Abstract
Rhophilin-1 is a Rho GTPase-interacting protein, the biologic function of which is largely unknown. Here, we identify and describe the functional role of Rhophilin-1 as a novel podocyte-specific protein of the kidney glomerulus. Rhophilin-1 knockout mice were phenotypically normal at birth but developed albuminuria at about 2 weeks of age. Kidneys from severely albuminuric mice revealed widespread podocyte foot process effacement, thickening of the glomerular basement membrane, and FSGS-like lesions. The absence of any overt changes in the expression of podocyte proteins at the onset of proteinuria suggested that the primary cause of podocyte abnormalities in Rhpn1-null mice was the result of cell-autonomous, Rhophilin-1–dependent signaling events. In culture, Rhophilin-1 was detected at the plasma membrane leading edge of primary podocytes, where it elicited remodeling of the actin cytoskeleton network. This effect of Rhophilin-1 on actin cytoskeleton organization associated with inhibitory effects on Rho-dependent phosphorylation of the myosin regulatory light chain and stress fiber formation. Conversely, phosphorylation of myosin regulatory light chain increased in podocyte foot processes of Rhpn1−/− mice, implicating altered actinomyosin contractility in foot process effacement and compromised filtration capacity. Targeted deletion of RhoA in podocytes of Rhophilin-1 knockout mice exacerbated the renal injury. Taken together, our results indicate that Rhophilin-1 is essential for the integrity of the glomerular filtration barrier and that this protein is a key determinant of podocyte cytoskeleton architecture.
Keywords: glomerulus, podocyte, albuminuria, FSGS
Glomerular damage is the primary cause of CKD and highlights the need to better understand the critical involvement of this highly specialized microvascular unit in renal disease etiology. The principal function of the glomerulus is to selectively filter blood across the capillary wall and produce an ultrafiltrate that can be secondarily modified by the renal tubule system. The filtration capacity of the glomerulus is defined by the functional properties of the glomerular filtration barrier (GFB), a trilaminar molecular sieve comprised of endothelial and podocyte cell types that medially elaborate the constituents of the glomerular basement membrane (GBM) situated between them. Although each individual component of the GFB is necessary for its concerted filtration capacity, damage to the podocyte is a hallmark of most glomerular diseases.1
The podocyte is a highly specialized epithelial cell with a unique morphologic design defined by a cell body that arborizes into primary processes that further furcate into more slender foot processes that enwrap the capillary wall. Juxtaposed between individual foot processes is the slit diaphragm, a unique cell–cell junction, the structural and signaling integrity of which is critically required to achieve efficient filtration capacity. Underlying the slit diaphragm is the actin cytoskeleton, the predominant structural element of foot processes that is implicitly involved in both the formation and maintenance of each of these structures. Notably, mutations in not only slit diaphragm-specific proteins, such as nephrin2 and podocin,3 but also, key actin cytoskeleton regulatory components, including α–actinin-4,4 CD2AP,5 and NCK1/2,6 lead to podocyte dysfunction, albuminuria, and associated glomerular disease. Mechanistically, the intimate and requisite relationship that exists between the slit diaphragm and the actin cytoskeleton of foot processes is exemplified by the molecular interconnectivity that links many of the proteins of these two structures together.5–8 A series of recent studies investigating the role of the canonical Rho GTPase family of actin cytoskeleton-regulatory proteins in podocyte biology has provided substantial direct evidence for the unifying involvement of this family of proteins in determining podocyte cytoskeletal dynamics and function.9–13 Continued investigation into the repertoire of individual protein species that characterize and control podocyte cytoskeletal dynamics is, therefore, a central component to furthering our understanding of both normal and pathologic podocyte-dependent glomerular function.
In this study, we identify and define the functional role of Rhophilin-1, a novel highly expressed podocyte protein. Although Rhophilin-1 was originally identified almost 20 years ago in a screen for RhoA GTPase-interacting partners,14 no physiologic function has been previously attributed to this protein. Our results indicate that Rhophilin-1 is essential for the maintenance of podocyte foot process architecture as well as the functional integrity of the GFB and establish Rhophilin-1 as a novel and necessary member of the molecular machinery that determines actin cytoskeleton dynamics in this unique cell type.
Results
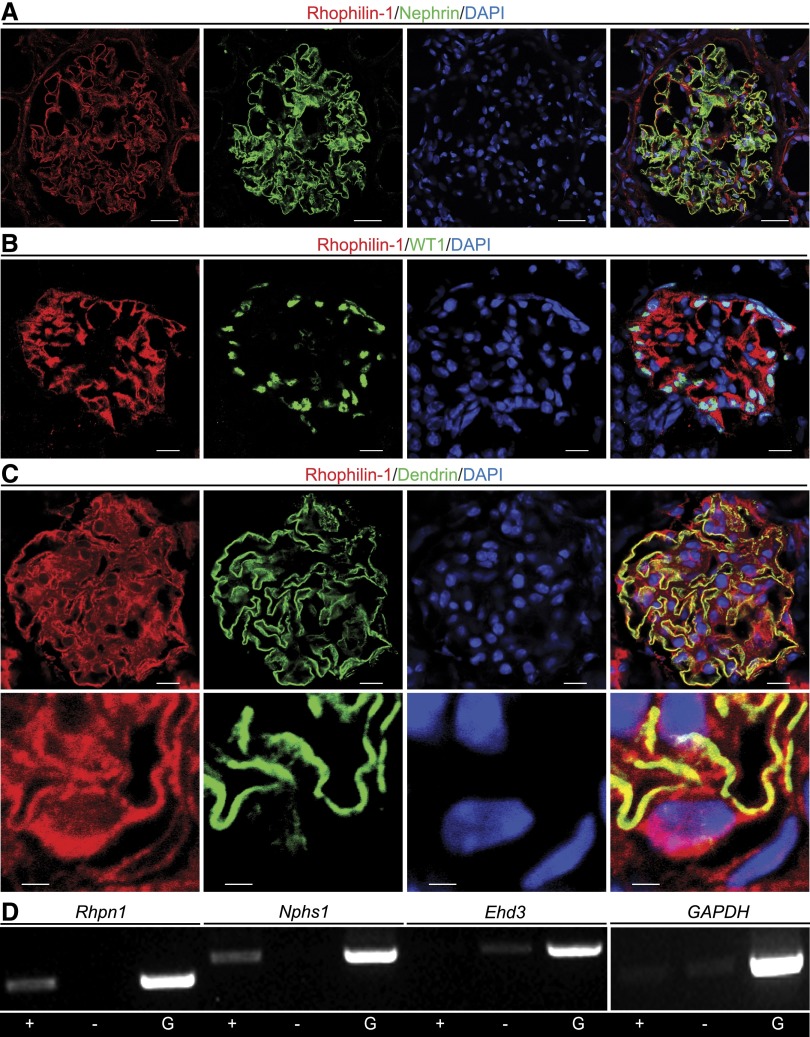
We previously identified Rhpn1 as a highly enriched transcript expressed in the mouse glomerulus but did not have any protein expression data to support its cellular expression.15 Here, we show by coimmunofluorescence in human frozen kidney sections that Rhophilin-1 is richly expressed in the renal glomerulus, where it localizes within the immediate vicinity of the podocyte-specific protein nephrin (Figure 1A). In mouse kidney, Rhophilin-1 expression was restricted to WT1-expressing podocytes, which decorated the periphery of glomeruli (Figure 1B). Costaining with Rhophilin-1 and dendrin, a podocyte protein that localizes to the slit diaphragm of podocyte foot processes,16 resulted in considerable overlap of the two proteins (Figure 1C). At high magnification, Rhophilin-1 colocalized with dendrin expression, which was spatially restricted to fine podocyte processes. Rhophilin-1 appeared additionally localized to podocyte extensions emanating from the cell body surrounding the nucleus (Figure 1C, lower panel). To further verify its restricted cellular expression to the podocyte lineage of the glomerulus, we performed FACS analysis of isolated single cells derived from glomeruli of bitransgenic mice harboring podocin promoter-driven Cre recombinase and ROSA26/yellow fluorescent protein (YFP) transgenes (Supplemental Figure 1). PCR analysis of cDNA samples derived from YFP-positive (podocyte fraction) or -negative cell populations (mesangial and endothelial fractions) indicated that Rhpn1 expression was confined to the YFP-positive podocyte fraction of cells that expresses nephrin (Figure 1D). Rhophilin-2, a paralog of Rhophilin-1 sharing 40% sequence identity, was only nominally detected in glomeruli (Supplemental Figure 2).
Figure 1.
Rhophilin-1 is expressed exclusively in podocytes of the kidney glomerulus. (A) In human glomeruli, Rhophilin-1 localizes extensively with the podocyte slit diaphragm protein nephrin. (B) In mouse, Rhophilin-1 is expressed in the glomerulus, where it surrounds and extends from WT1-expressing podocytes. (C) Rhophilin-1 colocalizes with the podocyte-specific protein dendrin in foot processes of the mouse glomerulus (high magnification in lower panel). (D) RT-PCR analysis of YFP-positive (+) and YFP-negative (−) cell populations obtained from FACS-sorted glomerular cells of Nphs2-cre–driven ROSA26/YFP reporter mice. Only + cells express Nphs1 and Rhpn1, whereas only − cells express the endothelial-specific transcript Ehd3. Glomeruli (G) express each of the transcripts. DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Scale bars, 20 μm in A; 10 μm in B and C, upper panel; 2 μm in C, lower panel.
