gene in the renin cell lineage prevented all morphologic and functional changes induced by deletion of pVHL protein in JG cells. For the renin-expressing cell phenotype, transcription factors such as recombination signal-binding protein Jκ, cAMP response element-binding protein, and Krueppel-like factor have been hypothesized as master regulators.5 If and how HIF-2 interferes with those factors is currently unknown. Their elucidation therefore remains a task for future research.
An elementary role of HIF-2 in the endocrine switch of JG cells also fits well with the common assumption that HIF-2 is the physiologic control factor of EPO gene expression.22,24,31 In line, pVHL deletion in cells considered as physiologic EPO production sites apart from renal fibroblasts such as hepatocytes,22 glial cells,24 or osteoblasts32 triggers EPO expression in a HIF-2–dependent fashion. How normal renin-expressing JG cells might be physiologically activated to produce EPO is currently difficult to answer. Our data are derived from renin cells with chronic HIF-2 stabilization. We do not yet know whether JG cells are instantaneously ready to produce EPO in response to HIF-2 accumulation or whether HIF-2–mediated cell transformation is required before acquiring the capability for EPO production. This issue has to be clarified in future experiments. In any case, HIF-2 stabilization will be required, which is commonly induced by hypoxia. Because of their close anatomic position to the afferent arteriolar lumen,33 it is less conceivable that JG cells at normal perfusion can become hypoxic enough to activate HIF-2 in states of hypoxia or anemia that are still compatible with life. Thus, it is more obvious to speculate about JG EPO production in less perfused renocortical areas as they occur in ischemic kidney diseases. Because potential JG EPO expression has not been sufficiently considered in localization studies of intrarenal EPO expression thus far, this issue has to be addressed in future studies.
In spite of continuous HIF-2 stabilization, EPO expression in pVHL-deleted JG cells was still regulatable to some extent. Interestingly, it was reversibly stimulated by maneuvers also known to stimulate renin gene expression such as challenges of BP and/or sodium homeostasis.34 This may indicate that signaling mechanisms making JG cells sensitive to decreases in BP or to sodium loss are still active even after deletion of pVHL. In fact, inhibitory modifier pathways of HIF-triggered EPO gene expression exist (e.g., NF-κB35 or oxygen radical formation36,37). Interestingly, both signaling pathways are also considered to inhibit renin gene expression.39–41
In summary, our data show that deletion of pVHL in the renin cell lineage causes an endocrine and phenotype shift of renal JG cells from a renin-secreting cell to a fibroblast-like EPO-expressing cell. The endocrine and phenotype switches are essentially triggered by accumulation of the HIF-2 transcription factor in JG cells. In the transformed JG cells, EPO expression is still modulated by parameters that also modulate renin expression in native JG cells.
Concise Methods
Animals
Renin cell-specific pVHL-deficient (Ren1+/Cre Vhlfl/fl) mice were generated by crossbreeding mice with loxP flanked Vhl alleles,9 and mice with targeted insertion of Cre recombinase into the renin-1d locus.10 The renin cell-specific double knockout mice for pVHL and HIF-2α (Ren1+/Cre Vhlfl/fl HIF-2αfl/fl) were generated by crossing the aforementioned mouse lines with mice with loxP flanked HIF-2α alleles.42 Mice with wild-type Vhl and HIF-2α alleles and one renin-1d Cre allele (Ren1+/Cre mice) served as control litters. Animals were maintained on standard rodent chow (0.6% NaCl; Ssniff, Germany) with free access to tap water. For a moderate RAS stimulation,43 mice were treated for 3 weeks with the angiotensin-converting enzyme inhibitor enalapril (on average, 10 mg/kg per day) added to the drinking water. For a strong RAS stimulation, mice were fed a low-salt diet (0.02% NaCl; Ssniff) combined with enalapril. To suppress the RAS, 21-day–release pellets containing 50 mg DOCA (Innovative Research of America) were implanted subcutaneously in the right flank in six mice of each genotype. Beginning with the first day of DOCA treatment, these animals were fed a high-salt diet (4.0% NaCl; Ssniff) in combination with distilled water containing 1% NaCl as drinking fluid. Animals used in this study were male mice aged between 8 and 12 weeks. All animal experiments were performed according to the US National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the local ethics committee.
