Abstract
The kidney has a major role in extracellular calcium homeostasis. Multiple genetic linkage and association studies identified three tight junction genes from the kidney—claudin-14, -16, and -19—as critical for calcium imbalance diseases. Despite the compelling biologic evidence that the claudin-14/16/19 proteins form a regulated paracellular pathway for calcium reabsorption, approaches to regulate this transport pathway are largely unavailable, hindering the development of therapies to correct calcium transport abnormalities. Here, we report that treatment with histone deacetylase (HDAC) inhibitors downregulates renal CLDN14 mRNA and dramatically reduces urinary calcium excretion in mice. Furthermore, treatment of mice with HDAC inhibitors stimulated the transcription of renal microRNA-9 (miR-9) and miR-374 genes, which have been shown to repress the expression of claudin-14, the negative regulator of the paracellular pathway. With renal clearance and tubule perfusion techniques, we showed that HDAC inhibitors transiently increase the paracellular cation conductance in the thick ascending limb. Genetic ablation of claudin-14 or the use of a loop diuretic in mice abrogated HDAC inhibitor-induced hypocalciuria. The genetic mutations in the calcium-sensing receptor from patients with autosomal dominant hypocalcemia (ADH) repressed the transcription of miR-9 and miR-374 genes, and treatment with an HDAC inhibitor rescued the phenotypes of cell and animal models of ADH. Furthermore, systemic treatment of mice with antagomiRs against these miRs relieved claudin-14 gene silencing and caused an ADH-like phenotype. Together, our findings provide proof of concept for a novel therapeutic principle on the basis of epigenetic regulation of renal miRs to treat hypercalciuric diseases.
Keywords: calcium, calcium-sensing receptor, kidney stones, mineral metabolism, molecular biology, hypercalciuria
Regulation of extracellular calcium homeostasis resides principally within the kidney. A monogenic renal disorder, autosomal recessive familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC; Online Mendelian Inheritance in Man [OMIM] #248250), is caused by mutations in the claudin genes: claudin-161 and claudin-19.2 The claudin-16 and claudin-19 genes are exclusively expressed in the thick ascending limb (TALH) of the nephron, where a major percentage of filtered divalent cations is reabsorbed paracellularly (30%–35% Ca++ and 50%–60% Mg++).3 A run of in vitro4,5 and in vivo6,7 studies has shown that claudin-16 and claudin-19 confer cation permeability to the tight junction (TJ), which (1) allows paracellular Ca++ and Mg++ reabsorption and (2) generates a lumen-positive diffusion potential that supplies the driving force for Ca++ and Mg++ reabsorption. A genome-wide association study has identified a novel claudin—claudin-14—as a major risk gene of hypercalciuric nephrolithiasis.8 Our recent publications have revealed the pathogenic basis for claudin-14 in the kidney: (1) claudin-14 interacts with claudin-16 and inhibits its permeability9; (2) transgenic overexpression of claudin-14 in the mouse kidney phenocopies FHHNC10; (3) claudin-14 is naturally silenced by two microRNA (miR) molecules—miR-9 and miR-3749; and (4) the expression levels of both miRs are physiologically regulated by the Ca++-sensing receptor (CaSR) by nuclear factor of activated T cells (NFAT) binding and nearby histone deacetylation.10 Such data have raised the tantalizing possibility that epigenetic regulation may represent a novel mechanism to correct physiologic abnormalities, such as hypercalciuria, and prevent kidney stone formation. Here, with a range of in vitro and in vivo approaches, we have shown that histone deacetylase (HDAC) inhibitors reduce renal Ca++ excretion through repression of the claudin-14 gene expression. The promoter of the claudin-14 gene itself was not regulated through histone deacetylation; instead, it was the two claudin-14–targeting miRs—miR-9 and miR-374—that were transcriptionally regulated by HDAC.
The renal tubules are composed of heterogeneous epithelia: the proximal tubule (PT), the loop of Henle, the distal convoluted tubule (DCT), and the collecting duct. The predominant Ca++ reabsorption sites are in the PT, the TALH, and the DCT.11,12 Although the PT and TALH use the paracellular pathway to handle Ca++,11 the DCT relies on a coordinated transcellular Ca++ transport pathway based on the apically localized transient receptor potential channel V member 5 (TRPV5) and the basolaterally localized Na+/Ca++ exchanger 1 (NCX1).12 Because HDAC is a global regulator of gene expression, its renal effects could stem from different tubular segments. Here, with ex vivo tubule perfusion approaches and using animal models defective for TALH Ca++ transport (CLDN14 knockout [KO] or furosemide-treated mice), we have conclusively shown that the TALH is the primary target segment of HDAC inhibition. We show that the GFR remains unaltered, whereas the DCT exhibits secondary adaptive attenuation of cellular Ca++ buffering and basolateral NCX1-driven Ca++ extrusion on the basis of an unaltered luminal Ca++ influx capability mediated by TRPV5.
The CaSR, a member of the G protein-coupled receptors, plays a critical role in extracellular Ca++ homeostasis.13 Its loss-of-function mutations cause two recessive genetic disorders—FHH (OMIM #145980) and neonatal severe hyperparathyroidism (OMIM #239200).14 Its gain-of-function mutations cause a dominant genetic disorder—autosomal dominant hypocalcemia (ADH; OMIM #146200).15,16 A severe form of ADH has been characterized in patients with hypocalcemia, hypomagnesemia, and urinary loss of Ca++ and Mg++, and it is accompanied by additional Bartter-like phenotypes.17,18 Despite the rapid expanding repertoire of known CaSR genetic mutations, it remains elusive how these mutations affect CaSR downstream signaling targets. Here, we studied three common CaSR mutations found in patients with ADH—E127A, F128L, and T151M—with quantitative RNA analyses. We found that the transcription of both miR-9 and miR-374 genes was consistently repressed by CaSR gain-of-function mutations in an overexpressing cell model. HDAC inhibitors accordingly were able to correct the CaSR mutational effect on miRs. In vivo antagomir treatments ablated endogenous miR-9 and miR-374 and induced ADH-like phenotypes in laboratory mice. In animals receiving the calcimimetic compound cinacalcet to mimic ADH conditions, HDAC inhibitors effectively ameliorated cinacalcet-induced hypercalciuria and hypermagnesiuria.
Results
An Epigenetic Program Regulates the miR–CLDN14 Axis in the Kidney
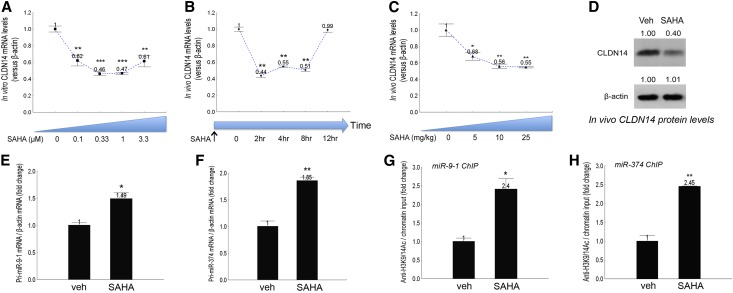