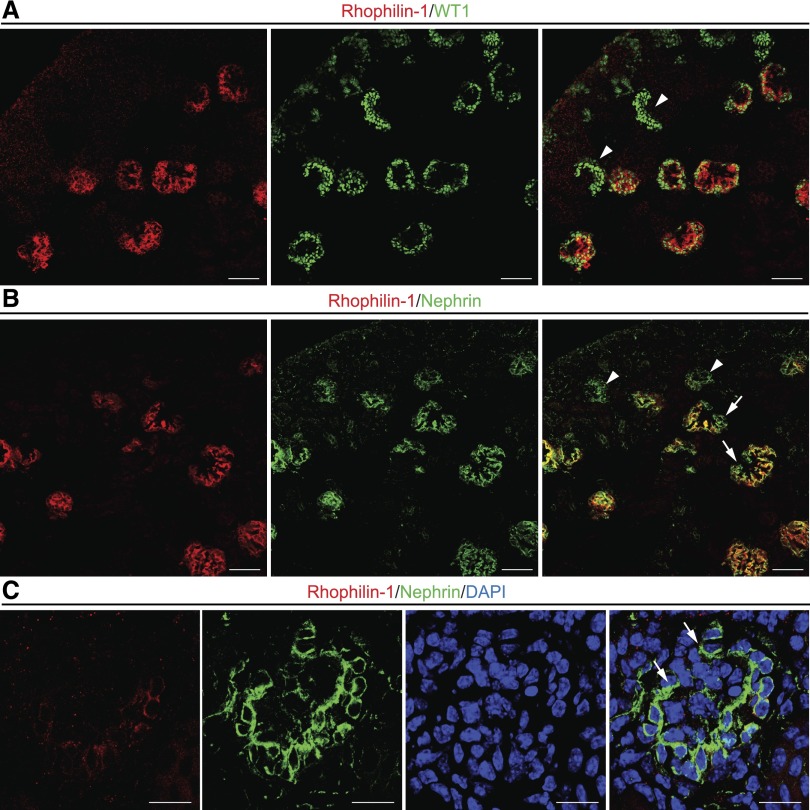
We then examined kidneys of newborn mice to delineate the spatiotemporal appearance of Rhophilin-1, because mice of this young age harbor each of the various developmental stages of glomerular development. Rhophilin-1 expression was only detected in late-stage, mature glomeruli, and it was not found in peripheral immature glomeruli that were uniquely demarcated by the expression of WT1 in early podocytes of comma- and S-shaped structures (Figure 2A). Nephrin is also a podocyte marker of mature glomeruli, and its timing of expression in podocytes during the capillary loop stage of nephrogenesis is implicated in the coordinated formation of foot processes and slit diaphragms as the podocyte adopts its articulated morphology and surrounds newly formed capillaries.17,18 Although the general expression pattern of Rhophilin-1 and nephrin overlapped considerably, nephrin-positive, early capillary loop glomeruli forming toward the cortex did not express detectable amounts of Rhophilin-1 (Figure 2B). On closer examination, Rhophilin-1 expression could be seen to lag spatiotemporally after nephrin expression was initiated in podocytes of the maturing glomerulus, because these cells predictably differentiate to enwrap the nascent capillary (Figure 2C).
Figure 2.
Rhophilin-1 is expressed in podocytes of mature glomeruli of newborn mice. (A) Rhophilin-1 is not expressed in cells of immature outer cortical glomeruli that stain positively for WT1, an early marker protein of the podocyte lineage (arrowheads). Rhophilin-1 expression is restricted to podocytes of late-stage capillary loop glomeruli located more centrally in the kidney. The peripheral edge of the newborn kidney cortex is located at the top left. (B and C) Rhophilin-1 partially colocalizes with nephrin in mature glomeruli. Note that nephrin expression spatiotemporally precedes that of Rhophilin-1 as the podocytes differentiate and foot processes are formed in maturing glomeruli. Arrowheads indicate nephrin-expressing podocytes that are negative for Rhophilin-1. Arrows highlight glomerular expression of nephrin in podocytes as they enclose around capillary loops before Rhophilin-1 expression is initiated. DAPI, 4′,6-diamidino-2-phenylindole. Scale bars, 50 μm in A and B; 20 μm in C.
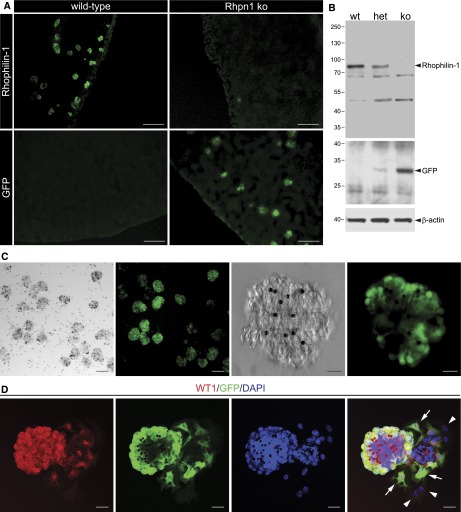
To examine the in vivo function of Rhophilin-1, we generated Rhpn1 null mice, wherein a green fluorescent protein (GFP) expression cassette was inserted in frame after the Rhpn1 start site (Supplemental Figure 3). Chimeric germ-line transmission and subsequent breeding of heterozygous mice gave rise to pups of each of the predicted genotypes born at the expected Mendelian frequency. Immunofluorescence of frozen sections from wild-type and Rhpn1−/− kidneys with anti–Rhophilin-1 and anti-GFP Abs as well as Western blotting of purified glomerular extracts derived from wild-type and targeted mice confirmed the authenticity of both Abs for their respective antigens as well as the absence of full-length Rhophilin-1 in mutant mice (Figure 3, A and B). GFP expression in glomeruli of Rhpn1 null reporter mice was readily detected in and restricted to podocytes as well as their extensions encompassing the glomerular tuft (Figure 3C). This result indicates that GFP expression is strictly controlled by the Rhpn1 promoter and that this fluorescent signal is a reliable surrogate marker of Rhophilin-1 expression in vivo. Additional support for this conclusion was provided by studying primary cultures of glomerular explants derived from Rhpn1 null mice. Under appropriate conditions, WT1-positive nuclei could be observed in cells attached to the glomerular core and also, adherent cells that had migrated away from the glomerulus and onto the substrate surface. GFP was coexpressed in only WT1-positive cells and absent in those adherent cells that did not express the podocyte nuclear protein (Figure 3D).
Figure 3.
Glomeruli of Rhpn1 ko mice do not express Rhophilin-1, and GFP can be used as a surrogate of its expression. (A) Glomerular-restricted expression of Rhophilin-1 in wild-type mice is absent in kidneys of Rhpn1 ko mice, which correspondingly display GFP expression confined to glomeruli. (B) Immunoblotting of glomerular lysates confirms expression of Rhophilin-1 in wild-type (wt) and heterozygous (het) mice and its deletion in Rhpn1 ko animals. GFP is expressed in a reciprocal manner. Molecular mass markers (kilodaltons) are indicated to the left. (C) Live phase contrast and GFP (green) images of purified glomerular suspensions isolated from Rhpn1 ko mice show the purity of glomerular preparations as well as GFP-restricted expression to podocytes and their processes. Magnetic beads (4.5 μm in diameter) are observed as opaque spheres within glomeruli. (D) Primary cultures of glomeruli isolated from Rhpn1 null mice give rise to WT1-expressing outgrown podocytes that stain positively for GFP (arrows). Outgrown cells that do not express WT1 do not express GFP (arrowheads). DAPI, 4′,6-diamidino-2-phenylindole. Scale bars, 100 μm in A; 50 μm in C, left panels; 10 μm in C, right panels; 20 μm in D.
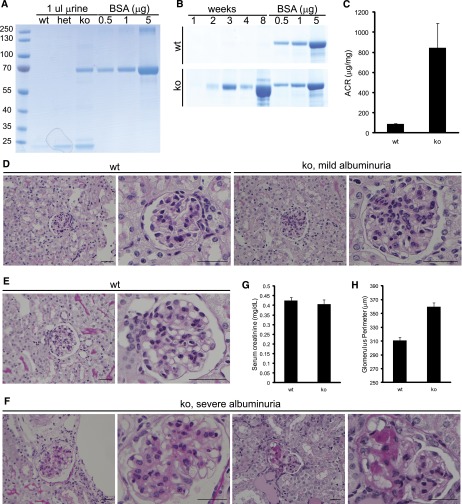
At 1 month of age, albuminuria was readily detected in urine collected from Rhpn1 null mice on a pure C57/BL6 background but absent in both wild-type and Rhpn1 heterozygous mice (Figure 4A). Newborn Rhpn1 null mice did not present albuminuria, but by 2 weeks of age, albuminuria could consistently be detected in all mutant mice. We observed heterogeneity in the progression of albuminuria in mice of mixed 129Sv/BL6 background, with severe albuminuria noted in a majority of mice by 2–4 months of age (Figure 4, B and C, Supplemental Figure 4). We did not observe any sex bias between males and females with regards to their progression and sensitivity to Rhophilin-1 deletion. Mice of mixed 129Sv/BL6 background were consistently more albuminuric than age-matched C57/BL6 mice, and this finding is consistent with the inherent tolerance to kidney damage that characterizes C57/BL6 mice. Notably, regardless of the severity of albuminuria, both mixed and pure C57/BL6 strains presented albuminuria by approximately 2 weeks of age. The kidney phenotype of Rhpn1/2 double knockout (ko) mice is shown in Supplemental Figure 2.
Figure 4.