Determination of Hematocrit, Plasma Renin, and Plasma EPO Levels
Blood samples (75 µl) were taken from the tail vein into capillary tubes containing 1 µl 125 mM EDTA to prevent clotting. Hematocrit values were determined after centrifugation (13,000×g for 7 minutes at room temperature). We measured the renin concentration in plasma samples based on the generation of AngI after the addition of plasma from bilaterally nephrectomized male rats as excess renin substrate. The generated AngI (in nanograms per milliliter per hour) was determined by RIA (Byk & DiaSorin Diagnostics, Germany). EPO protein concentration was determined in plasma samples using the Quantikine Mouse EPO ELISA kit (R&D Systems, Germany) according to the manufacturer’s protocol.
Magnetic Isolation of Glomeruli
Glomeruli were isolated according to our previously published procedure.8 Mice were anesthetized and kidneys were perfused through the abdominal aorta with 2×109 Dynabeads M-450 epoxy/PBS. One-half of the left kidney was snap-frozen in liquid nitrogen for subsequent mRNA analysis. The remaining half of the left kidney plus the right kidney were cut into small pieces of approximately 0.75 mm3 and digested in collagenase A at 37°C for 30 minutes. After digestion, tissue was gently pressed through a 100-µm cell strainer using a flattened pestle. The filtered cells were then transferred in a Falcon tube, which was placed at a magnet (BD Becton Dickinson GmbH, Heidelberg, Germany). In this way, glomeruli containing Dynabeads could be captured. After several washing procedures, glomerulus preparations were snap-frozen in liquid nitrogen for mRNA, gDNA, or protein analysis.
Determination of mRNA Expression by Real-Time PCR
Total RNA was isolated from kidneys and from glomerulus preparations as described by Chomczynski and Sacchi44 and quantified by a photometer. One microgram of the resulting RNA was used for RT. The cDNA was synthesized by Moloney murine leukemia virus RT (Life Technologies). For quantification of mRNA expression, real-time PCR was performed using a Light Cycler Instrument and the LightCycler 480 SYBR Green I Master Kit (Roche Diagnostics, Germany) and glyceraldehyde-3-phosphate dehydrogenase or ribosomal protein L32 as a control. Table 1 lists the primer sequences.
Table 1.
Primer sequences
| Genes | Sequence (5′-3′) | Product Size (bp) |
|---|---|---|
| GAPDH | Forward: CACCAGGGCTGCCATTTGCA | 294 |
| Reverse: GCTCCACCCTTCAAGTGG | ||
| RPL32 | Forward: TTAAGCGAAACTGGCGGAAAC | 100 |
| Reverse: TTGTTGCTCCCATAACCGATG | ||
| EPO | Forward: AATGGAGGTGGAAGAACAGG | 174 |
| Reverse: ACCCGAAGCAGTGAAGTGA | ||
| Renin | Forward: ATGAAGGGGGTGTCTGTGGGGTC | 194 |
| Reverse: ATGTCGGGGAGGGTGGGCACCTG |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RPL32, ribosomal protein L32.
Perfusion Fixation for Immunohistochemistry, In Situ Hybridization, and Transmission Electron Microscopy
For immunohistochemistry and in situ hybridization, kidneys were perfusion fixed with 3% paraformaldehyde/PBS, dehydrated, and embedded in paraffin. CD73 staining was performed on cryosections of perfusion-fixed kidneys. For transmission electron microscopy, kidneys were perfused with 2% glutaraldehyde/PBS, cut in 1-mm3–wide blocks, and embedded in epoxide resin using an automatic microwave.