The epigenetic drugs acting to regulate chromatin remodeling and gene transcription have gained considerable recognition in the treatments of various types of cancers,19,20 neurologic disorders,21 HIV infections,22 cardiovascular and inflammatory diseases,23 and others. To systematically screen for the epigenetic effects on CLDN14 gene expression, we treated the primary cultures of mouse TALH cells (freshly isolated from the C57BL/6 mice described before)9,10 in vitro for 16 hours with the DNA methyltransferase inhibitor 5-Aza-2′-deoxycytidine, the histone-lysine methyltransferase inhibitor BIX 01294,24 the histone demethylase inhibitor tranylcypromine,25 and the HDAC inhibitors trichostatin A (TsA) and suberanilohydroxamic acid26 (SAHA; approved by the Food and Drug Administration as Vorinostat). 5-Aza-2′-deoxycytidine, BIX 01294, and tranylcypromine were without significant effects on claudin-14 mRNA levels (Supplemental Figure 1). By contrast, SAHA significantly downregulated CLDN14 mRNA levels by 38% at 0.1 μM (P<0.01, n=3 versus vehicle) (Figure 1A); the downregulation persisted through 0.33–3.3 μM and reached the maximal level of 54% at 0.33 μM (Figure 1A). The CLDN14 expression was more sensitive to TsA treatments. At concentrations as low as 0.01 μM, TsA significantly reduced CLDN14 mRNA levels by 64% (P<0.001, n=3 versus vehicle) (Supplemental Figure 2A); the reduction was significant through 0.033–0.33 μM (Supplemental Figure 2A). To examine the in vivo effects of HDAC inhibitors on CLDN14, wild-type C57BL/6 mice were treated with SAHA or TsA over a range of doses and durations. The in vivo effects of SAHA and TsA were particularly fast. A single dose of SAHA at 25 mg/kg body wt−1 significantly reduced CLDN14 mRNA levels in the kidney by 56% (P<0.01, n=5 versus vehicle) (Figure 1B) at 2 hours; by 4 hours and through to 8 hours, the reduction of CLDN14 expression persisted in the kidney (Figure 1B). By 12 hours, the effects of SAHA completely dissipated (Figure 1B). We then varied the SAHA treatment doses from 5 to 25 mg/kg and found significant downregulation of CLDN14 at a dosage as low as 5 mg/kg (P<0.05, n=5 versus vehicle) and through 10–25 mg/kg at 4 hours (Figure 1C). The CLDN14 protein levels were measured in freshly isolated TALH cells pooled from SAHA or vehicle-treated mouse kidneys (n=5). SAHA treatment of 25 mg/kg body wt−1 profoundly reduced the CLDN14 protein levels by 60% when assayed at 4 hours (Figure 1D). TsA was more effective in suppressing CLDN14 expression. At 1 mg/kg body wt−1 and the 4-hour time point, TsA decreased CLDN14 mRNA levels in the kidney significantly by 71% (P<0.001, n=5 versus vehicle) (Supplemental Figure 2B). There are two mechanisms to explain the CLDN14 gene regulation by HDAC inhibitor—cis regulation on the level of CLDN14 promoter and trans regulation through miRs-based silencing.9,10 Because the claudin-14 KO/Reporter mouse line was generated with an lacZ reporter gene replacing the last exon of the claudin-14 gene (including the entire coding region and the 3′ untranslated region),27 the expression levels of the lacZ gene serve as a faithful measurement for endogenous CLDN14 promoter activity, regardless of any miR-based regulation. The lacZ mRNA levels in the KO mouse kidneys showed no significant change 4 hours after receiving 25 mg/kg body wt−1 SAHA treatment (Supplemental Figure 3). By contrast, the CLDN14 targeting miRs miR-9 and miR-374 were both upregulated by SAHA in the kidney. At the same dosage and time point, SAHA significantly increased the transcriptional levels of the miR-9–1 gene by 1.49-fold (P<0.05, n=5 versus vehicle) (Figure 1E) and the miR-374 gene by 1.85-fold (P<0.01, n=5 versus vehicle) (Figure 1F). To determine if SAHA caused direct histone acetylation on the miR promoters, we analyzed the previously discovered promoter regions in miR-9–1 and miR-374 genes containing the NFAT binding site (AGGAAAAT) located 1–2 kb upstream of the respective miR hairpin sequence,10 where the histone acetylation was vividly regulated through CaSR signaling. Chromatin immunoprecipitation (ChIP) assays revealed that the same promoter region experienced significant increases in histone H3 Lysine-9 (H3K9) and Lysine-14 (H3K14) acetylation levels 4 hours after receiving SAHA at 25 mg/kg body−1—by 2.40-fold for miR-9–1 (P<0.05, n=5) (Figure 1G) and 2.45-fold for miR-374 (P<0.01, n=5) (Figure 1H). The NFAT binding to the miR-9–1 or miR-374 promoter was not affected by SAHA (Supplemental Figure 4). Together, these data have established a molecular basis for SAHA regulation of CLDN14 in the kidney.
Figure 1.
HDAC inhibitor regulation of CLDN14 and miR gene expression in the kidney. (A) The dose response of HDAC inhibitor SAHA effects on CLDN14 mRNA levels in primary cultures of mouse TALH. (B) The time response of SAHA effects on CLDN14 mRNA levels in mouse kidneys. (C) The dose response of SAHA effects on CLDN14 mRNA levels in mouse kidneys. (D) CLDN14 proteins levels in SAHA- or vehicle-treated freshly isolated mouse TALH tubules. (E and F) The effects of SAHA on miR transcription measured for (E) pri-miR-9–1 and (F) pri-miR-374 levels. (G and H) The effects of SAHA on histone acetylation levels over the miR gene promoters measured for (G) miR-9–1 and (H) miR-374 genes with ChIP. Veh, vehicle. *P<0.05; **P<0.01; ***P<0.001.
The Short-Term Effects of HDAC Inhibitors on Urinary Calcium and Magnesium Excretion
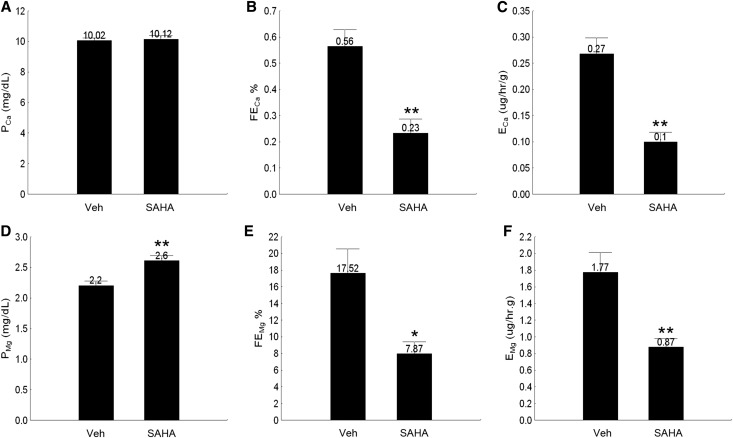
Because CLDN14 is a negative regulator of CLDN169 and Ca++ reabsorption10 in the kidney, curbing CLDN14 expression by SAHA may provide a novel route to lower urinary Ca++ excretion. To test this hypothesis, we treated wild-type C57BL/6 mice with SAHA at 5 mg/kg body wt−1 and traced urinary Ca++ and Mg++ levels (as ratios to creatinine) from 4 to 12 hours in spot urine collections. Consistent with the downregulation of CLDN14 gene expression at 4 hours, the urinary excretion of Ca++ and Mg++ was significantly reduced by 47% and 40%, respectively (P<0.01, n=5 versus vehicle) (Supplemental Figure 5). By 8 hours, hypocalciuria was still pronounced in SAHA-treated animals (Supplemental Figure 5A), but urinary magnesium excretion recovered to the vehicle level (Supplemental Figure 5B). The SAHA effects on urinary Ca++ and Mg++ levels were completely lost after 12 hours (Supplemental Figure 5). To capture time-sensitive changes in renal tubular transport function, we performed 1-hour renal clearance measurements on age- and sex-matched wild-type mice infused with FITC-inulin (Concise Methods) starting at 4 hours after a single dose of 5 mg/kg body wt−1 SAHA. The plasma Ca++ level (PCa) (Figure 2A) was not significantly altered by SAHA at 4 hours, despite pronounced reduction of both fractional Ca++ excretion level (FECa) (Figure 2B, Table 1) and absolute Ca++ excretion level (ECa) (Figure 2C, Table 1) in SAHA-treated animals compared with vehicle-treated animals (P<0.01, n=6). The plasma Mg++ level (PMg) (Figure 2D) was evidently higher in the SAHA group than the vehicle group (SAHA: 2.60±0.09 mg/dl versus vehicle: 2.20±0.08 mg/dl; P<0.01, n=6) (Table 1) owing to significant decreases in fractional and absolute Mg++ excretion levels (FEMg and EMg, respectively) (Figure 2, E and F) induced by SAHA. The GFR based on FITC-inulin clearance (Table 1) was not significantly different between SAHA- and vehicle-treated animals, and there was no significant difference in urinary volume (UV) between the two groups (Table 1). Together, these data indicate a renal tubular-specific effect of HDAC inhibitor on Ca++ and Mg++ excretion, which is independent of glomerular hemodynamics.
Figure 2.
Renal clearance analyses of plasma and urine electrolyte levels in wild-type mice treated with SAHA. (A–C) The effects of SAHA on (A) plasma Ca++ levels, (B) fractional excretion rates, and (C) absolute excretion rates of Ca++ in 1-hour urine collections. (D and E) The effects of SAHA on (D) plasma Mg++ levels, (E) fractional excretion rates, and (F) absolute excretion rates of Mg++ in 1-hour urine collections. Veh, vehicle. *P<0.05; **P<0.01.
Table 1.
Plasma and urine electrolyte levels in wild-type animals receiving SAHA treatments measured by renal clearance
| Group | Vehicle | SAHA | Significance |
|---|---|---|---|
| Weight, g | 21.73±0.64 | 21.89±1.05 | NS |
| UV, μl/h⋅g | 6.14±0.84 | 5.63±0.92 | NS |
| GFR, ml/h⋅g | 0.51±0.09 | 0.47±0.07 | NS |
| PCa, mg/dl | 10.02±0.22 | 10.12±0.28 | NS |
| PMg, mg/dl | 2.20±0.08 | 2.60±0.09 | P<0.01 |
| FECa, % | 0.56±0.07 | 0.23±0.06 | P<0.01 |
| ECa, μg/h⋅g | 0.27±0.03 | 0.10±0.02 | P<0.01 |
| FEMg, % | 17.52±3.02 | 7.87±1.53 | P<0.05 |
| EMg, μg/h⋅g | 1.77±0.24 | 0.87±0.11 | P<0.01 |
Values are expressed as means±SEMs; n=6 male animals ages 10–12 weeks.