Rhophilin-1 ko mice are albuminuric and develop FSGS-like lesions. (A) One-month-old C57/BL6 Rhophilin-1 ko mice present with albuminuria, whereas wild-type (wt) and heterozygous (het) mice do not when spot urine was analyzed by SDS-PAGE and Coomassie Blue staining. BSA was used as standard. (B) Coomassie Blue-stained gels of serially collected spot urine samples showing the temporal progression to severe albuminuria in Rhophilin-1 ko mice of mixed 129SV/BL6 background compared with wt littermates. (C) Albumin/creatinine (ACR) was elevated in Rhophilin-1 ko mice 2–6 months age (n=8, ±SEM). (D–F) Representative periodic acid–Schiff staining of kidneys. (D) Glomeruli of mildly albuminuric ko mice at 2 months of age have patent capillary loops and expansion of the mesangium in the absence of any overt tubular changes. Littermate wt controls are shown for comparison. (F) Mesangial expansion is more robust in glomeruli of severely albuminuric Rhophilin-1 ko mice. Focal and segmental sclerosis of glomeruli, loss of glomerular cellularity, fusion to Bowman’s capsule, and protein-filled tubular casts were commonly observed. Histologic images in E and F are from the same mice examined in B when euthanized at 2 months of age. (G) Serum creatinine levels were unchanged in wt and ko mice euthanized at 9–12 months of age ( n=8, ±SEM). (H) Glomerular size was quantitatively increased in Rhophilin-1 ko mice compared with littermate controls 2–6 months age (n=150 glomeruli measured in three animals of each genotype, ±SEM). Scale bars, 50 μm.
Histologic examination of renal specimens obtained from mildly to severely albuminuric mice gave a progressive pathologic picture of glomerular damage that was both focal and segmental in nature. The most prominent early histologic feature observed was mesangial expansion within an otherwise healthy-appearing glomerulus (Figure 4D). There were no readily observable changes in the tubular compartment of kidneys with mild albuminuria at this stage of glomerular disease. Glomeruli of 2-month-old mutant mice with severe albuminuria could be distinguished from wild-type littermates by increased mesangial expansion, synechia, and prominent sclerotic lesions consistent with FSGS (Figure 4, E and F). Protein casts could be readily identified in tubular lumens of mice with macroalbuminuria. There was no increased mortality in Rhophilin-1 ko mice, and serum creatinine levels were unchanged (Figure 4G). A significant increase in glomerular size was also noted in kidneys of Rhpn1 null mice before glomeruli became atrophic and sclerotic (Figure 4H). The increase in glomerular size was mirrored by an increase in kidney size (left kidney weight/body weight [milligrams/gram]: wild-type=4.81±0.26; ko=5.69±0.17; n=8, ±SEM).
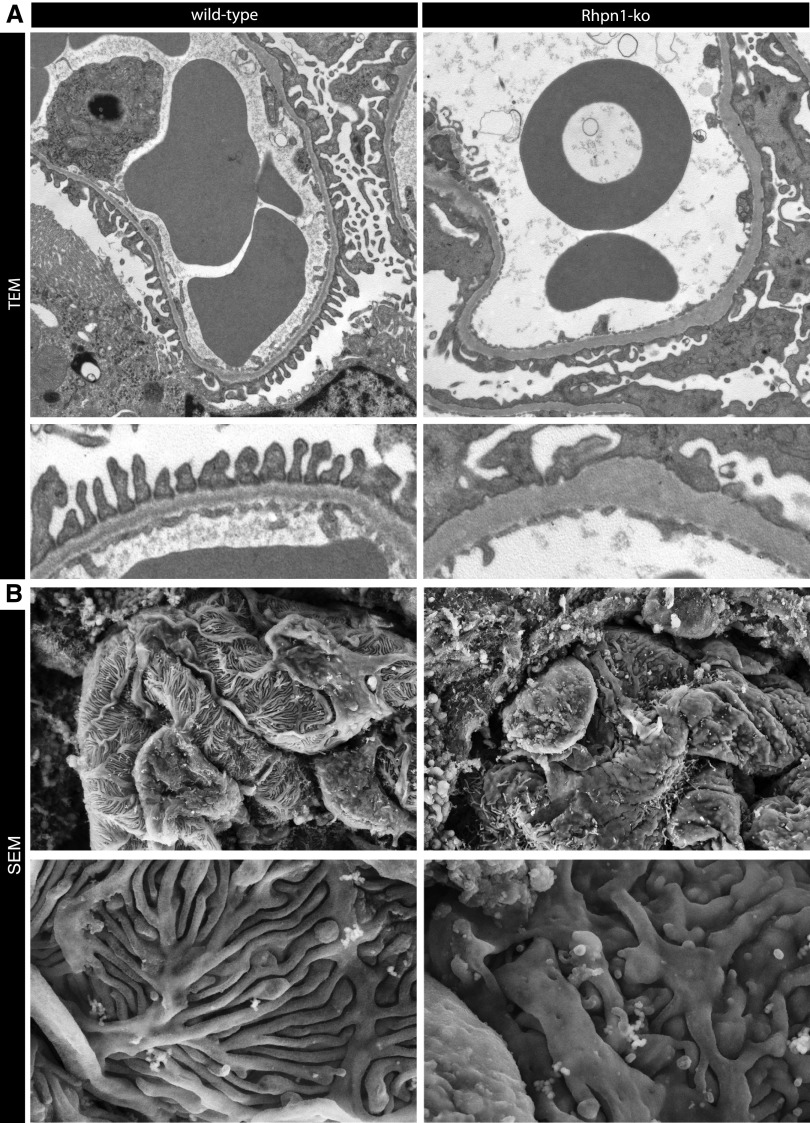
Ultrastructural visualization of glomeruli by electron microscopy revealed prominent effacement of podocyte foot processes (Figure 5A) and a significant reduction in the number of slits circumnavigating capillary loops in Rhpn1 null mice compared with wild-type mice, which is consistent with a primary podocyte challenge in these mice (number of slits per micrometer GBM: wild-type=1.99±0.12; ko=0.63±0.07; n=8, ±SEM). Notably, slit diaphragms were occasionally observed spanning the gap between two adjacent foot processes in Rhophilin-1 ko mice (Supplemental Figure 5). On higher magnification, at the basal aspect of podocyte processes abutting the GBM, membrane invaginations and irregularities could be observed in mutant mice (Figure 5A, lower panel). Also, equally evident in glomeruli of Rhpn1 mutant mice was a considerable thickening and remodeling of the GBM that accompanied foot process effacement (Figure 5A) (GBM thickness [nanometers]: wild-type=192.9±6.5, ko=313±29.5; n=8, ±SEM). Glomeruli from 1-week-old mutant mice tended to have areas of foot process effacement and a reduction in slit frequency that was more prominent than GBM changes (Supplemental Figure 5). This finding suggests that the initial effects of Rhophilin-1 deletion on albuminuria may be contributed to primarily by podocyte-intrinsic defects to the GFB, because GBM changes seem to occur secondarily. Scanning electron microscopy of severely albuminuric mutant mice revealed a loss of the slender foot processes normally apparent in wild-type mice and their replacement with broader, stubby processes that also had prominent microvillous transformation on the apical surface (Figure 5B).
Figure 5.
Ultrastructural changes to podocytes and the GBM characterize the glomerular phenotype of Rhophilin-1 null mice. (A) Transmission electron microscopy (TEM) reveals widespread foot process effacement around capillary loops of Rhophilin-1 ko glomeruli. Also note that the basal surface of podocytes that juxtaposes the outer surface of the GBM shows membrane irregularities and invaginations. The GBM of ko mice is thickened, electron-dense, and homogeneous without clearly defined structural layers. (B) Scanning electron microscopy (SEM) images of the outer surface of capillary loops show a replacement of thin interdigitating podocyte foot processes of wild-type mice with flattened epithelial sheets and stubby foot processes. Microvillous transformation can also been seen on the apical surface of podocytes of Rhophilin-1 ko glomeruli. Magnification, ×10,000 in A, upper panel; ×25,000 in A, lower panel; ×7000 in B, upper panel; ×20,000 in B, lower panel.