Immunohistochemistry
Sagittal sections (5 µm) were blocked with 10% horse serum/1% BSA in PBS before the primary antibodies were applied. After three washes, sections were stained with Cy2, Cy5, tetramethylrhodamine B isothiocyanate, and Alexa Fluor 350 secondary antibodies. Slices were mounted with DakoCytomation Glycergel mounting medium and were viewed with an Axiovert Microscope. Table 2 lists the primary antibodies used for immunohistochemistry. Immunohistochemistry of HIF-1α and HIF-2α was performed as described by Schley et al.45
Table 2.
Antibodies used for immunohistochemistry
| Antigene | Provider | Catalog No. | Type |
|---|---|---|---|
| α-SMA | Abcam, Inc. (Cambridge, UK) | ab7817 | Monoclonal |
| Akr1b7 | Santa Cruz Biotechnology (Dallas, TX) | sc-27763 | Polyclonal |
| Calponin | Abcam, Inc. | ab46794 | Monoclonal |
| CD73 | BD Pharmingen (Franklin Lakes, NJ) | 550738 | Monoclonal |
| Collagen I | Abcam, Inc. | ab34710 | Polyclonal |
| Cx40 | Biotrend (Koeln, Germany) | Cx40-A | Polyclonal |
| HIF-1α | Novus Biologicals (Littleton, CO) | α67 | Monoclonal |
| HIF-2α | Rosenberger et al. (ref. 48) | PM9 | |
| Transgelin | Abcam, Inc. | ab10135 | Polyclonal |
| PDGFR-β | Abcam, Inc. | ab32570 | Monoclonal |
| Renin | Davids Biotechnology (Regensburg, Germany) | Polyclonal |
Three-Dimensional Reconstruction
Digitalization of the antibody-stained serial sections (100 sections) was performed using an AxioCam MRm camera (Carl Zeiss) mounted on an Axiovert 200M microscope (Carl Zeiss) with fluorescence filters for renin and α-SMA (tetramethylrhodamine B isothiocyanate, filter set 43; Cy2, filter set 38 HE; Carl Zeiss). After acquisition, a stack of equal-size images was built using the ImageJ graphic tool (Wayne Rasband; National Institutes of Health, Bethesda, MD). The equalized data were then imported into Amira 4.1 visualization software (Mercury Computer Systems, Chelmsford, MA) on a Dell Precision 690 computer system and split into the renin and α-SMA channels. After this step, the renin and α-SMA channels were aligned. In the segmentation step, the α-SMA and renin data sets served as a scaffold and were spanned manually or automatically using grayscale values. Matrices, volume surfaces, and statistics were generated from these segments.46 From each of the genotypes, two kidneys were partially reconstructed.
In Situ Hybridization
Localization of EPO mRNA synthesis was studied by nonradioactive mRNA in situ hybridization using a probe covering base pairs 312–602 of the rat EPO cDNA (NM_0171001).17 Digoxigenin-labeled UTP and T7 or SP6 RNA polymerase were used to generate sense and antisense RNA probes. In situ hybridization was performed as previously described,43,47 and the hybridized RNA probe was visualized using 4-nitroblue tetrazolium chloride. Sections were examined using a Leica DMRB microscope equipped with an interference contrast module (Leica, Wetzlar, Germany). Bright-field images were acquired using a Moticam 2300 digital camera and the Motic Images 2.0 imaging system (Motic, Xiamen, China).
Transmission Electron Microscopy
One-micrometer sections were cut and stained with Azure A for light microscopy. Selected areas were then sectioned into 70-nm thick slices with a diamond knife and placed on copper grids coated with pioloform. Slices were contrasted using a 4% uranyl acetate solution and a 0.5% lead citrate solution. For acquisition of the images, we used a transmission electron microscope (Phillips CM12 TEM; Fei & Co, Netherlands) with a LaB5 cathode and an acceleration voltage of 120 keV. The digitalization was carried out with a TEM-1000 slow-scan charge-coupled device camera and the EM-Menu 4.0 program (both from TVIPS-Tietz GmbH, Germany).