The Long-Term Effects of HDAC Inhibitors on Urinary Calcium and Magnesium Excretion
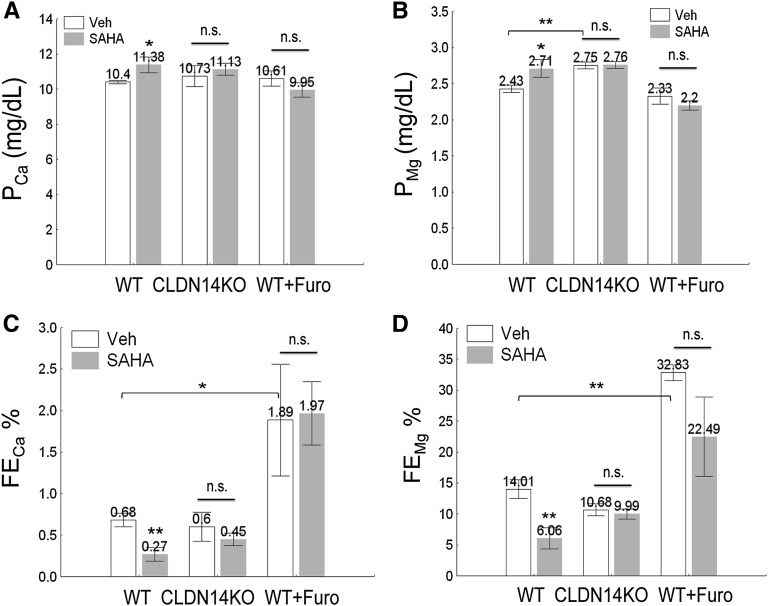
To reveal the renal transport function over a chronic phase, we performed 24-hour urinalysis on age (8–10 weeks old) and sex (male) matched wild-type mice receiving SAHA at 10 mg/kg body wt−1 per day. The PCa and PMg levels in SAHA-treated animals were both significantly higher than in the vehicle group (PCa: SAHA group: 11.38±0.44 mg/dl versus control: 10.40±0.10 mg/dl [Figure 3A]; PMg: SAHA group: 2.71±0.12 mg/dl versus control: 2.43±0.05 mg/dl [Figure 3B]; P<0.05, n=9) (Table 2), compatible with the observation of significantly reduced FECa (P<0.01, n=9) (Figure 3C, Table 2) and FEMg (P<0.01, n=9) (Figure 3D, Table 2) in the SAHA group. The ECa and EMg levels were also significantly lowered by SAHA (P<0.05, n=9) (Table 2), despite unchanged GFR and UV levels (Table 2). The plasma levels of Na+, K+, and phosphate were not affected by SAHA, and there was no significant change in urinary excretion of Na+, K+, and phosphate (Supplemental Figure 6). TsA at 1 mg/kg body wt−1 per day exerted similar effects on renal Ca++ and Mg++ metabolism. We then asked whether SAHA affected other Ca++-related hormonal or genetic pathways in the kidney. Because chronic SAHA treatment induced mild hypercalcemia, the circulating parathyroid hormone (PTH), 1,25-(OH)2-vitD3, and fibroblast growth factor 23 (FGF23) levels were measured to determine if hypercalcemia originated from changes in these hormonal systems. None of these hormones changed during SAHA treatments (Supplemental Figure 7), and there was no significant change in the expression levels of other major Ca++/Mg++ transporters or regulators from the kidney—CLDN16, CLDN19, TRPV5, TRPM6, NKCC2, ROMK, TSC, Klotho, and VDR (Supplemental Figure 8). Together, these results have revealed a Ca++/Mg++-specific effect for the HDAC inhibitor in the kidney, which is independent of systemic endocrine factors.
Figure 3.
Metabolic analyses of plasma and urine electrolyte levels in wild-type (WT) and CLDN14 KO mice treated with SAHA and/or furosemide. (A and B) The effects of SAHA on plasma levels of (A) Ca++ and (B) Mg++ in WT versus CLDN14 KO mice or mice pretreated with furosemide. (C and D) The effects of SAHA on fractional excretion rates for (C) Ca++ and (D) Mg++ in WT versus CLDN14 KO mice or mice pretreated with furosemide in 24-hour urine collections. Furo, furosemide; n.s., not significant; veh, vehicle. *P<0.05; **P<0.01.
Table 2.
The 24-hour plasma and urine electrolyte levels in wild-type and claudin-14 KO animals under SAHA and furosemide treatments
| Group | WT+Veh | WT+SAHA | CLDN14KO+Veh | CLDN14KO+SAHA | WT+Veh+Furo | WT+SAHA+Furo |
|---|---|---|---|---|---|---|
| Weight, g | 19.15±0.42 | 19.41±0.56 | 18.64±1.93 | 19.18±0.56 | 19.17±0.28 | 18.30±0.43 |
| UV, μl/24 h⋅g | 44.95±3.96 | 37.11±4.54 | 39.83±5.78 | 44.03±2.07 | 137.11±15.95a | 110.59±9.03 |
| GFR, ml/24 h⋅g | 2.82±0.36 | 2.09±0.51 | 1.97±0.32 | 2.42±0.29 | 1.77±0.44 | 1.69±0.13 |
| PCa, mg/dl | 10.40±0.10 | 11.38±0.44b | 10.73±0.59 | 11.13±0.34 | 10.61±0.43 | 9.95±0.41 |
| PMg, mg/dl | 2.43±0.05 | 2.71±0.12b | 2.75±0.05a | 2.76±0.05 | 2.33±0.11 | 2.20±0.06 |
| FECa, % | 0.68±0.08 | 0.27±0.08c | 0.60±0.18 | 0.45±0.07 | 1.89±0.67d | 1.97±0.38 |
| ECa, μg/24 h⋅g | 1.96±0.33 | 0.72±0.26b | 1.11±0.20 | 1.20±0.24 | 2.84±0.78 | 3.37±0.75 |
| FEMg, % | 14.01±1.53 | 6.06±1.71c | 10.68±1.00 | 9.90±0.83 | 32.83±1.30a | 22.50±6.40 |
| EMg, μg/24 h⋅g | 9.41±1.18 | 3.16±0.80c | 5.75±1.01 | 6.50±0.55 | 13.24±3.13 | 8.34±2.15 |
Values are expressed as means±SEMs; n=5–9 male animals ages 8–10 weeks. WT, wild type; Veh, vehicle; Furo, furosemide.
P<0.01 versus the WT group with Veh treatment.
P<0.05 versus Veh treatment in the same group.
P<0.01 versus Veh treatment in the same group.
P<0.05 versus the WT group with Veh treatment.
The Genetic and Cellular Origins of HDAC Inhibitor-Induced Hypocalciuria in the Kidney
To elucidate the genetic origin of SAHA effects, the CLDN14 KO mice (previously backcrossed to C57BL/6 background)9,10 were treated with the same SAHA regimen as described elsewhere for wild-type animals. In contrast to the wild-type mice, the CLDN14 KO animals were refractory toward SAHA-induced hypercalcemia and hypermagnesemia (Figure 3, A and B, Table 2), mindful that the CLDN14 KO was already hypermagnesemic (Figure 3B) because of reduced baseline levels of urinary Mg++ excretion.10 The urinary Ca++ and Mg++ changes were both abolished in CLDN14 KO (Figure 3, C and D, Table 2), suggesting that SAHA primarily acted on the kidney where CLDN14 was exclusively localized. To elucidate the tubular origin of SAHA effects, we pretreated wild-type animals with furosemide at 40 mg/kg body wt−1 per day. Furosemide, by inhibiting the NKCC2 transporter in the TALH, induces natriuresis and diuresis, which in turn, abolished the transepithelial voltage for paracellular Mg++ and Ca++ reabsorption.3,28–30 The furosemide treatment significantly increased UV, FECa, and FEMg (Figure 3, C and D, Table 2). In addition to the elevated baseline levels, furosemide abolished the SAHA effects on plasma (Figure 3, A and B) and urine (Figure 3, C and D) Ca++ and Mg++ levels, identifying the TALH as the major nephron segment for SAHA function. Together, these results indicate that HDAC inhibitors act on CLDN14 to regulate Ca++ and Mg++ reabsorption in the TALH of the kidney.