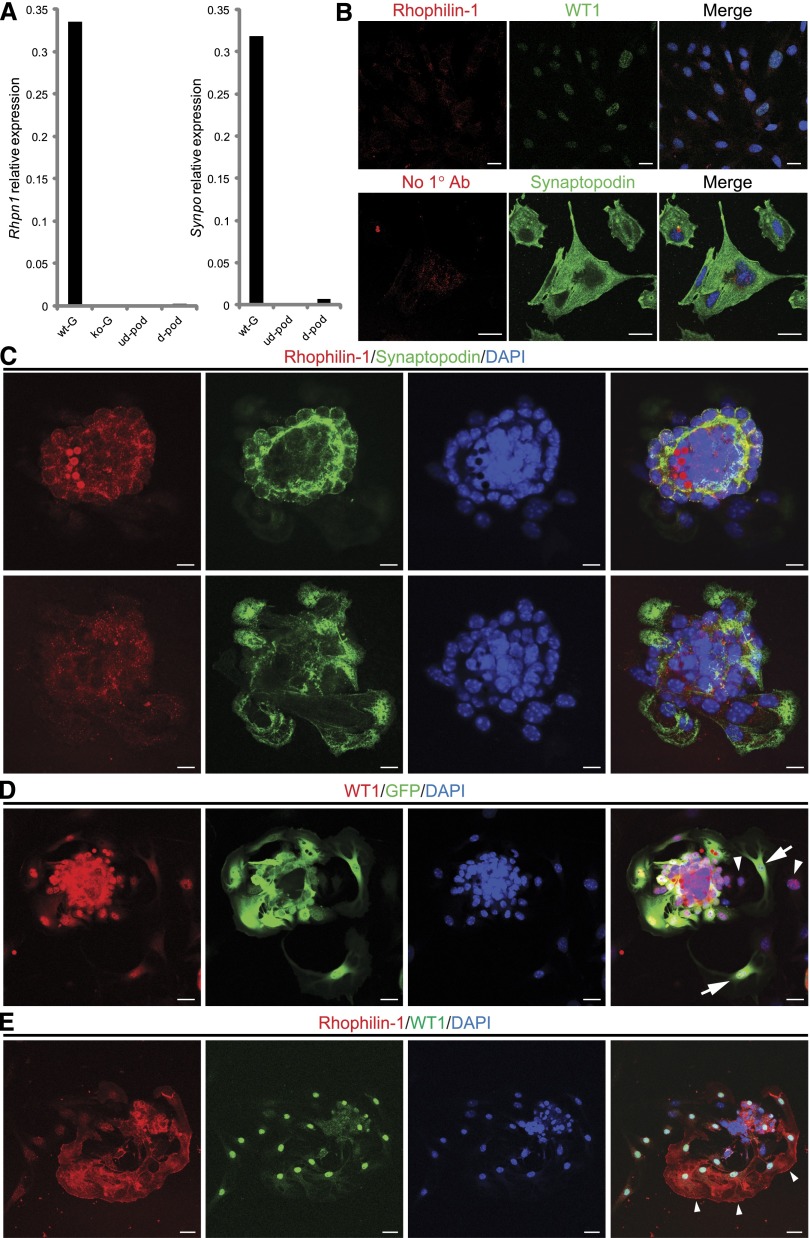
Because we could not readily identify any dramatic change in the expression of a host of podocyte-specific proteins in the kidneys of young Rhpn1 null mice before the onset of heavy proteinuria (Supplemental Figure 6), we hypothesized that Rhophilin-1 deletion may have more specific autonomous effects on podocyte function. As a first attempt to define a putative mechanism for the podocyte phenotypic changes seen in Rhpn1 null mice, we examined the established murine and human immortalized podocyte cell lines. In our hands, however, neither undifferentiated nor differentiated podocytes exhibited any robust Rhophilin-1 gene or protein expression (Figure 6A). This marked downregulation of Rhophilin-1 is also supported by data obtained from unbiased transcriptome profiling of cultured podocytes (K. Endlich, personal communication). However, differentiation-dependent expression of synaptopodin at the gene and protein level was noted under nonpermissive growth conditions (Figure 6, A and B). At the transcript level, Synpo expression was increased over 700-fold relative to that of Rhpn1 on differentiation. Also striking, however, was the considerably limited differentiation capacity of cultured podocytes to express Synpo compared with those levels noted in podocytes of freshly isolated glomeruli. To try and circumvent this issue, we, therefore, investigated endogenous Rhophilin-1 expression in primary podocytes of wild-type glomeruli, because our initial studies performed using GFP visualization as a surrogate reporter for Rhophilin-1 expression had suggested that the Rhpn1 promoter was active under these culture conditions (Figure 3D). Our first analyses, however, of single outgrown primary podocytes did not reveal any discernible cellular expression of Rhophilin-1, despite the conserved differentiation status of these cells as conveyed by their abundant synaptopodin expression (Figure 6C). Rhophilin-1 and synaptopodin expressions were, however, readily noted in glomerular cores that appeared as acini with budding podocytes visible at the periphery. Indeed, re-examination of Rhpn1 null glomerular explants for GFP expression indicated that, although GFP-positive cells were present, there was, in fact, considerable dedifferentiation of podocytes, seen as a loss of GFP expression in otherwise WT1-positive cells, as they migrated out from the glomerular core (Figure 6D). With this in mind, on a more stringent analysis of primary podocyte cultures, we could identify rare but definitive Rhophilin-1 expression at the plasma membrane region of early podocytes migrating out from the wild-type glomerular cores (Figure 6E). Consistently, in those podocytes that continued to express Rhophilin-1, the protein was primarily expressed at the leading edge plasmalemma of convex-shaped cells that appeared to be migrating away from the glomerulus.
Figure 6.
Rhophilin-1 expression in cell lines and primary cultures of glomerular explants. (A) Quantitative analysis of representative Rhpn1 gene expression in glomeruli isolated from wild-type (wt-G) and Rhpn1 null (ko-G) mice compared with undifferentiated (ud-pod) and differentiated (d-pod) cultured mouse podocytes. (B) Differentiation of cultured podocytes induces synaptopodin expression (lower panel) but does not elicit a similar increase in Rhophilin-1 expression (upper panel). Differentiated podocytes stain positively for WT1 and synaptopodin. Immunostaining in the presence or absence of Rhophilin-1 primary Ab gave similar background signal. Merge images are presented with 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstain. (C) Primary outgrown podocytes from wt glomeruli express synaptopodin but do not show significant Rhophilin-1 expression. Both Rhophilin-1 and synaptopodin are readily detected and colocalize in podocytes of the glomerular core (upper panel). Changing the plane of focus (lower panel) reveals outgrown primary podocytes that stain positive for synaptopodin but lack Rhophilin-1. (D) Primary cultures of glomeruli isolated from Rhpn1 null mice show podocyte dedifferentiation. Outgrown cells of the podocyte lineage that express WT1 can be found to costain with GFP (a reporter of Rhpn1 promoter activity; arrows). Some WT1-expressing outgrown podocytes have lost expression of GFP, indicating that the Rhpn1 promoter that drives GFP expression has been silenced and that significant dedifferentiation has occurred (arrowheads). (E) Endogenous Rhophilin-1 expression is observed in a subpopulation of primary outgrown podocytes that stain positively for WT1 (arrowheads). Scale bars, 20 μm in B, D, and E; 10 μm in C.
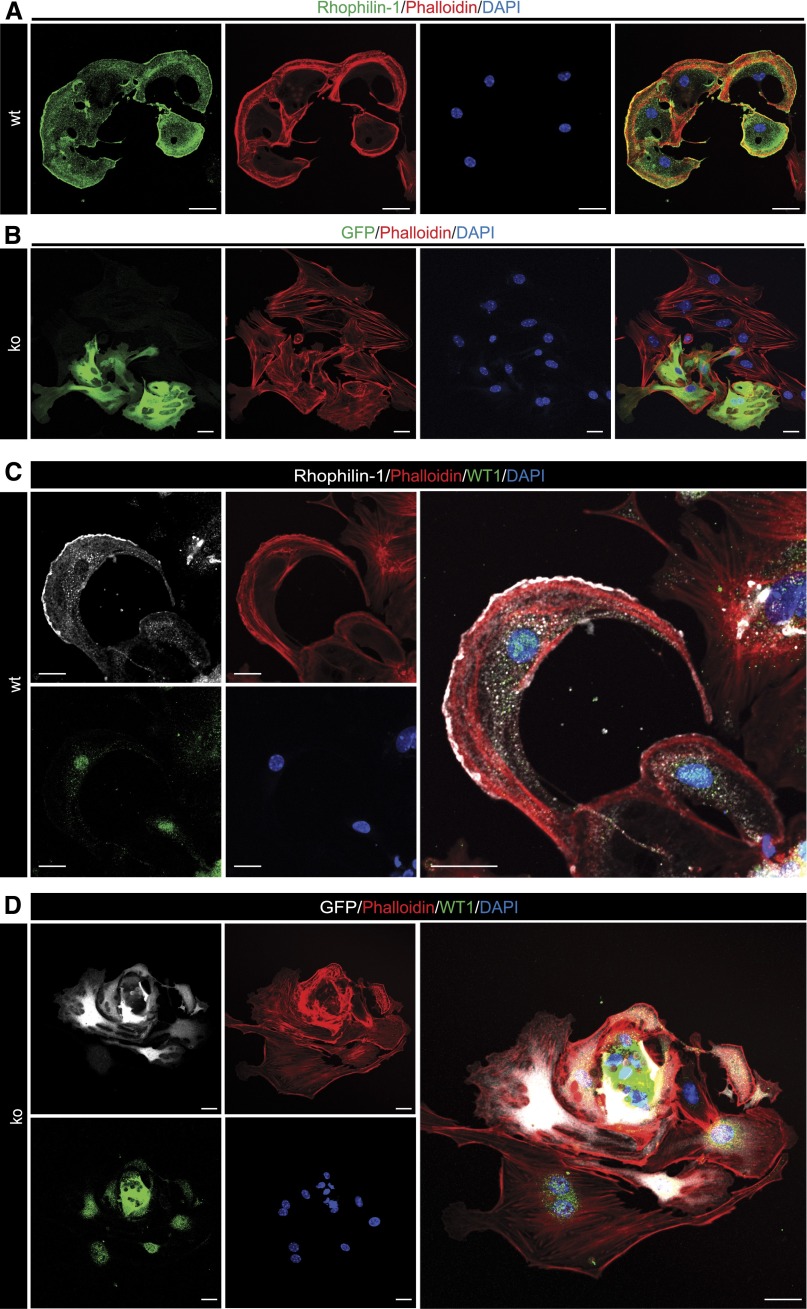
Rhophilin-1 is currently recognized as an adaptor protein for Rho,14 but there is currently no published account of the cellular role of endogenously expressed Rhophilin-1. Given the recently recognized importance of Rho GTPase signaling in determining podocyte cytoskeleton dynamics, we examined the actin cytoskeleton of Rhophilin-1–expressing primary podocytes. Invariably, wild-type podocytes expressing Rhophilin-1 at the plasma membrane displayed a pattern of filamentous actin that was uniquely conserved in these cells and that was not noted in podocytes that lacked Rhophilin-1 (Figure 7). In wild-type cells expressing Rhophilin-1, actin formed a conspicuous polarized network of branched actin bundles that was concentrated toward the cortical regions of the cell where Rhophilin-1 was localized. Also remarkable was the absence of stress fibers throughout the distal portion of the cell body of podocytes, where Rhophilin-1 expression was less obvious. Primary podocytes derived from Rhophilin-1 ko mice that were positive for GFP (indicative of a relatively high degree of differentiation) displayed ventral stress fibers that typically spanned across the cell body and were never seen to adopt the same convex morphology and concentrated peripheral actin network as Rhophilin-1–expressing cells.