Immunoblot Analyses for HIF-2α and β-Actin
Glomerulus preparations were sonicated in lysis buffer. After a centrifugation step for 5 minutes at 10,000 rpm (4°C), protein concentrations were determined from the supernatant. Aliquots of 50 µg of protein for the HIF-2α and 5 µg of protein for the β-actin immunoblot were electrophoretically separated on 10% polyacrylamide gels and transferred to nitrocellulose membranes, which were blocked overnight in 5% nonfat dry milk diluted in Tris-buffered saline with 0.1% Tween-20. The membranes were then incubated for 1 hour at room temperature with antibodies against HIF-2α (NB100-122, 1:500; Novus Biologicals) or β-actin (A5316, 1:5000; Sigma-Aldrich). After washing, the membranes were incubated for 2 hours with corresponding secondary antibodies (1:2000; Santa Cruz Biotechnology) and were subjected to a chemiluminescence detection system. Quantitative assessment of band densities was performed densitometrically.
Statistical Analyses
All data are presented as the mean±SEM. Statistical significance was determined by the t test for intraindividual comparison, and by ANOVA and Bonferroni’s adjustment for multiple comparisons between experimental groups. P<0.05 was considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Anita Zuegner, Ramona Mogge, and Rosmarie Heydn for their expert technical assistance. We also thank Alexander Paliege (Department of Anatomy, Charité University of Medicine Berlin, Berlin, Germany) for performing EPO in situ hybridizations, and Carsten Willam (Department of Nephrology and Hypertension, Friedrich-Alexander-University Erlangen-Nuremberg, Erlangen, Germany) for performing HIF stainings.
Mice with targeted insertion of Cre recombinase into the renin-1d locus were kindly provided by R. Ariel Gomez and Maria Luisa S. Sequeira-Lopez (Department of Pediatrics, University of Virginia School of Medicine, Charlottesville, VA), and mice with loxP flanked HIF-2α alleles were kindly provided by Ian J. Frew (Institute of Molecular Health Sciences, ETH Zurich, and Institute of Physiology, University of Zurich, Zurich, Switzerland).
This work was supported by grants from the German Research Foundation (Ku 859/15-1 and SFB 699).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013111152/-/DCSupplemental.
References
- 1.Sequeira Lopez ML, Pentz ES, Robert B, Abrahamson DR, Gomez RA: Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol Renal Physiol 281: F345–F356, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Richoux JP, Amsaguine S, Grignon G, Bouhnik J, Menard J, Corvol P: Earliest renin containing cell differentiation during ontogenesis in the rat. An immunocytochemical study. Histochemistry 88: 41–46, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Minuth M, Hackenthal E, Poulsen K, Rix E, Taugner R: Renin immunocytochemistry of the differentiating juxtaglomerular apparatus. Anat Embryol (Berl) 162: 173–181, 1981 [DOI] [PubMed] [Google Scholar]
- 4.Celio MR, Groscurth P, Inagami T: Ontogeny of renin immunoreactive cells in the human kidney. Anat Embryol (Berl) 173: 149–155, 1985 [DOI] [PubMed] [Google Scholar]
- 5.Brunskill EW, Sequeira-Lopez ML, Pentz ES, Lin E, Yu J, Aronow BJ, Potter SS, Gomez RA: Genes that confer the identity of the renin cell. J Am Soc Nephrol 22: 2213–2225, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantin M, Araujo-Nascimento MD, Benchimol S, Desormeaux Y: Metaplasia of smooth muscle cells into juxtaglomerular cells in the juxtaglomerular apparatus, arteries, and arterioles of the ischemic (endocrine) kidney. An ultrastructural-cytochemical and autoradiographic study. Am J Pathol 87: 581–602, 1977 [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez RA, Lynch KR, Sturgill BC, Elwood JP, Chevalier RL, Carey RM, Peach MJ: Distribution of renin mRNA and its protein in the developing kidney. Am J Physiol 257: F850–F858, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Kurt B, Paliege A, Willam C, Schwarzensteiner I, Schucht K, Neymeyer H, Sequeira-Lopez ML, Bachmann S, Gomez RA, Eckardt KU, Kurtz A: Deletion