The Tubular Differences in HDAC Inhibitor Effects
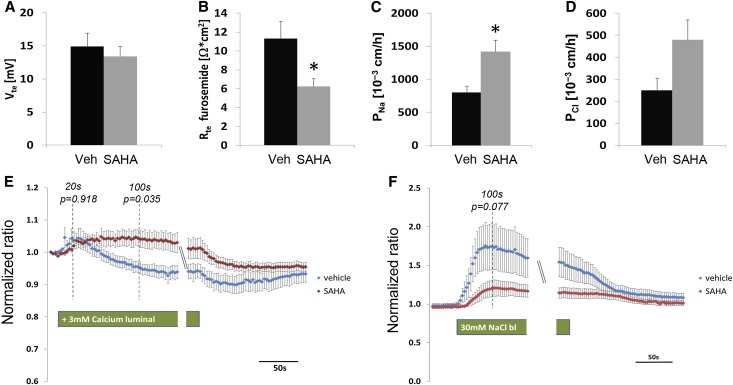
To reveal the tubular specific contribution to observed HDAC inhibitor effects, we studied the Ca++ transport pathways in freshly isolated ex vivo perfused TALH and DCTs from SAHA-treated animals. At 4 hours after a single dose of 5 mg/kg body wt−1 SAHA or vehicle treatment, age- and sex-matched mice were euthanized for immediate kidney harvest followed by manual tubule isolation. On average, more than five tubules were perfused for each animal. Five to six animals were studied in each treatment group. The tubule perfusion protocol for TALH had been described before by us.6 The TALHs from SAHA- or vehicle-treated animals showed similar lumen-positive potential (Vte) (Figure 4A) that was fully inhabitable by luminal furosemide (0.05 mM), suggesting that the transcellular pathway mediated through NKCC2 was not affected by SAHA. The transepithelial resistance (Rte) in the presence of furosemide was significantly reduced by SAHA (P<0.05, n=5–6) (Figure 4B), suggesting its role in stimulating paracellular conductance. To determine the ion-specific permeabilities of the TJ, changes in the junctional diffusion potential produced by a NaCl gradient of 145 versus 30 mM were recorded in the absence of active transport (inhibited by furosemide). The Goldman equation allowed calculation of paracellular Na+ (PNa) and Cl− (PCl) permeabilities from the diffusion potential. The PNa in the SAHA group was approximately 2-fold higher than that in the vehicle group (P<0.05; n=5–6) (Figure 4C); the PCl in the SAHA group showed increasing trend but did not reach significance (Figure 4D). Together, these results are consistent with our previous recording that CLDN14 primarily affected CLDN16 cation permeability through protein interaction.9 The TRPV5-mediated transcellular pathway in the DCT is another major component of renal Ca++ transport machinery. Because endogenous TRPV5 or TRPM6 conductance has never been captured from native DCT epithelia, we adopted intracellular calcium imaging as a tool to report transcellular calcium transport levels for perfused DCTs (Concise Methods). A ratiometric fluorescence dye (Fura-2) was used to report intracellular calcium levels (Supplemental Figure 9) independent of dye concentration, imaging parameters, or cell volume. The normalized ratio of fluorescence emission at 340 versus 380 nm for perfused DCTs from SAHA- or vehicle-treated animal groups is shown in Figure 4E. A high luminal Ca++ solution (3 mM) was used to elicit changes in intracellular Ca++ levels. The intracellular Ca++ changes measured at 20 seconds after high Ca++ challenge were regarded as TRPV5-mediated effect. Shown in Figure 4E, the initial peak in intracellular Ca++ levels was similar in magnitude between SAHA and vehicle groups (P=0.92, n=5–6), which can be fully inhibited by ruthenium red (a TRPV5 inhibitor) at 1 μM, suggesting that TRPV5 activity was not affected by SAHA. However, the intracellular Ca++ level in SAHA group became different from that in the vehicle group after 50 seconds, and the difference reached significance at 100 seconds (Figure 4E) (P=0.04, n=5–6). Unlike the vehicle-treated animals, the DCT cells from the SAHA group were not able to remove the intracellular Ca++ surge induced by the luminal Ca++ load (Figure 4E), suggesting defects in the basolateral Ca++ efflux. The major Ca++ efflux mechanism in the DCT is through the NCX1, which can be inhibited by low extracellular Na+ levels. When perfused DCTs were bathed with low Na+ solution (30 mM), intracellular Ca++ levels exhibited a transient increase caused by inhibition of Ca++ efflux (Figure 4F). The intracellular Ca++ peak (measured at 100 seconds) was clearly lower in SAHA-treated animals than in vehicle treatment animals (Figure 4F), suggesting that the NCX1 activity was already blunted by SAHA. Because low extracellular Na+ caused unexpected fluctuation in intracellular Ca++ (Figure 4F), the statistical significance value (P) for comparing SAHA and vehicle treatments was 0.08 (n=5–6). Using a more specific NCX1 inhibitor, such as KB-R7943 mesylate,31 although not within the scope of this study, may clarify its role in mediating SAHA effects. Together, these results indicate that SAHA reduces the transcellular transport capacity for Ca++ in the DCT that likely results from reduced luminal Ca++ delivery because of increased reabsorption in the TALH.
Figure 4.
Characterization of major Ca++ transport pathways in perfused TALHs and DCTs. (A) The transepithelial voltage Vte, (B) transepithelial resistance Rte, (C) paracellular Na+ permeability PNa, and (D) paracellular Cl− permeability PCl values in ex vivo-perfused TALH tubules from wild-type mice treated with SAHA or vehicle. (E and F) Normalized Fura-2 fluorescence ratio in DCTs from wild-type mice treated with SAHA or vehicle perfused with (E) luminal high Ca++ buffer (+3 mM) or (F) basolateral low Na+ buffer (30 mM). Veh, vehicle. *P<0.05.
HDAC Inhibitors Correct CaSR Mutational Effects in ADH Disease
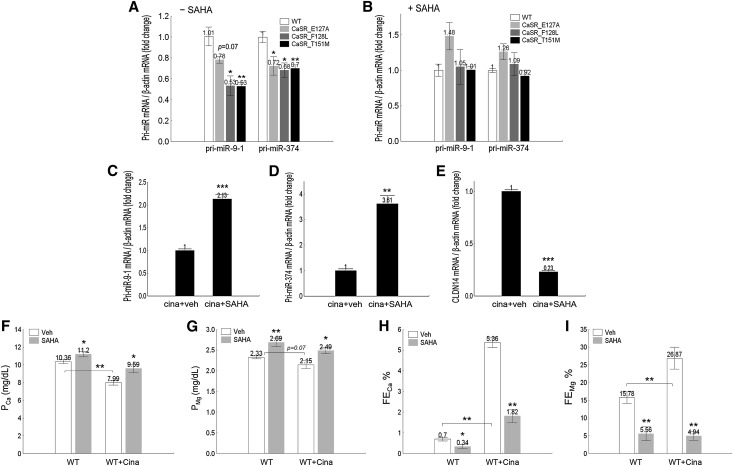
ADH is caused by activating mutations in the CaSR gene. Knowing that miRs are an important second messenger of CaSR signaling,9 we asked whether CaSR mutations deregulated miR abundance as a potential causal mechanism and whether HDAC inhibitors corrected the CaSR mutational effects as a novel therapeutic approach for ADH disease. We generated three common ADH mutations in the human CaSR gene (E127A, F128L, and T151M previously described by Pollak et al.15 and Pearce et al.16), transfected the wild-type CaSR gene and its mutants into a well established cell model for studying CaSR signaling (HEK293 cells that expressed no endogenous CaSR),17 and quantified the transcriptional levels of four miR genes—miR-9–1, miR-9–2, miR-9–3 (all three loci transcribing mature miR-9 hairpin sequence), and miR-374. Among them, the miR-9–1 and miR-374 genes were transcriptionally suppressed by the CaSR mutants. The cellular abundance of the primary miR-9-1 (pri-miR-9-1) transcript was significantly reduced to 53% of the wild-type level by the F128L and T151M mutations (P<0.05, n=4) (Figure 5A); the E127A mutation elicited a decreasing trend in miR-9–1 transcription (P=0.07, n=4) (Figure 5A). All three mutations significantly reduced the pri-miR-374 transcript levels by 30% compared with wild-type CaSR (P<0.05, n=4) (Figure 5A). Pretreating the HEK293 cells with SAHA at 0.33 μM completely abolished the CaSR mutational effect on miR gene transcription (Figure 5B). To mimic the ADH disease in vivo, we pretreated age- (8–10 weeks old) and sex-matched (male) wild-type mice (strain C57BL/6) with the calcimimetic compound cinacalcet at 30 mg/kg body wt−1 per day and examined the SAHA effect on miR gene expression (at 4 hours after receiving 25-mg/kg body wt−1 SAHA treatment). The transcript levels of pri-miR-9–1 and pri-miR-374 in isolated TALH tubules were both profoundly increased by SAHA to 2.13-fold (P<0.001, n=5) (Figure 5C) and 3.61-fold (P<0.01, n=5) (Figure 5D) of the vehicle level, respectively, which led to a reciprocal decrease in renal CLDN14 mRNA levels (P<0.001, n=5) (Figure 5E). Notably, the SAHA effect size on miRNA and CLDN14 was much higher in cinacalcet pretreated animals (Figure 5, C–E) than not treated animals (Figure 1, C, E, and F). Cinacalcet significantly reduced the baseline level of PCa (to 7.99±0.27 mg/dl; P<0.01, n=7) (Figure 5F, Table 3) compared with that in animals not treated with cinacalcet (PCa: 10.36±0.22 mg/dl); baseline PMg showed a clear decreasing trend (P=0.07, n=7) (Figure 5G, Table 3), consistent with our previous observation.10 SAHA reversed the cinacalcet effect by increasing PCa and PMg (Figure 5, F and G) in cinacalcet pretreated animals. PMg recovered to above the control level with no cinacalcet treatment, but PCa was still below the normal range. The cinacalcet-induced hypercalciuria (FECa) (Figure 5H, Table 3) and hypermagnesiuria (FEMg) (Figure 5I, Table 3) were both effectively reversed by SAHA. FECa was 1.82±0.31% in animals receiving both cinacalcet and SAHA (only 2.60-fold higher than animals receiving no treatment), whereas cinacalcet treatment alone increased FECa by 7.66-fold (Figure 5H, Table 3). The SAHA effect on FEMg in cinacalcet pretreated animals was even more dramatic. Despite a 1.70-fold increase of baseline FEMg by cinacalcet (Figure 5I), animals receiving both cinacalcet and SAHA had even lower FEMg levels (4.94±1.21%) (Figure 5I, Table 3) compared with those receiving no treatment at all (15.78±1.64%; P<0.01, n=7) (Figure 5I, Table 3). Together, these results have revealed a novel pathogenic mechanism for ADH disease based on miR deregulation and suggested a novel therapeutic approach using an HDAC inhibitor to reverse miR abnormalities.