Figure 7.
Endogenous Rhophilin-1 is expressed at the plasma membrane of podocytes and induces remodeling of the actin cytoskeleton. (A and C) Subcellular expression of endogenous Rhophilin-1 is concentrated at the convex plasmembrane surface of wild-type (wt) primary podocytes. The actin cytoskeleton, identified by phalloidin staining, is correspondingly remodeled toward the leading edge of Rhophilin-1–expressing podocytes. Intense cortical stress fibers made up of long and branched actin filaments predominated at the leading edge. There was a conspicuous absence of stress fibers toward the cell body and rear of the cell. (B and D) Age-matched glomerular explants from Rhophilin-1 ko mice. Rhophilin-1 null primary podocyte outgrowths (ko), positive for GFP expression, did not adopt a similar convex morphology as seen in Rhophilin-1–expressing wt podocytes but instead, appeared more stellate and less curved at the plasma membrane. These ko podocytes were typified by ventral actin stress fibers that spanned across the cell body. Note in D that Rhophilin-1 ko podocytes, regardless of the extent of their differentiation status (both GFP- positive and -negative WT1-expressing podocytes are present), have a similar network of stress fibers. DAPI, 4′,6-diamidino-2-phenylindole. Scale bars, 20 μm.
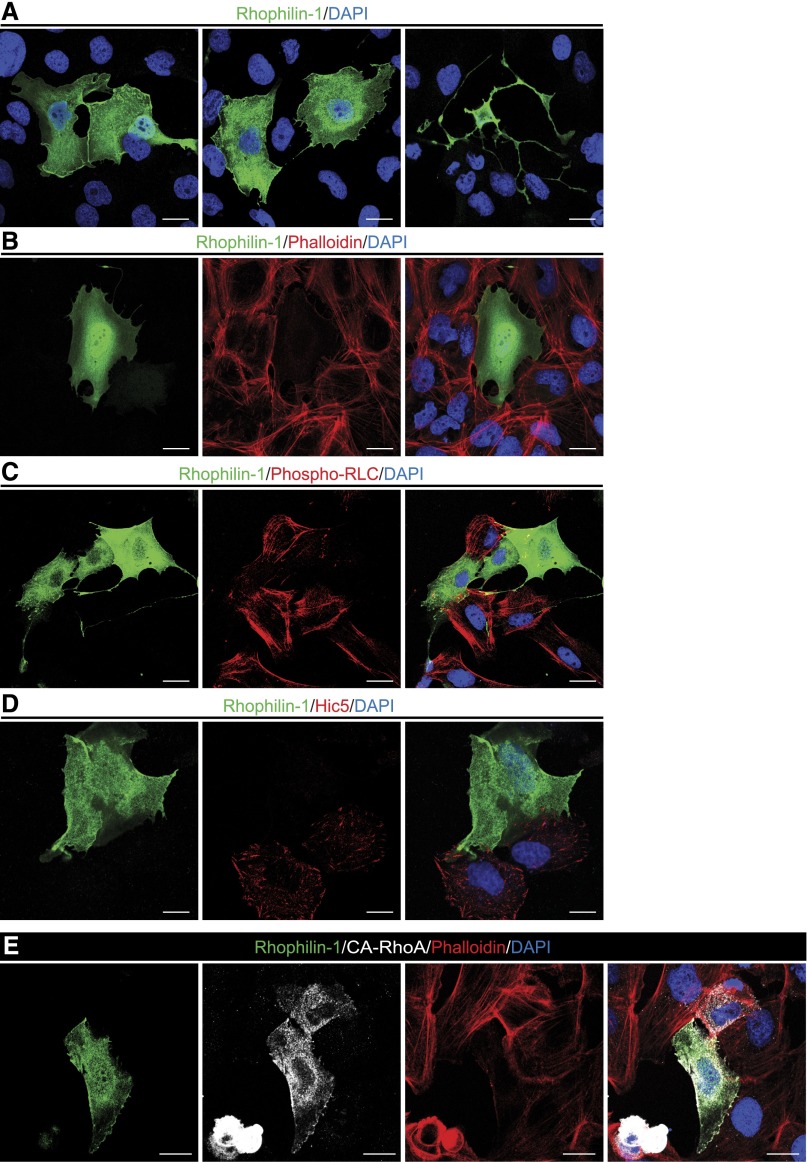
Because of the rarity with which we could detect endogenous Rhophilin-1 expression in primary podocytes and to study further the mechanism of its action, we evaluated to what extent we could recapitulate the observed effects of endogenous Rhophilin-1 on the actin cytoskeleton in immortalized human podocytes transiently transfected with Rhophilin-1. Microscopically, exogenous Rhophilin-1 localized throughout the entire cell body and also showed a considerable degree of plasma membrane enrichment both at sites of cell–cell contact and in single cells (Figure 8A, Supplemental Figure 7). Rhophilin-1 expression elicited dramatic morphologic changes in podocyte cell shape (Figure 8A). Concomitant with these changes, heterologous Rhophilin-1 expression greatly reduced stress fiber formation through the cell body while maintaining a thin peripheral cytoskeleton toward the plasma membrane (Figure 8B, Supplemental Figure 7). Of the Rho GTPase family of cytoskeleton-regulatory proteins, members of the Rho subfamily are well recognized determinants of stress fiber formation in cells.19 To test this finding, we analyzed the activation status of the downstream Rho substrate nonmuscle myosin II regulatory light chain (NM II RLC), the regulatory subunit of NM that facilitates stabilization and formation of myosin filaments that link actin filaments together into thick bundles that form cellular structures, such as stress fibers.20 Under control vector-transfected conditions, podocytes lack Rhophilin-1, have prevalent thick stress fibers, and stain abundantly for NM II RLC phosphorylated at a residue critical for its activity. In contrast, Rhophilin-1 expression-induced reduction of stress fibers was associated with a marked reduction in myosin RLC phosphorylation (Figure 8C), a result suggesting that Rhophilin-1 invoked its effects on actin cytoskeleton remodeling by interfering with Rho-dependent stress fiber formation. The other principal cellular response to Rho activation is focal adhesion (FA) formation.19 Similar to its inhibitory effect on myosin RLC phosphorylation and stress fiber formation, Rhophilin-1–expressing podocytes also had fewer FAs, which are detected using Hic5, an FA-associated protein abundantly expressed in podocytes (Figure 8D). To test whether Rhophilin-1 could inhibit RhoA-dependent stress fiber formation, we evaluated the effect of its expression on the contractile phenotype induced by constitutively active (CA)-RhoA (Supplemental Figure 7). In support of the propensity of Rhophilin-1 to interfere with downstream Rho-dependent signaling, we found that Rhophilin prevented the effects of CA-RhoA on stress fiber formation (Figure 8E).
Figure 8.
Rhophilin-1 expression in human podocytes inhibits Rho-dependent signaling. (A) Heterologously expressed Rhophilin-1 partially localizes at the cell membrane and sites of cell–cell contact. (B) The actin stress fiber network (visualized with phalloidin) is disrupted in Rhophilin-1–expressing cells. (C) Rhophilin-1 inhibits phosphorylation of the RhoA substrate myosin RLC. (D) FAs (Hic5) are markedly reduced in Rhophilin-1–expressing cells. (E) Rhophilin-1 prevents CA-RhoA–dependent stress fiber formation and contractile phenotype. Note the smaller cell size and increased stress fiber formation in CA-RhoA–expressing cells compared with Rhophilin-1/CA-RhoA–expressing cells. DAPI, 4′,6-diamidino-2-phenylindole. Scale bars, 20 μm.
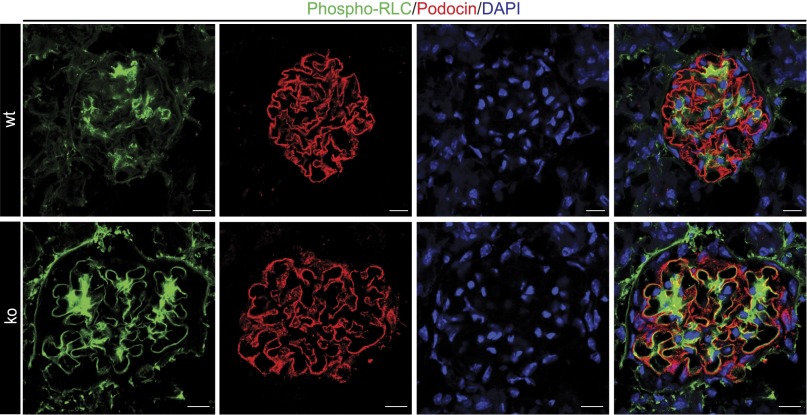
Taken together, these results indicate that Rhophilin-1 is an inhibitor of Rho-dependent stress fiber formation in vitro. To extend these observations back to the in vivo situation, it follows that podocytes of Rhophilin-1 null mice should have elevated levels of phosphorylated NM II RLC if, in fact, Rhophilin-1 is a major determinant of its activity and foot process architecture. In situ analysis of freshly isolated and fixed kidney sections indicated a remarkable increase in phosphorylated myosin RLC in the periphery of capillary loops of Rhophilin-1 ko mice compared with wild-type, where its expression partially overlapped with podocin (Figure 9). Also notable was an increase in phospho-RLC in the mesangial compartment. Coimmunofluorescence of glomeruli indicated areas of synaptopodin expression in foot processes that occurred in the absence of phospho-RLC (Supplemental Figure 8).