of von Hippel-Lindau protein converts renin-producing cells into erythropoietin-producing cells. J Am Soc Nephrol 24: 433–444, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haase VH, Glickman JN, Socolovsky M, Jaenisch R: Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A 98: 1583–1588, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sequeira López ML, Pentz ES, Nomasa T, Smithies O, Gomez RA: Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Koury ST, Bondurant MC, Koury MJ: Localization of erythropoietin synthesizing cells in murine kidneys by in situ hybridization. Blood 71: 524–527, 1988 [PubMed] [Google Scholar]
- 12.Bachmann S, Le Hir M, Eckardt KU: Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem 41: 335–341, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Maxwell PH, Osmond MK, Pugh CW, Heryet A, Nicholls LG, Tan CC, Doe BG, Ferguson DJP, Johnson MH, Ratcliffe PJ: Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int 44: 1149–1162, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Obara N, Suzuki N, Kim K, Nagasawa T, Imagawa S, Yamamoto M: Repression via the GATA box is essential for tissue-specific erythropoietin gene expression. Blood 111: 5223–5232, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Pan X, Suzuki N, Hirano I, Yamazaki S, Minegishi N, Yamamoto M: Isolation and characterization of renal erythropoietin-producing cells from genetically produced anemia mice. PLoS ONE 6: e25839, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frede S, Freitag P, Geuting L, Konietzny R, Fandrey J: Oxygen-regulated expression of the erythropoietin gene in the human renal cell line REPC. Blood 117: 4905–4914, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Paliege A, Rosenberger C, Bondke A, Sciesielski L, Shina A, Heyman SN, Flippin LA, Arend M, Klaus SJ, Bachmann S: Hypoxia-inducible factor-2alpha-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int 77: 312–318, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Machura K, Iankilevitch E, Neubauer B, Theuring F, Kurtz A: The aldo-keto reductase AKR1B7 coexpresses with renin without influencing renin production and secretion. Am J Physiol Renal Physiol 304: F578–F584, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Kurtz L, Janssen-Bienhold U, Kurtz A, Wagner C: Connexin expression in renin-producing cells. J Am Soc Nephrol 20: 506–512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivan M, Kaelin WG, Jr: The von Hippel-Lindau tumor suppressor protein. Curr Opin Genet Dev 11: 27–34, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Chavez JC, Baranova O, Lin J, Pichiule P: The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J Neurosci 26: 9471–9481, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH: Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 117: 1068–1077, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, Eckardt KU: Differentiating the functional role of hypoxia-inducible factor (HIF)-1 alpha and HIF-2 alpha (EPAS-1) by the use of RNA interference: Erythropoietin is a HIF-2 alpha target gene in Hep3B and Kelly cells. FASEB J 18: 1462–1464, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Weidemann A, Kerdiles YM, Knaup KX, Rafie CA, Boutin AT, Stockmann C, Takeda N, Scadeng M, Shih AY, Haase VH, Simon MC, Kleinfeld D, Johnson RS: The glial cell response is an essential component of hypoxia-induced erythropoiesis in mice. J Clin Invest 119: 3373–3383, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hackenthal E, Paul M, Ganten D, Taugner R: Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 70: 1067–1116, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Castrop H, Klar J, Wagner C, Hocherl K, Kurtz A: General inhibition of renocortical cyclooxygenase-2 expression by the renin-angiotensin system. Am J Physiol Renal Physiol 284: F518–F524, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC, Shen J, Chen YM, Wu KD, Tsai TJ, Duffield JS, Lin SL: Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 80: 1170–1181, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Gandhi