Figure 5.
Activation of CaSR effects on miR and CLDN14 gene expressions and plasma and urine electrolyte levels in cell and animal models. (A and B) Pri-miR transcript levels in HEK293 cells transfected with CaSR mutations found from patients with ADH in the (A) absence or (B) presence of SAHA. (C–E) The effects of SAHA on (C) pri-miR-9–1, (D) pri-miR-374, or (E) CLDN14 transcript levels in the TALH of the mouse kidneys pretreated with cinacalcet. (F and G) The effects of SAHA on plasma levels of (F) Ca++ and (G) Mg++ in wild-type (WT) mice pretreated with cinacalcet. (H and I) The effects of SAHA on fractional excretion rates for (H) Ca++ and (I) Mg++ in WT mice pretreated with cinacalcet in 24-hour urine collections. Cina, cinacalcet; veh, vehicle. *P<0.05; **P<0.01; ***P<0.001.
Table 3.
The 24-hour plasma and urine electrolyte levels in wild-type animals under SAHA and cinacalcet treatments
| Group | WT+Veh | WT+SAHA | WT+Veh+Cina | WT+SAHA+Cina |
|---|---|---|---|---|
| Weight, g | 20.23±0.23 | 19.89±0.41 | 20.90±0.71 | 20.48±0.33 |
| UV, μl/24 h⋅g | 41.28±3.40 | 41.51±5.03 | 40.93±6.60 | 33.84±4.11 |
| GFR, ml/24 h⋅g | 2.53±0.16 | 2.23±0.44 | 2.56±0.51 | 2.78±0.40 |
| PCa, mg/dl | 10.36±0.22 | 11.20±0.29a | 7.99±0.27b | 9.59±0.47a |
| PMg, mg/dl | 2.33±0.04 | 2.69±0.10c | 2.15±0.10d | 2.49±0.07a |
| FECa, % | 0.70±0.09 | 0.34±0.10a | 5.36±0.23b | 1.82±0.31c |
| ECa, μg/24 h⋅g | 1.79±0.21 | 0.95±0.29a | 10.66±1.86b | 4.52±0.63a |
| FEMg, % | 15.78±1.64 | 5.56±1.96c | 26.87±3.10b | 4.94±1.21c |
| EMg, μg/24 h⋅g | 9.50±1.27 | 3.31±1.28c | 14.89±3.43 | 3.01±0.72c |
Values are expressed as means±SEMs; n=7 male animals ages 8–10 weeks. WT, wild type; Veh, vehicle; Cina, cinacalcet.
P<0.05 versus Veh treatment in the same group.
P<0.01 versus the WT group with Veh treatment.
P<0.05 versus Veh treatment in the same group.
P=0.07 versus the WT group with Veh treatment.
miR Manipulation Provides Therapeutic Principles for HDAC Inhibitors
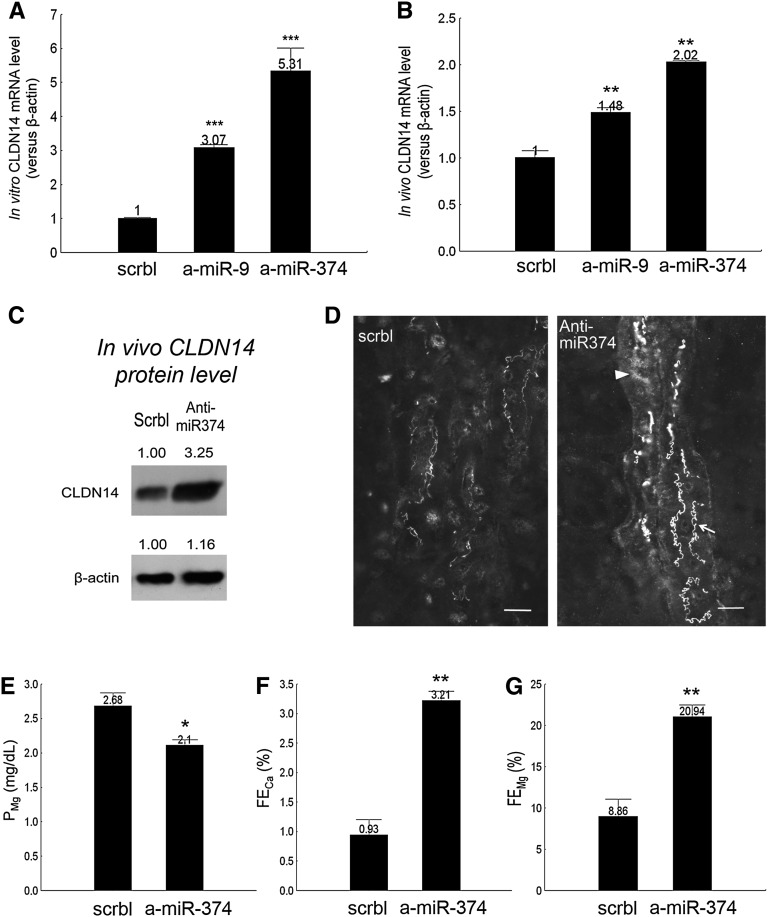
Because the SAHA effect and the ADH disease mechanism intersected at the miRs, we asked whether manipulation of miRs per se was sufficient to induce changes in renal calcium metabolism. Since our previous discovery of the CLDN14 targeting miRs miR-9 and miR-374,9 attempts to knockdown miR expression have been made with antagomirs32 based on the locked nucleic acids (LNAs). We have screened for LNA sequences ranging from 8 to 23 nucleotides long33–35 using cultured TALH cells and found the most effective sequences for miR-9 and miR-374 (Concise Methods). In vitro in the primary TALH cultures, transfection with anti–miR-9 or anti–miR-374 but not scrambled antagomir induced significant increases in CLDN14 gene expression by 3.07-fold and 5.31-fold, respectively (P<0.001, n=4) (Figure 6A). The in vivo knockdown seemed to be much more difficult. Although several studies have reported effective ablation of glomerular miRs from the mesangial cells36 or the podocytes37 with systemic injection of antagomirs, we found that systemic injection of anti–miR-9 or anti–miR-374 was not able to knockdown miR in renal tubules throughout the dosage range of 1–25 mg/kg body wt−1 in mice. Instead, when the LNA antagomirs were coinjected with the in vivo jetPEI Delivery Reagent (based on the polyethylenimine cationic polymers), a single dose of anti–miR-9 or anti–miR-374 at 2.5 mg/kg body wt−1 significantly increased the CLDN14 mRNA level in the kidney 24 hours after injection by 1.48-fold and 2.02-fold, respectively, relative to the scrambled antagomir (P<0.01, n=5) (Figure 6B). Because anti–miR-374 was more effective than anti–miR-9 and miR-9 had known tumorigenic implication through targeting E-cadherin,38 we, thus, focused on miR-374 to study its role in renal tubular functions. We gave wild-type C57BL/6 mice anti–miR-374 injections at 2.5 mg/kg body wt−1 per day and measured CLDN14 protein abundance, localization, and plasma and urinary electrolytes levels. In freshly isolated TALH cells pooled from antagomir-treated mouse kidneys (n=5), the CLDN14 protein level was 3.25-fold higher with anti–miR-374 than scrambled antagomir treatment (Figure 6C). The change in CLDN14 levels at the TJ was particularly striking. The thin interdigitated TJ lines faintly stained for CLDN14 in control mouse kidneys (Figure 6D) were profoundly strengthened in anti–miR-374–treated mouse kidneys (Figure 6D, arrow), with occasional subapical staining spotted in some TALH cells (Figure 6D, arrowhead). To reveal the functional effects of antagomir treatment in the kidney, we performed 24-hour urinalysis on age- (7–8 weeks old) and sex-matched (male) wild-type mice receiving anti–miR-374 injections at 2.5 mg/kg body wt−1 per day. The PCa was not changed by anti–miR-374, but the PMg was significantly lower in the anti–miR-374 group than the scrambled (P<0.05, n=6) (Figure 6E, Table 4). The fractional excretion rates for Ca++ and Mg++ were both significantly increased by anti–miR-374 in 24-hour urine collections (FECa: 3.45-fold; FEMg: 2.36-fold versus scrambled [Figure 6, F and G]; P<0.01, n=6) (Table 4). Other renal parameters, such as GFR and UV, were not changed by anti–miR-374 (Table 4), indicating a direct tubular effect. Together, the anti–miR-374 knockdown animals phenocopied the CLDN14 transgenic overexpression animals described by us before,10 establishing a therapeutic principle for using miRs to manipulate CLDN14 expression and urinary calcium excretion.