Figure 9.
NM II RLC is phosphorylated in glomeruli of Rhophilin-1 ko mice. Myosin RLC phosphorylation in the periphery of capillary loops and the mesangial compartment of Rhophillin-1 ko mice is increased compared with wild-type (wt). DAPI, 4′,6-diamidino-2-phenylindole. Scale bars, 10 μm.
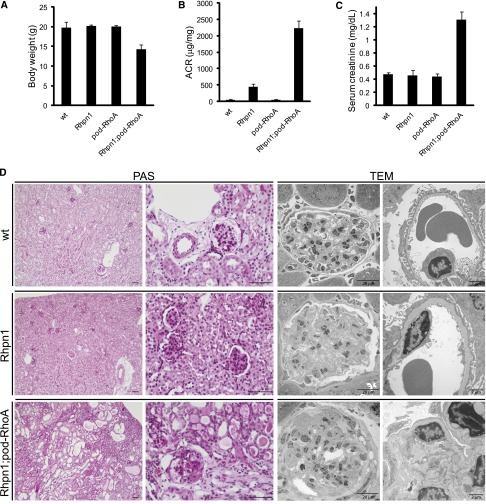
The in vivo involvement of podocyte-specific RhoA in mediating the renal phenotype of Rhophilin-1 null mice was then examined using triple transgenic animals (Rhpn1−/−; Nphs2cre+/−; RhoAfl/fl). To our surprise, whereas podocyte-specific RhoA deletion mutants (pod-RhoA) showed no renal phenotype in Rhpn1+/+ and Rhpn1+/− mice (result not shown),11 Rhophilin-1/pod-RhoA double ko animals presented a much more severe kidney injury (compared with Rhophilin-1 ko) characterized by segmental and global FSGS, complete podocyte effacement, tubule dilation and protein casts, interstitial fibrosis, elevated serum creatinine, and reduced body weight by 3 months of age (Figure 10). These results confirm the existence of a tightly coordinated, physiologically relevant signaling relationship between Rhophilin-1 and RhoA in podocytes.
Figure 10.
Podocyte-specific RhoA deletion exacerbates the kidney phenotype of Rhophilin-1 null mice. (A–C) Body weights, albumin/creatinine (ACR), and serum creatinine of mice of the indicated genotypes at approximately 3 months of age (n=3 for each group). (D) Representative periodic acid–Schiff (PAS) staining and transmission electron microscopy (TEM) images of kidneys isolated from mice of the indicated genotype at 3 months of age. Rhophilin-1 null glomeruli showed mesangial expansion and regions of foot process effacement. Kidneys from Rhophilin-1/pod-RhoA double ko mice presented glomerular segmental and global sclerosis accompanied by complete foot process effacement. Widespread tubule dilation and protein casts as well as focal areas of interstitial fibrosis were also evident. wt, wild-type. Scale bars, 50 μm in PAS images.
Discussion
The Rho GTPases are major determinants of actin cytoskeletal dynamics that control a wide variety of morphogenic events.21–23 In principle, dysfunction at any level along this regulated signaling cascade could alter cytoskeletal dynamics and with respect to the podocyte, result in a compromised ability to form and maintain foot processes. At the top of the Rho GTPase signaling cascade, such a role has been investigated by knocking out each of the classic Rho GTPases (RhoA, Rac1, and Cdc42) in the podocyte lineage using conditional gene targeting strategies in the mouse.11 Although there are additional factors to consider when interpreting these results,24 the findings of this study confirm the unequivocal involvement of Cdc42 in podocyte biology. At first glance, RhoA and Rac1 seem to be dispensable, because it is the inappropriate activation of these Rho GTPases that is more commonly associated with podocyte dysfunction in a number of murine models of glomerular disease.12,13,25–28 Be that as it may, however, more recent examination of podocyte-specific Rac1 null mice with acute and chronic forms of kidney injury10 combined with results obtained here using Rhophilin-1/pod-RhoA null mice suggest that it is the intracellular signaling context of each Rho GTPase species in healthy and disease states that determines their obligate involvement in regulating podocyte function. It is, therefore, clear that each of the classic Rho GTPases plays important and coordinated roles in controlling podocyte function and that care should be taken when considering their individual contributions to phenotype determination.
Phosphorylation of the RLC of NM II facilitates the interaction of polymerized actin filaments with bipolar myosin filaments to form actomyosin cables.20 These structures, also commonly known as stress fibers, are found at the base of the cell and mediate contractile forces that exert tension or produce movement. On the basis of the results of electron microscopy studies, it has been suggested that the replacement of podocyte foot processes with broad epithelial sheets in glomerular disease is dependent on the altered contractility afforded by rearrangement of their predominantly thin actin filaments into thick actomyosin bundles.29,30 In healthy podocytes, we predict that Rhophilin-1 controls actin cytoskeletal dynamics by primarily restricting Rho activity. The presence of Rhophilin-1, as suggested by our observations with primary cultures, may be necessary to maintain subcortical actin in a structural network that is required to provide the appropriate resistance to mechanical forces that take place in the glomerulus. In the absence of Rhophilin-1, the increased association of actin filaments with myosin multimers is predicted to alter podocyte contractility and mechanical stability in a manner that prohibits the maintenance of slender podocyte extensions. Taken together, these observations may provide a possible explanation for the surprising lack of foot process effacement and albuminuria that accompanies podocyte-specific deletion of NM IIA in the mouse,31 because our results suggest that it is the inappropriate activation of this protein and not its inactivation that elicits podocyte foot process effacement. Such a scenario is supported by the ability of the GFB to tolerate podocyte-specific RhoA deletion but not its overactivation.11,12
Our in vitro results obtained using immortalized human podocytes and primary cultures of podocytes derived from glomerular explants support the conceptual framework of a Rhophilin-1–regulated cytoskeleton axis in maintaining foot process structure in vivo. With respect to the phenotype observed in primary podocytes, the subcellular localization and restricted expression of endogenous Rhophilin-1 to the plasma membrane leading edge and resultant actin network are particularly noteworthy. Subcellular localization of endogenous Rhophilin-1 has only been previously detected in the fibrous sheath of the sperm tail, where its functional significance remains unknown.32 Rhophilin-1 is currently considered a cytosolic adapter protein linking Rho to other proteins, and our results provide the first indication of its in vivo subcellular localization and function in somatic cells. Because of the rarity, with which we were able to reliably detect endogenous Rhophilin-1 expression in primary podocyte cultures, we have not been able to determine what factors mediate its expression at the leading edge using this model. Furthermore, heterologous expression of Rhophilin-1 in human podocytes did not faithfully recapitulate the plasma membrane localization seen in primary podocytes, although some enrichment at the plasma membrane was noted under the conditions used here. These results suggest that the spatial targeting of Rhophilin-1 is dependent on additional factors present in primary cultures that are missing in cell lines that do not achieve sufficient differentiation to mimic the primary podocyte phenotype that may more closely phenocopy the situation in vivo. Prenylation of Rho facilitates its membrane association, and it may, in part, participate in the targeting of Rhophilin-1, because Rhophilin-1 and Rho directly interact with one another.14 Additionally, it has recently been shown that the PDZ domain of Rhophilin-2 possesses binding properties that facilitate association with both protein-binding partners as well as membrane surfaces, including the plasma membrane.33
The loss of ventral stress fibers and accumulation of transverse stress fibers coupled with process extension observed in podocytes expressing Rhophilin-1 are consistent with the involvement of Rhophilin-1 in Rho GTPase signaling and parallel the findings of pioneering studies indicating that Rho regulates the assembly of stress fibers and FAs and that Rac mediates lamellipodia formation.19,34 Primary podocytes expressing Rhophilin-1 present a dual cytoskeletal phenotype that also points to the complexity and coordinated nature of Rho GTPase activities. At the leading edge of primary podocytes, Rhophilin-1 expression at this subcellular site coincides with the appearance of a highly branched subcortical actin network and is consistent with a putative Rhophilin-1–mediated inhibition of Rho-induced long stress fiber formation and an antagonistic Rac-induced branching of actin filaments and lamellipodial extension. Such a similar reciprocal pattern of Rho-mediated regulation of Rac signaling has been mechanistically ascribed to Arhgap24, a Rho-regulated Rac GTPase-activating protein.35 Of significant interest, this Rac GTPase-activating protein has been identified in podocytes, where it was shown to influence podocyte cell shape and membrane dynamics.9
In addition to podocyte cytoskeletal changes and foot process effacement, one of the most striking ultrastructural features observed in the glomeruli of Rhophilin-1 ko mice is thickening and remodeling of the GBM. The Rhophilin-1–dependent mechanisms leading to these changes in glycoprotein and proteoglycan matrix composition of the GBM remain unknown, but preliminary results suggest that there is significant alteration in the population of extracellular matrix components (collagen, laminin, and nidogen transcripts) detected in RNA-sequenced glomeruli of wild-type and Rhophilin-1 null mice (M.A. Lal et al., unpublished data). Such podocyte-induced changes in the organization of the GBM bare similarities to established models of fibrillogenesis, where molecules, such as NM II, are used by the cell to form and remodel the extracellular matrix during periods of tissue morphogenesis and injury repair.20,36 It is not unlikely that, in Rhophilin-1 ko mice, increased NM II-mediated stress fiber formation and altered contractility in podocytes may result in altered integrin-mediated adhesion and secondary GBM modification. Furthermore, it is also likely that the final overt glomerular damage that occurs in Rhophilin-1 ko mice is a result of the composite integration of communication between different cell types. Expansion of the mesangium and deposition of matrix are hallmarks of glomerular disease pathology, but the mechanisms accounting for the prevalence of such changes in glomerular disease of podocyte origin remain incompletely understood. In the case of Rhophilin-1 null mice, it is possible that the podocyte-mediated remodeling of the GBM may contribute to changes in the mesangium as a result of their biophysical connection to each other.37
In summary, our results place Rhophilin-1 in the class of podocyte-specific actin-regulatory molecules required for governing the highly ordered three-dimensional cytoskeletal structure of these cells. Additional elucidation of the molecular mechanisms that predicate Rhophilin-1 indispensability for maintaining podocyte foot process morphology will require additional in vivo manipulations as well as the refinement and improvement of podocyte cell culture strategies that more faithfully recapitulate the properties of the in vivo podocyte and its foot process extensions that define its function.