R, Le Hir M, Kaissling B: Immunolocalization of ecto-5′-nucleotidase in the kidney by a monoclonal antibody. Histochemistry 95: 165–174, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sequeira Lopez ML, Gomez RA: Development of the renal arterioles. J Am Soc Nephrol 22: 2156–2165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, Keith B, Epstein JA, Moores SL, Erickson-Miller CL, Haase VH: Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood 116: 3039–3048, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rankin EB, Wu C, Khatri R, Wilson TLS, Andersen R, Araldi E, Rankin AL, Yuan J, Kuo CJ, Schipani E, Giaccia AJ: The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell 149: 63–74, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taugner RA, Hackenthal E: The Juxtaglomerular Apparatus: Structure and Function, Heidelberg, Germany, Springer Verlag, 1989 [Google Scholar]
- 34.Castrop H, Höcherl K, Kurtz A, Schweda F, Todorov V, Wagner C: Physiology of kidney renin. Physiol Rev 90: 607–673, 2010 [DOI] [PubMed] [Google Scholar]
- 35.La Ferla K, Reimann C, Jelkmann W, Hellwig-Burgel T: Inhibition of erythropoietin gene expression signaling involves the transcription factors GATA-2 and NF-kappa B. FASEB J 18: 1811–1813, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Fandrey J, Frede S, Ehleben W, Porwol T, Acker H, Jelkmann W: Cobalt chloride and desferrioxamine antagonize the inhibition of erythropoietin production by reactive oxygen species. Kidney Int 51: 492–496, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Fandrey J, Frede S, Jelkmann W: Role of hydrogen peroxide in hypoxia-induced erythropoietin production. Biochem J 303: 507–510, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Todorov V, Müller M, Schweda F, Kurtz A: Tumor necrosis factor-alpha inhibits renin gene expression. Am J Physiol Regul Integr Comp Physiol 283: R1046–R1051, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Todorov VT, Völkl S, Friedrich J, Kunz-Schughart LA, Hehlgans T, Vermeulen L, Haegeman G, Schmitz ML, Kurtz A: Role of CREB1 and NFkappaB-p65 in the down-regulation of renin gene expression by tumor necrosis factor alpha. J Biol Chem 280: 24356–24362, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Todorov VT, Völkl S, Müller M, Bohla A, Klar J, Kunz-Schughart LA, Hehlgans T, Kurtz A: Tumor necrosis factor-alpha activates NFkappaB to inhibit renin transcription by targeting cAMP-responsive element. J Biol Chem 279: 1458–1467, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Itani H, Liu X, Sarsour EH, Goswami PC, Born E, Keen HL, Sigmund CD: Regulation of renin gene expression by oxidative stress. Hypertension 53: 1070–1076, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC: Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A 104: 2301–2306, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bachmann S, Mutig K, Bates J, Welker P, Geist B, Gross V, Luft FC, Alenina N, Bader M, Thiele BJ, Prasadan K, Raffi HS, Kumar S: Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. Am J Physiol Renal Physiol 288: F559–F567, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 45.Schley G, Klanke B, Schödel J, Forstreuter F, Shukla D, Kurtz A, Amann K, Wiesener MS, Rosen S, Eckardt KU, Maxwell PH, Willam C: Hypoxia-inducible transcription factors stabilization in the thick ascending limb protects against ischemic acute kidney injury. J Am Soc Nephrol 22: 2004–2015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauter A, Machura K, Neubauer B, Kurtz A, Wagner C: Development of renin expression in the mouse kidney. Kidney Int 73: 43–51, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Paliege A, Pasumarthy A, Mizel D, Yang T, Schnermann J, Bachmann S: Effect of apocynin treatment on renal expression of COX-2, NOS1, and renin in Wistar-Kyoto and spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 290: R694–R700, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Rosenberger C, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU: Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.