Figure 6.
The renal effects of antagomir treatments. (A) CLDN14 mRNA levels in primary TALH cultures transfected with antagomirs against miR-9 or miR-374. (B) CLDN14 mRNA levels in mouse kidneys treated with antagomirs against miR-9 or miR-374. (C) CLDN14 protein levels in freshly isolated TALHs from mouse kidneys treated with anti–miR-374 versus scrambled antagomir. (D) Mouse kidney sagittal sections immunostained for CLDN14 from anti–miR-374 versus scrambled treatments. Scale bar, 10 μm. (E) Plasma Mg++ and fractional excretion of (F) Ca++ and (G) Mg++ levels in anti–miR-374 versus scrambled animal groups. a-miR, anti-miR; scrbl, scrambled. *P<0.05; **P<0.01; ***P<0.001.
Table 4.
Plasma and urine electrolyte levels in antagomir-injected animals
| Group | Scrambled | Anti–miR-374 | Significance |
|---|---|---|---|
| Weight, g | 17.75±0.42 | 17.84±0.72 | NS |
| UV, μl/24 h⋅g | 34.81±3.22 | 41.65±7.67 | NS |
| GFR, ml/24 h⋅g | 3.30±0.56 | 2.75±0.47 | NS |
| PCa, mg/dl | 10.43±0.23 | 10.50±0.22 | NS |
| PMg, mg/dl | 2.68±0.20 | 2.10±0.09 | P<0.05 |
| FECa, % | 0.93±0.27 | 3.21±0.16 | P<0.01 |
| ECa, μg/24 h⋅g | 2.67±0.36 | 9.11±1.38 | P<0.01 |
| FEMg, % | 8.86±2.23 | 20.94±1.53 | P<0.01 |
| EMg, μg/24 h⋅g | 7.61±1.98 | 11.92±2.25 | NS |
Values are expressed as means±SEMs; n=6 male animals ages 7–8 weeks.
Discussion
This work has identified a novel approach based on epigenetic drugs and miR manipulation to reduce urinary calcium excretion. For many years, the only available therapy for hypercalciuria was through the use of diuretics, such as thiazide and amiloride. Although it is still debated, the physiologic mechanism of diuretic-induced hypocalciuria is considered secondary to extracellular volume depletion and reduced GFR, which increase paracellular Ca++ reabsorption in the PTs.39 Since the discovery of CLDN16 and CLDN19 from the FHHNC disease, the possibility of directly manipulating loop Ca++ reabsorption has emerged. The CLDN16- and CLDN19-deficient animals that we have generated using small interfering RNAs (siRNAs)6,7 have proven a principle to induce hypercalciuria in humans based on siRNA treatments. Nevertheless, because there was no gain-of-function mutation ever found for CLDN16 or CLDN19, therapies relying on this pathway to reduce Ca++ excretion have been difficult until our recent discovery of the inhibitory subunit CLDN14.9,10 Because miR-9 and miR-374 directly target CLDN14 in the kidney, approaches acting to regulate these miRs are specific to the kidney and avoid the unwanted effects from manipulating CLDN14 itself using approaches, such as siRNA, that can also affect the inner ear, where CLDN14 is important for the generation of endocochlear potential.40 The HDAC inhibitors acting farther upstream of miRs will be particularly important. Although generally considered as a global regulator, pharmacologic inhibition of HDACs alters the expression of only 2% of all transcribed genes.41 Our animal studies have strengthened this view that the HDAC inhibitors only affect the miR–CLDN14 pathway in the kidney, with no promiscuous effect on other Ca++/Mg++ and Na+/K+ transporters in the kidney (Supplemental Figure 8). Because HDAC inhibitor does not alter GFR, the glomerulotubular balance is well maintained. Functional recordings from perfused renal tubules further clarified the HDAC inhibitor effects by showing that increased Ca++ reabsorption came from the TALH but not the DCT.
The antitumor ability of SAHA was first shown in several in vivo animal models of cancer using a dosage range of 50–100 mg/kg body wt−1 (equivalent to 150–300 mg/m2 surface area [SA]−1), including a xenograft mouse model of human prostate cancer42 and a transgenic mouse model of leukemia.43 Initial toxicology studies in rodents showed that the maximal administered dose of 3000 mg/m2 produced no organ toxicity.44 Based on these preclinical data, phase I and II trials have been carried out to test SAHA as a novel treatment for patients with refractory cutaneous T cell lymphoma at a dosage of 300 mg/m2.44,45 The common adverse effects (AEs) include fatigue, diarrhea, nausea, thrombocytopenia, and hyperglycemia.46 The AE frequency increased with the dosage, but the pattern remained the same. No abnormality in serum or urine Ca++ level has been reported for SAHA, although hypocalcemia was found in patients receiving other types of HDAC inhibitors, such as Entinostat47 or Romidepsin.48 In this study, we report using an extremely low dose of SAHA (at 15 mg/m2) to promote positive Ca++ homeostasis. Because of the low dosage, AEs can be significantly reduced. The physiologic outcome of SAHA stems from its direct and specific effect on augmenting the TJ permeability in the TALH. From a cell biology stand, the SAHA effect proves that TJ function can be modulated transiently and reversibly with pharmacologic reagents, supporting a novel view of a drugable TJ. SAHA is a paninhibitor for class I HDAC, which includes HDAC1, -2, -3, -6, and -8.21 Additional refinement of this class of inhibitors with improved substrate specificity will allow synthesis of drugs against each specific HDAC member and significantly improve safety. It is not know which specific HDAC member mediates miR transcriptional regulation and promotes Ca++ reabsorption in the kidney.
Since the discovery of CaSR, studies of its downstream signaling pathways have attracted immense research interest. The classic CaSR signaling pathway is mediated through the binding to the G proteins, which stimulate the production of diacylglycerol and inositol 1,4,5-trisphosphate and increase the intracellular Ca++ levels.49 These second messengers (inositol 1,4,5-trisphosphate and intracellular Ca++) have been broadly used to assess the genetic mutations of CaSR in Xenopus oocytes and HEK293 cells.15–18 Our study has revealed a novel second messenger based on miRs that is particularly useful for assessing the activating mutations of CaSR found in ADH disease. The common ADH mutations invariably repress the transcription of miR-9 and miR-374 genes, which can be relieved by HDAC inhibitors. Ablation of endogenous miR-374 with antagomirs generated ADH-like phenotypes in mice, emphasizing the pathogenic role of miR in ADH development. The HDAC inhibitors were particularly effective to ameliorate hypomagnesemia, hypercalciuria, and hypermagnesiuria in a pharmacologic ADH animal model. Hypocalcemia was only partially corrected, largely owing to the continuous suppression of PTH secretion in these animals. It is not known whether HDAC inhibitors act on the parathyroid glands. An elegant in vitro study has shown direct binding of type 1 HDAC to the PTH gene promoter and shown that HDAC inhibitors stimulate the PTH gene transcription in several cell models.50 In vivo, the HDAC inhibitor treatment had no overt effect on circulating PTH levels (Supplemental Figure 7), despite mild hypercalcemia.
Concise Methods
Reagents, Abs, Cell Lines, and Animals
The following Abs were used in this study: rabbit polyclonal anti-Tamm–Horsfall protein (Biomedical Technologies), rabbit polyclonal anti-CLDN14 (against RAPSVTSAAHSGYRLNDYV), rabbit polyclonal anti-CLDN16 (against SYSAPRTETAKMYAVDTRV), and rabbit polyclonal anti-CLDN19 (against NSIPQPYRSGPSTAAREYV). Human HEK293 cells (Joan Brugge) were cultured in DMEM (Invitrogen) supplemented with 10% FBS, penicillin/streptomycin, and 1 mM sodium pyruvate. All mice were bred and maintained according to the Washington University animal research requirements, and all procedures were approved by the Institutional Animal Research and Care Committee. Wild-type C57BL/6 mice were from Charles River Laboratory. The CLDN14+/lacZ reporter/KO mice were from Tamar Ben-Yosef and backcrossed to the C57BL/6 background for seven generations.
Pharmacologic Manipulation in Experimental Animals
SAHA and TsA were dissolved in 5% (wt/vol) ethanol/0.9% saline solution and fed to mice with gavage syringe. Furosemide was dissolved in 5% (wt/vol) DMSO/0.9% saline solution and intraperitoneally (ip) injected to animals. All animals had free access to water and were housed under a 12-hour light cycle. Blood samples were taken by cardiac puncture rapidly after initiation of terminal anesthesia and centrifuged at 4°C for 10 minutes. Kidney were dissected out and immediately frozen at −80°C.