Concise Methods
Abs
The following primary Abs were used. Abs to β-actin (ab8227; Abcam, Inc.), GFP (A11122; Invitrogen), Hic5 (611164; BD Transduction Laboratories), c-myc (M4439, C3956; Sigma-Aldrich), phospho-Myosin Light Chain 2 (Ser19, 3675; Cell Signaling Technology), nephrin (BP5030; Acris), Rhophilin-1 (sc-1940; Santa Cruz Biotechnology and SAB1408228; Sigma-Aldrich), Synaptopodin (sc-50459; Santa Cruz Biotechnology), and WT1 (CA1026; Calbiochem) were obtained commercially. A polyclonal Ab to Rhophilin-1 was generated by immunizing NZW rabbits with a recombinant GST protein fused to amino acids 42–105 of murine Rhophilin-1 (pGEX 4T-3; Amersham Biosciences). Additional Abs to dendrin and nephrin were described previously.16 Secondary Alexa Fluor Abs were from Invitrogen. Filamentous actin was visualized using rhodamine-phalloidin (Life Technologies), and nuclei were counterstained using 4′,6-diamidino-2-phenylindole (Invitrogen).
Cell Culture and Transfection
Conditionally immortalized human podocytes developed by transfection with the temperature-sensitive SV40-T gene were propagated and seeded at 33°C and 5% CO2 in RPMI supplemented with 10% FBS (Invitrogen), 1× insulin-transferring-selenium A supplement (Invitrogen), and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin; Invitrogen). When cells had reached approximately 60% confluence, they were transferred to 37.5°C for 14 days for differentiation. Transient transfections were performed using Lipofectamine 2000 (Invitrogen) or electroporation (Amaxa Nucleofector; Lonza) according to the manufacturer’s recommendations. Human Rhpn1 was expressed in mammalian cells using the pcDNA4/TO/myc-His-A expression vector (Invitrogen) containing an Myc epitope tag (EQKLISEEDL) at the C terminus. The construct was prepared as described here. Full-length cDNA encoding human Rhpn1 was amplified by PCR from a corresponding IMAGE cDNA clone IRATp970F0343D and subsequently cloned into the EcoR1-XbaI sites of pcDNA4/TO/myc-His-A. The integrity of the construct was confirmed by DNA sequencing. Details of how the plasmid was constructed are available on request. CA-RhoA plasmid was used as previously described.
For culture of primary cells, glomeruli isolated from wild-type or Rhpn1 ko mice were seeded at 500 glomeruli/well in 24-well tissue culture dishes with 12-mm glass coverslips and cultivated in DMEM/F12 media (Invitrogen) supplemented with 5% FBS (Invitrogen), 1× insulin-transferrin-selenium A supplement (Invitrogen), and antibiotics (100 units/ml penicillin and 100 μg/ml streptomycin; Invitrogen). After 24 hours in culture, nonadherent glomeruli were removed by aspiration, and fresh media was reintroduced. Under such conditions, podocytes could be seen to begin budding out from glomerular cores within 24 hours. After a sufficient number of individual cells had expanded on the coverslips (typically between 48 and 96 hours), cells were processed for immunofluorescence.
Mice
Rhpn1 ko mice were generated by homologous recombination in 129×1/SvJ RW4 embryonic stem cells using a targeting construct that deletes exons 1–4 of the native Rhpn1 gene and inserts GFP in frame and downstream of the ATG start codon. Correctly targeted embryonic stem cells were identified by long PCR of the short and long arms as well as Southern blotting using appropriate restriction enzymes and hybridization probes located outside the targeting construct (Supplemental Figure 3). Chimerism and germ-line transmission were confirmed in offspring of 129;C57BL6(J) mixed strain mice. Mice were additionally backcrossed onto a pure C57BL6J background for >10 generations.
For urine analysis, spot urine samples were collected and analyzed by SDS-PAGE followed by Coomassie Brilliant Blue or competitive mouse albumin ELISA (Exocell). Urine and serum creatinine were measured using the QuantiChrom Creatinine Assay Kit (BioAssay Systems).
R26-stop-enhanced YFP (R26-stop-EYFP) mice (The Jackson Laboratory) with a loxP-flanked STOP sequence followed by the EYFP gene inserted into the Gt(ROSA)26Sor locus were bred to mice expressing Cre recombinase under the control of the Nphs2 promoter (The Jackson Laboratory). Cre-mediated recombination in the podocyte cell lineage of offspring mice was detected by immunofluorescence and glomerular FACS analysis.
Rhophilin-1/Rhophilin-2 double transgenic mice were generated by intercrossing Rhpn1+/− and Rhpn2+/− mice38 of a mixed C57BL/6, 129Sv, and CD1 genetic background.
Podocyte-specific deletion of RhoA39 on a Rhophilin-1 null background was achieved by intercrossing triple transgenic heterozygous mice (Rhpn+/−; Nphs2cre/+; RhoAfl/+) of a mixed C57BL/6 and 129Sv genetic background.
Breeding and genotyping were performed according to standard procedures. Primers for genotyping the various mouse mutants are available on request. Animal experiments were conducted in accordance with Swedish animal regulations after approval by the Stockholm regional animal ethics committee.
Glomerular Preparation and Podocyte Single-Cell Isolation
Glomeruli were purified from renal cortices of mice by magnetic bead perfusion and particle collection as previously described. To obtain single cells from glomeruli of individual Nphs2-cre;R26-stop-EYFP or Rhpn1/GFP transgenic mice, glomeruli were incubated with trypsin solution containing 0.2% trypsin-EDTA, 100 μg/ml heparin, and 100 units/ml DNase I (Invitrogen) in PBS for 30 minutes at 37°C on a rotisserie followed by mechanical disruption by triturating the glomerular suspension multiple times through a 27-gauge needle. Single cells were separated from remnant glomerular cores by magnetic particle collection of the latter and further purified by size selection through a 40-μm pore size cell strainer. Single cells were subsequently concentrated by centrifugation at 500×g for 5 minutes and resuspended in 400 μl PBS containing 0.1% BSA. To separate podocytes from the remaining glomerular cell population, the glomerular single-cell suspension was FACS sorted according to YFP or GFP using a MoFlo XDP cell sorter. The resultant single-cell fractions were subsequently normalized to the same cell concentration and then pelleted at 500×g for 5 minutes followed by RNA extraction using the Absolutely RNA Nanoprep Kit (Stratagene). RT-PCR was then performed according to standard protocols.
Immunofluorescence and Histochemistry
For immunofluorescence of protein expression in cell culture or frozen kidney sections (prepared in Tissue-Tek OCT compound; Sakura Finetek), specimens were fixed with ice cold 4% paraformaldehyde for 15 minutes, acetone, methanol, or 1:1 methanol/acetone and then additionally permeabilized with 0.1% Triton X-100/PBS for 5 minutes followed by extensive washing. Blocking of nonspecific epitopes was done using 10% normal donkey serum (The Jackson Laboratory) for 30 minutes. Primary Abs were typically used at 1:200 for 1 hour at room temperature or 4°C overnight. Specimens were subsequently incubated with suitable fluorescent secondary Abs for 1 hour and mounted in fluorescent mounting medium (Dako). Where appropriate, F-actin and nuclei were visualized using rhodamine-phalloidin and 4,′6-diamidino-2-phenylindole, respectively. Imaging was performed using a Zeiss LSM510 META confocal microscope (Carl Zeiss AG). For all multiple fluorescent imaging, individual channels were scanned sequentially.
For human kidney, normal renal tissue was obtained from unaffected kidneys surgically removed because of localized carcinoma. All materials were used in agreement with the local Board of Ethics. Frozen human kidney biopsies were sectioned at 8 μm and stained as described above with appropriate Abs.
For histologic examination of mouse kidney, biopsies were formalin-fixed and paraffin-embedded followed by sectioning at 4 μm. The tissue sections were then stained with hematoxylin/eosin or periodic acid–Schiff by standard protocols. Estimation of changes in glomerular size was determined by measuring the perimeter of glomeruli using ImageJ software. Evaluation of glomerular histologic changes was done in a blinded manner.
Transmission Electron Microscopy
Kidney was dissected, and small pieces were fixed in 2% glutaraldehyde and 1% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at room temperature for 30 minutes and stored at 4°C. Specimens were rinsed in 0.1 M phosphate buffer (pH 7.4), postfixed in 2% osmium tetroxide and 0.1 M phosphate buffer (pH 7.4) at 4°C for 2 hours, dehydrated in ethanol followed by acetone, and embedded in LX-112 (Ladd, Burlington, VT). Semithin sections were cut and stained with Toluidine blue O and used for light microscopic analysis. Ultrathin sections (approximately 40–50 nm) were cut by a Leica Ultracut UCT (Leica, Wien, Austria), contrasted with uranyl acetate followed by lead citrate, and examined in a Tecnai 12 Spirit Bio TWIN Transmission Electron Microscope (FEI Company, Eindhoven, The Netherlands) at 100 kV. Digital images were taken by using a Veleta Camera (Olympus Soft Imaging Solutions; GmbH, Münster, Germany).
For semiquantification, three locations per glomerulus were systematically chosen with a random start at low magnification along the glomerular capillaries. Three images were systematically taken from each location, including areas with or without foot process effacement (no sclerotic areas), giving a minimum of nine quantified images per specimen. To distinguish between areas with and without foot process effacement, the number of individual podocyte foot processes (or slits) covering a measured length of the underlying GBM was determined. GBM thickness (nanometers) was determined at three unique sites using the same length of GBM.
Scanning Electron Microscopy
Specimens were fixed by immersion in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). The kidney was cut in 1-mm slices, briefly rinsed in distilled water, and placed in 70% ethanol for 10 minutes, 95% ethanol for 10 minutes, absolute ethanol for 15 minutes at room temperature, and pure acetone for 10 minutes. Specimens were then dried using a critical point dryer (Balzer, CPD 010) using carbon dioxide. After drying, specimens were mounted on an aluminum stub and coated with carbon (Bal-Tec MED 010). The specimens were analyzed in an Ultra 55 Field Emission Scanning Electron Microscope (Carl Zeiss, Oberkochen, Germany) at 3 kV.
Quantitative PCR and Western Blot
Total RNA from mouse glomeruli and differentiated/undifferentiated immortalized human podocytes were isolated using the Qiagen RNeasy Mini Kit. The first-strand cDNA synthesis was carried out according to the manufacturer´s protocol (BioRad). TaqMan probes were purchased (Applied Biosystems), and quantitative PCR was performed using the ABI PRISM 7300 Sequence Detection System (Applied Biosystems). Each sample was done in triplicate. The comparative threshold method was then used for the relative quantification of gene expression.
For Western blotting, cells or kidney tissues were homogenized in 150 mM Tris buffer supplemented with 1% Triton X-100 and protease inhibitor cocktail (Roche Diagnostics) and passed multiple times through a 23-gauge needle. Detergent soluble proteins were subsequently obtained by centrifugation at 10,000×g for 10 minutes. Supernatants were resuspended in NuPAGE LDS sample buffer (Invitrogen) supplemented with β-mercaptoethanol (1:20) and then separated by SDS-PAGE followed by blotting to polyvinylidene difluoride or nitrocellulose membranes. Staining of membranes for protein expression was done according to standard procedures using appropriate Abs and detection reagents (GE Healthcare).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Berit Rydlander for technical assistance and the other members of the Matrix division for helpful scientific discussions. We also appreciate the skilled assistance of the animal caretakers at the Karolinska Institutet Scheele Facility.
This work was supported, in part, by grants from the Svenska Sällskapet för Medicinsk Forskning (to M.A.L.), the Knut and Alice Wallenberg Foundation, the Swedish Research Council, the Swedish Foundation for Strategic Research, and the Novo Nordisk Foundation (to K.T.), a regional agreement on medical training and clinical research/ALF between the Stockholm County Council and the Karolinska Institutet, the Swedish Association of Kidney Patients, Stig and Gunborg Westman's Foundation, and Magnus Bergvall's Foundation (to A.W.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013111195/-/DCSupplemental.
References
- 1.Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 354: 1387–1401, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodríguez-Pérez JC, Allen PG, Beggs AH, Pollak MR: Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet 24: 251–256, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Huber TB, Kwoh C, Wu H, Asanuma K, Gödel M, Hartleben B, Blumer KJ, Miner JH, Mundel P, Shaw AS: Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin. J Clin Invest 116: 1337–1345, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz K, Simons M, Reiser J, Saleem MA, Faul C, Kriz W, Shaw AS, Holzman LB, Mundel P: Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest 108: 1621–1629, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akilesh S, Suleiman H, Yu H, Stander MC, Lavin P, Gbadegesin R, Antignac C, Pollak M, Kopp JB, Winn MP, Shaw AS: Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest 121: 4127–4137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blattner SM, Hodgin JB, Nishio M, Wylie SA, Saha J, Soofi AA, Vining C, Randolph A, Herbach N, Wanke R, Atkins KB, Gyung Kang H, Henger A, Brakebusch C, Holzman LB, Kretzler M: Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int 84: 920–930, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott RP, Hawley SP, Ruston J, Du J, Brakebusch C, Jones N, Pawson T: Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J Am Soc Nephrol 23: 1149–1154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Ellis MJ, Gomez JA, Eisner W, Fennell W, Howell DN, Ruiz P, Fields TA, Spurney RF: Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int 81: 1075–1085, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu L, Jiang R, Aoudjit L, Jones N, Takano T: Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1621–1630, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe G, Saito Y, Madaule P, Ishizaki T, Fujisawa K, Morii N, Mukai H, Ono Y, Kakizuka A, Narumiya S: Protein kinase N (PKN) and PKN-related protein rhophilin as targets of small GTPase Rho. Science 271: 645–648, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Takemoto M, He L, Norlin J, Patrakka J, Xiao Z, Petrova T, Bondjers C, Asp J, Wallgard E, Sun Y, Samuelsson T, Mostad P, Lundin S, Miura N, Sado Y, Alitalo K, Quaggin SE, Tryggvason K, Betsholtz C: Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J 25: 1160–1174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patrakka J, Xiao Z, Nukui M, Takemoto M, He L, Oddsson A, Perisic L, Kaukinen A, Szigyarto CA, Uhlén M, Jalanko H, Betsholtz C, Tryggvason K: Expression and subcellular distribution of novel glomerulus-associated proteins dendrin, ehd3, sh2d4a, plekhh2, and 2310066E14Rik. J Am Soc Nephrol 18: 689–697, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Li C, Ruotsalainen V, Tryggvason K, Shaw AS, Miner JH: CD2AP is expressed with nephrin in developing podocytes and is found widely in mature kidney and elsewhere. Am J Physiol Renal Physiol 279: F785–F792, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Quaggin SE, Kreidberg JA: Development of the renal glomerulus: Good neighbors and good fences. Development 135: 609–620, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Burridge K, Wennerberg K: Rho and Rac take center stage. Cell 116: 167–179, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR: Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778–790, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heasman SJ, Ridley AJ: Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 9: 690–701, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Iden S, Collard JG: Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 9: 846–859, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Pertz O, Hodgson L, Klemke RL, Hahn KM: Spatiotemporal dynamics of RhoA activity in migrating cells. Nature 440: 1069–1072, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Lal MA, Tryggvason K: Knocking out podocyte rho GTPases: And the winner is.... J Am Soc Nephrol 23: 1128–1129, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, Takai Y, Ishikawa A, Shimosawa T, Ando K, Nagase M, Fujita T: Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest 121: 3233–3243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata S, Nagase M, Fujita T: Fluvastatin ameliorates podocyte injury in proteinuric rats via modulation of excessive Rho signaling. J Am Soc Nephrol 17: 754–764, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T: Modification of mineralocorticoid receptor function by Rac1 GTPase: Implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR: Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab 15: 186–200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirato I: Podocyte process effacement in vivo. Microsc Res Tech 57: 241–246, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Shirato I, Sakai T, Kimura K, Tomino Y, Kriz W: Cytoskeletal changes in podocytes associated with foot process effacement in Masugi nephritis. Am J Pathol 148: 1283–1296, 1996 [PMC free article] [PubMed] [Google Scholar]
- 31.Johnstone DB, Zhang J, George B, Léon C, Gachet C, Wong H, Parekh R, Holzman LB: Podocyte-specific deletion of Myh9 encoding nonmuscle myosin heavy chain 2A predisposes mice to glomerulopathy. Mol Cell Biol 31: 2162–2170, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura K, Fujita A, Murata T, Watanabe G, Mori C, Fujita J, Watanabe N, Ishizaki T, Yoshida O, Narumiya S: Rhophilin, a small GTPase Rho-binding protein, is abundantly expressed in the mouse testis and localized in the principal piece of the sperm tail. FEBS Lett 445: 9–13, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Sheng R, Källberg M, Silkov A, Tun MP, Bhardwaj N, Kurilova S, Hall RA, Honig B, Lu H, Cho W: Genome-wide functional annotation of dual-specificity protein- and lipid-binding modules that regulate protein interactions. Mol Cell 46: 226–237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellegrin S, Mellor H: Actin stress fibres. J Cell Sci 120: 3491–3499, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Ohta Y, Hartwig JH, Stossel TP: FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol 8: 803–814, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Nakaya Y, Sukowati EW, Wu Y, Sheng G: RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol 10: 765–775, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Sakai T, Kriz W: The structural relationship between mesangial cells and basement membrane of the renal glomerulus. Anat Embryol (Berl) 176: 373–386, 1987 [DOI] [PubMed] [Google Scholar]
- 38.Behrends J, Clément S, Pajak B, Pohl V, Maenhaut C, Dumont JE, Schurmans S: Normal thyroid structure and function in rhophilin 2-deficient mice. Mol Cell Biol 25: 2846–2852, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson B, Peyrollier K, Pedersen E, Basse A, Karlsson R, Wang Z, Lefever T, Ochsenbein AM, Schmidt G, Aktories K, Stanley A, Quondamatteo F, Ladwein M, Rottner K, van Hengel J, Brakebusch C: RhoA is dispensable for skin development, but crucial for contraction and directed migration of keratinocytes. Mol Biol Cell 22: 593–605, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.