Animal Metabolic Studies
Mice were housed individually in metabolic cages (Harvard Apparatus) with free access to water and food for 24 hours. Urine was collected under mineral oil. The plasma and urine Ca++ and Mg++ levels were determined with an atomic flame absorption spectrophotometer (PerkinElmer). The creatinine levels were measured with an enzymatic method that was independent of plasma chromogens.51 The fractional excretion of electrolytes was calculated using the following equation: FEion=volume (V)×urineion (Uion)/(GFR×Pion), where GFR was calculated according to the clearance rate of creatinine (GFR=V×Ucreatinine/Pcreatinine).
Renal Clearance Protocol
The method for performing renal clearance measurements in laboratory mice has been described by Hou et al.6 and Gong and Hou.10 Mice were anesthetized by ip injection of Inactin (100 mg/kg; Sigma-Aldrich). The jugular vein was catheterized for intravenous infusion of 0.9% saline at 2 μl/min, with 1% FITC-inulin included in the infusate. After an equilibration period of 60 minutes, renal clearance measurements were carried out for a 60-minute period. Urine was collected under mineral oil, and a 30-μl blood sample was taken at hourly intervals. Urine and plasma Ca++ and Mg++ concentrations were measured by atomic flame absorption spectrophotometer (PerkinElmer). Urine and plasma FITC-inulin levels were measured in 100 mM Hepes buffer (pH 7.0) with fluorescence spectrophotometer (BioTek). The fractional excretion of electrolytes was calculated using the following equation: FEion=V×Uion/(GFR×Pion), where GFR was calculated according to the clearance rate of FITC-inulin (GFR=V×Uinulin/Pinulin).
Establishing Primary Cultures of Mouse TALH Cells
We used an immunomagnetic separation method to isolate the TALH cells from the mouse kidney.9 Abs against the TALH cell-specific surface antigen Tamm–Horsfall protein (a glycosylphosphatidylinositol [GPI]-anchored protein that is exclusively expressed in the TALH and the early part of the DCT) were coated onto the paramagnetic polystyrene beads (Dynabeads M-280; Dynal), allowing immunoprecipitation of the TALH cells from collagenase-digested mouse kidneys. The isolated cells were plated in DMEM medium supplemented with 10% FBS, penicillin/streptomycin, and 1 mM sodium pyruvate for 16 hours followed by immediate pharmacologic treatment.
TALH Tubule Perfusion and Electrophysiologic Recording
The methods for perfusion and transepithelial measurements in freshly isolated mouse TALH segments were performed as described previously.6 The TALH tubule was held and perfused by a concentric glass pipette system. The perfusion pipette was double-barreled, with an outer diameter of 10–12 μm. One barrel was used for perfusion, fluid exchange, and voltage measurement. The second barrel was used for constant current injection (13 nA). The collection side consisted of a glass pipette with an inner diameter of 45 μm. Cable equations as described52 were used appropriately to calculate transepithelial resistance Rte. Equivalent short circuit current Isc was calculated from Rte and Vte according to Ohms law. The rates of perfusion were 10–20 nl/min. The bath was thermostated at 37°C. Continuous bath perfusion at 3–5 ml/min was obtained by gravity perfusion. Relative paracellular permeabilities were calculated from the observed transepithelial diffusion potentials in the presence of 0.1 mM furosemide according to the Goldman equation.
DCT Perfusion and Intracellular Ca++ Imaging
DCTs were identified by morphology and cut at the macula densa and approximately 300–500 µm downstream using a dissection microscope (Leica MZ16) at 4°C. DCTs were loaded at 37°C for 30–45 minutes with 5 µM Fura-2-AM in the presence of 0.09% (vol/vol) pluronic. DCTs were then transferred into the bath on a heated microscope stage. The bath was thermostated at 37°C and continuously perfused at 8–10 ml/min with control solution (140 mmol/L NaCl, 0.4 mmol/L KH2PO4, 1.6 mmol/L K2HPO4, 1 mmol/L MgCl2, 5 mmol/L glucose, and 1.3 mmol/L Ca-gluconate at pH 7.4). Tubules were held and perfused (10–20 nl/min) by a concentric glass pipette system as described.52 The perfusion pipette was double-barreled and allowed the alternate perfusion of control solution, control solution plus an additional 3 mM Ca-gluconate (+3C), or control solution plus an additional 3 mM Ca-gluconate and 1 µM ruthenium red (+3C+RuR). Only well perfused DCTs with well definable, and open lumens were used. Fluorescence was monitored by a digital imaging system (Visitron Systems GmbH) and analyzed by MetaFluor software. Ratio images (340:380 nm) were obtained every 3 seconds and stored for offline analysis. After a short equilibration period, luminal perfusion was switched to either +3C or +3C+RuR for 3 minutes. The luminal calcium load was subsequently washed out, and DCTs were challenged with a basolateral exchange to iso-osmolar low NaCl (30 NaCl) solution (30 mmol/L NaCl, 0.4 mmol/L KH2PO4, 1.6 mmol/L K2HPO4, 1 mmol/L MgCl2, 5 mmol/L glucose, 230 mmol/L mannitol, and 1.3 mmol/L Ca-gluconate at pH 7.4) for another 3 minutes.
Real-Time PCR Quantification
Total RNA, including miR, was extracted using Trizol (Invitrogen). Cellular mRNA was reverse transcribed using the Superscript-III Kit (Invitrogen) followed by real-time PCR amplification using SYBR Green PCR Master Mix (Bio-Rad) and the Eppendorf Realplex2S System. The design of claudin-14 primers was described before by Gong et al.9 The design of pri-miR primers and ChIP primers was according to Gong and Hou.10 Results were expressed as 2−∆Ct values with ∆CT=Ctgene−Ctβ-actin.
ChIP
Freshly isolated TALH cells or primary TALH cell cultures were crosslinked in 1% paraformaldehyde (pH 7.0) for 10 minutes at room temperature. The crosslinked cells were lysed following the instructions of the MAGnify ChIP System (Invitrogen). Chromatin was sonicated into 500-bp fragments with the Bioruptor UCD-200 (high power, 15 cycles of 30 seconds on and 30 seconds off; Diagenode). Sheared chromatin was precipitated with Ab-bound Dynabeads (Invitrogen). After reverse crosslink and DNA extraction, Ab-bound DNA was eluted and analyzed with real-time PCR. Two criteria for normalization were applied: fold enrichment over anti-IgG background signal and input control over chromatin input signal.
Immunolabeling and Fluorescence Microscopy
For viewing claudin localization in the kidney, fresh cryostat sections (10 μm) were fixed with cold methanol at −20°C followed by blocking with PBS containing 10% FBS and incubation with primary Ab (1:300) and FITC-labeled secondary Ab (1:200). After washing with PBS, slides were mounted with Mowiol (Calbiochem). Epifluorescence images were taken with a Nikon 80i Photomicroscope equipped with a DS-Qi1Mc Digital Camera. All images were converted to TIFF format and arranged using Photoshop CS4 (Adobe Systems, Inc.).
Antagomir Treatments
Antagomirs for miR-9, miR-374, and scrambled control miR were synthesized by Exiqon using LNAs using the following sequences: anti–miR-9: A*T*+A*C*A*+G*C*T*+A*G*A*+T*A*A*+C*C*A*+A*A*G and anti–miR-374: A*C*+T*T*A*+G*C*A*+G*G*T*+T*G*T*+A*T*T*+A*T*A (DNA base: G, A, T, C; LNA base: +G, +A, +T, +C; phosphorothioated DNA base: G*, A*, T*, C*). In vitro, 30 pmol each antagomir was transfected to the primary cultures of TALH in 12-well culture dishes using Lipofectamine 2000 (Invitrogen). In vivo, each antagomir was mixed with the in vivo jetPEI Delivery Reagent (VWR) according to the manufacturer’s guidelines followed by ip injection to animals at a dose of 2.5 mg/kg body wt−1.
Statistical Analyses
The significance of differences between groups was tested by ANOVA (Statistica 6.0; Statsoft). When the all-effect F value was significant (P<0.05), post hoc analysis of differences between individual groups was made with the Newman–Keuls test. Values were expressed as means±SEMs unless otherwise stated.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Drs. Tamar Ben-Yosef (Technion Israel Institute of Technology), Feng Chen (Washington University), and Joan Brugge (Harvard Medical School) for kindly sharing reagents and cell and animal models. We thank Daniel Martin (Washington University O’Brien Center) and Dr. Tong Wang (Yale University O’Brien Center) for assistance in plasma and urine electrolyte analyses.
This work was supported by National Institutes of Health Grants R01-DK084059 and P30-DK079333 and American Heart Association Grant 0930050N.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014020129/-/DCSupplemental.
References
- 1.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP: Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285: 103–106, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S: Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79: 949–957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greger R: Ion transport mechanisms in thick ascending limb of Henle’s loop of mammalian nephron. Physiol Rev 65: 760–797, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Hou J, Paul DL, Goodenough DA: Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci 118: 5109–5118, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA: Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 118: 619–628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou J, Shan Q, Wang T, Gomes AS, Yan Q, Paul DL, Bleich M, Goodenough DA: Transgenic RNAi depletion of claudin-16 and the renal handling of magnesium. J Biol Chem 282: 17114–17122, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Hou J, Renigunta A, Gomes AS, Hou M, Paul DL, Waldegger S, Goodenough DA: Claudin-16 and claudin-19 interaction is required for their assembly into tight junctions and for renal reabsorption of magnesium. Proc Natl Acad Sci U S A 106: 15350–15355, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, Sulem P, Halldorsson BV, de Vegt F, d’Ancona FC, den Heijer M, Franzson L, Christiansen C, Alexandersen P, Rafnar T, Kristjansson K, Sigurdsson G, Kiemeney LA, Bodvarsson M, Indridason OS, Palsson R, Kong A, Thorsteinsdottir U, Stefansson K: Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet 41: 926–930, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J: Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 31: 1999–2012, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Y, Hou J: Claudin-14 underlies Ca++-sensing receptor-mediated Ca++ metabolism via NFAT-microRNA-based mechanisms. J Am Soc Nephrol 25: 745–760, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou J, Rajagopal M, Yu AS: Claudins and the kidney. Annu Rev Physiol 75: 479–501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimke H, Hoenderop JG, Bindels RJ: Molecular basis of epithelial Ca2+ and Mg2+ transport: Insights from the TRP channel family. J Physiol 589: 1535–1542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC: Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Pollak MR, Brown EM, Chou YH, Hebert SC, Marx SJ, Steinmann B, Levi T, Seidman CE, Seidman JG: Mutations in the human Ca(2+)-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 75: 1297–1303, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Pollak MR, Brown EM, Estep HL, McLaine PN, Kifor O, Park J, Hebert SC, Seidman CE, Seidman JG: Autosomal dominant hypocalcaemia caused by a Ca(2+)-sensing receptor gene mutation. Nat Genet 8: 303–307, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Pearce SH, Williamson C, Kifor O, Bai M, Coulthard MG, Davies M, Lewis-Barned N, McCredie D, Powell H, Kendall-Taylor P, Brown EM, Thakker RV: A familial syndrome of hypocalcemia with hypercalciuria due to mutations in the calcium-sensing receptor. N Engl J Med 335: 1115–1122, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaître X, Paillard M, Planelles G, Déchaux M, Miller RT, Antignac C: Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol 13: 2259–2266, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita Y, Hori M, Taguchi M, Watanabe S, Fukumoto S: Functional activities of mutant calcium-sensing receptors determine clinical presentations in patients with autosomal dominant hypocalcemia. J Clin Endocrinol Metab 99: E363–E368, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Johnstone RW: Histone-deacetylase inhibitors: Novel drugs for the treatment of cancer. Nat Rev Drug Discov 1: 287–299, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Helin K, Dhanak D: Chromatin proteins and modifications as drug targets. Nature 502: 480–488, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Pipalia NH, Cosner CC, Huang A, Chatterjee A, Bourbon P, Farley N, Helquist P, Wiest O, Maxfield FR: Histone deacetylase inhibitor treatment dramatically reduces cholesterol accumulation in Niemann-Pick type C1 mutant human fibroblasts. Proc Natl Acad Sci U S A 108: 5620–5625, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM: Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487: 482–485, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haberland M, Montgomery RL, Olson EN: The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat Rev Genet 10: 32–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coward WR, Watts K, Feghali-Bostwick CA, Jenkins G, Pang L: Repression of IP-10 by interactions between histone deacetylation and hypermethylation in idiopathic pulmonary fibrosis. Mol Cell Biol 30: 2874–2886, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R: Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem Biol 13: 563–567, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Marks PA, Breslow R: Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol 25: 84–90, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, Rasmussen J, Anderson JM, Dolan DF, Forge A, Raphael Y, Camper SA, Friedman TB: Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum Mol Genet 12: 2049–2061, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Ryan MP, Devane J, Ryan MF, Counihan TB: Effects of diuretics on the renal handling of magnesium. Drugs 28[Suppl 1]: 167–181, 1984 [DOI] [PubMed] [Google Scholar]
- 29.Ryan MP: Diuretics and potassium/magnesium depletion. Directions for treatment. Am J Med 82: 38–47, 1987 [DOI] [PubMed] [Google Scholar]
- 30.Breiderhoff T, Himmerkus N, Stuiver M, Mutig K, Will C, Meij IC, Bachmann S, Bleich M, Willnow TE, Müller D: Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci U S A 109: 14241–14246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naro F, De Arcangelis V, Coletti D, Molinaro M, Zani B, Vassanelli S, Reggiani C, Teti A, Adamo S: Increase in cytosolic Ca2+ induced by elevation of extracellular Ca2+ in skeletal myogenic cells. Am J Physiol Cell Physiol 284: C969–C976, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M: Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438: 685–689, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Obad S, dos Santos CO, Petri A, Heidenblad M, Broom O, Ruse C, Fu C, Lindow M, Stenvang J, Straarup EM, Hansen HF, Koch T, Pappin D, Hannon GJ, Kauppinen S: Silencing of microRNA families by seed-targeting tiny LNAs. Nat Genet 43: 371–378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elmén J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjärn M, Hansen JB, Hansen HF, Straarup EM, McCullagh K, Kearney P, Kauppinen S: Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res 36: 1153–1162, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castoldi M, Vujic Spasic M, Altamura S, Elmén J, Lindow M, Kiss J, Stolte J, Sparla R, D’Alessandro LA, Klingmüller U, Fleming RE, Longerich T, Gröne HJ, Benes V, Kauppinen S, Hentze MW, Muckenthaler MU: The liver-specific microRNA miR-122 controls systemic iron homeostasis in mice. J Clin Invest 121: 1386–1396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R: TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 11: 881–889, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebeshuber CA, Kornauth C, Dong L, Sierig R, Seibler J, Reiss M, Tauber S, Bilban M, Wang S, Kain R, Böhmig GA, Moeller MJ, Gröne HJ, Englert C, Martinez J, Kerjaschki D: Focal segmental glomerulosclerosis is induced by microRNA-193a and its downregulation of WT1. Nat Med 19: 481–487, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA: miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol 12: 247–256, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ: Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 115: 1651–1658, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, Belyantseva I, Ben-Yosef T, Liburd NA, Morell RJ, Kachar B, Wu DK, Griffith AJ, Riazuddin S, Friedman TB: Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104: 165–172, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Van Lint C, Emiliani S, Verdin E: The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr 5: 245–253, 1996 [PMC free article] [PubMed] [Google Scholar]
- 42.Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, Thaler HT, Rifkind RA, Marks PA, Richon VM: Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res 60: 5165–5170, 2000 [PubMed] [Google Scholar]
- 43.Cohen LA, Amin S, Marks PA, Rifkind RA, Desai D, Richon VM: Chemoprevention of carcinogen-induced mammary tumorigenesis by the hybrid polar cytodifferentiation agent, suberanilohydroxamic acid (SAHA). Anticancer Res 19: 4999–5005, 1999 [PubMed] [Google Scholar]
- 44.Kelly WK, Richon VM, O'Connor O, Curley T, MacGregor-Curtelli B, Tong W, Klang M, Schwartz L, Richardson S, Rosa E, Drobnjak M, Cordon-Cordo C, Chiao JH, Rifkind R, Marks PA, Scher H: Phase I clinical trial of histone deacetylase inhibitor: Suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res 9[10 Pt 1]: 3578–3588, 2003 [PubMed] [Google Scholar]
- 45.Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, Frankel SR: Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 109: 31–39, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fraczek J, Vanhaecke T, Rogiers V: Toxicological and metabolic considerations for histone deacetylase inhibitors. Expert Opin Drug Metab Toxicol 9: 441–457, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Shah MH, Binkley P, Chan K, Xiao J, Arbogast D, Collamore M, Farra Y, Young D, Grever M: Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clin Cancer Res 12: 3997–4003, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Vansteenkiste J, Van Cutsem E, Dumez H, Chen C, Ricker JL, Randolph SS, Schöffski P: Early phase II trial of oral vorinostat in relapsed or refractory breast, colorectal, or non-small cell lung cancer. Invest New Drugs 26: 483–488, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Riccardi D, Brown EM: Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol 298: F485–F499, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhakat KK, Izumi T, Yang SH, Hazra TK, Mitra S: Role of acetylated human AP-endonuclease (APE1/Ref-1) in regulation of the parathyroid hormone gene. EMBO J 22: 6299–6309, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Himmerkus N, Shan Q, Goerke B, Hou J, Goodenough DA, Bleich M: Salt and acid-base metabolism in claudin-16 knockdown mice: Impact for the pathophysiology of FHHNC patients. Am J Physiol Renal Physiol 295: F1641–F1647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Greger R: Cation selectivity of the isolated perfused cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch 390: 30–37, 1981 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.