Abstract
In the current immunosuppressive therapy era, vessel thrombosis is the most common cause of early graft loss after renal transplantation. The prevalence of IgA anti–β2-glycoprotein I antibodies (IgA-aB2GPI-ab) in patients on dialysis is elevated (>30%), and these antibodies correlate with mortality and cardiovascular morbidity. To evaluate the effect of IgA-aB2GPI-ab in patients with transplants, we followed all patients transplanted from 2000 to 2002 in the Hospital 12 de Octubre prospectively for 10 years. Presence of IgA-aB2GPI-ab in pretransplant serum was examined retrospectively. Of 269 patients, 89 patients were positive for IgA-aB2GPI-ab (33%; group 1), and the remaining patients were negative (67%; group 2). Graft loss at 6 months post-transplant was significantly higher in group 1 (10 of 89 versus 3 of 180 patients in group 2; P=0.002). The most frequent cause of graft loss was thrombosis of the vessels, which was observed only in group 1 (8 of 10 versus 0 of 3 patients in group 2; P=0.04). Multivariate analysis showed that the presence of IgA-aB2GPI-ab was an independent risk factor for early graft loss (P=0.04) and delayed graft function (P=0.04). There were no significant differences regarding patient survival between the two groups. Graft survival was similar in both groups after 6 months. In conclusion, patients with pretransplant IgA-aB2GPI-ab have a high risk of early graft loss caused by thrombosis and a high risk of delayed graft function. Therefore, pretransplant IgA-aB2GPI-ab may have a detrimental effect on early clinical outcomes after renal transplantation.
Keywords: survival, immunology, kidney transplantation
Renal transplantation outcomes have improved in the modern era, particularly in the first post-transplant year, because of several factors. These factors include more awareness and better testing for donor-specific anti-HLA antibody, better detection of positive crossmatches, and better immunosuppression.1 Therefore, the establishment of a calcineurin inhibitor-based protocol, most frequently tacrolimus (TAC), mycophenolate mofetil (MMF), and steroids with or without induction, has successfully reduced acute rejection (AR) rates.2 Although late graft failures are now the main problem after transplantation, it is important to consider that 5%–8% of grafts are lost in the first months.3 These losses are partially caused by postoperative complications, AR, and especially, thrombotic events.4 In a series of 2600 patients, we have shown that venous and/or arterial thrombosis is the first cause of graft loss in the early post-transplant period.3,5
For >30 years, it has been known that the presence of antibodies against HLA antigens before transplantation is associated with early rejection of kidney grafts.6 However, other antibodies against minor histocompatibility antigens, endothelial cells, or autoantigens have also been recognized as harmful for renal allograft outcomes.7–9
Antiphospholipid (aPL) antibodies are a group of autoantibodies against phospholipids, phospholipids binding proteins, or both that are localized on membranes of endothelial and other cells involved in the coagulation cascade.10,11 Antibodies against phospholipids per se are associated with infectious diseases, whereas autoantibodies associated with vascular pathology are directed against β2-glycoprotein I (B2GPI),12 a serum protein that is synthesized in the liver, bowel, and kidney.13 B2GPI is also localized in platelets, endothelial cells,14 and kidney15 tubular epithelial16 membranes.
aPL syndrome (APS) is defined by the persistent presence of aPLs associated with thrombosis and/or pregnancy morbidity.17 International consensus by APS diagnosis considers diagnostic aPL as the presence of lupus anticoagulant, anticardiolipin (aCL), and anti-B2GPI (aB2GPI) antibodies. Only IgG or IgM isotypes are included in the international consensus.
Over the past few years, much attention has been focused on the diagnostic value of IgA isotype aPL antibodies. Isolated IgA aB2GPI antibodies have been associated with APS in patients with SLE.18 In a recent work, these antibodies were considered to be the most prevalent aPLs in patients with well defined clinical criteria of APS (C-APS). Only 14% of patients with C-APS were positive for any consensus antibody, whereas the presence of isolated IgA aB2GPI antibodies was found in 22% of patients with C-APS.19 Although most of the works highlighted the value of IgA aB2GPI antibodies in the diagnosis of APS, the meaning of the presence of aB2GPI IgA antibodies is still under debate in the literature, because the available diagnostic tools have not been sufficiently standardized and the diagnostic assays used in some works with negative results were not optimal.20
aPL antibodies of isotypes IgG and IgM are more frequent in patients with CKD than the general population.21–23 Our group has recently described an increased prevalence of IgA aB2GPI in patients on hemodialysis (33%) and their association with thrombotic events and mortality.24 This finding was subsequently confirmed by another study.25 However, the influence of these autoantibodies after renal transplantation is unknown, particularly in the first postoperative period when thrombotic events are more frequent. Our study has aimed to investigate the possible influence of preformed IgA aB2GPI antibodies on early and late renal graft outcomes.
Results
aPL Antibodies
The average pretransplant levels of aCL antibodies were IgM of 10.0±1.1 units/ml (mean±SEM), IgG of 6.0±0.4 units/ml, and IgA of 10.0±1.1 units/ml. aB2GPI antibodies were IgM of 7.4±1.3 units/ml. IgGs were 6.8±0.7 units/ml, and IgAs were 23.3±33.0 units/ml (Supplemental Figure 1). Patients whose antibody levels exceeded the cutoff were considered positive. Prevalence of patients positive for aCL was 4.5% for IgM, 1.5% for IgG, and 0.5% for IgA. Prevalence of aB2GPI antibodies in patients was 1.1% for IgM and 2.6% for IgG.
Patients with IgA aB2GPI Antibodies
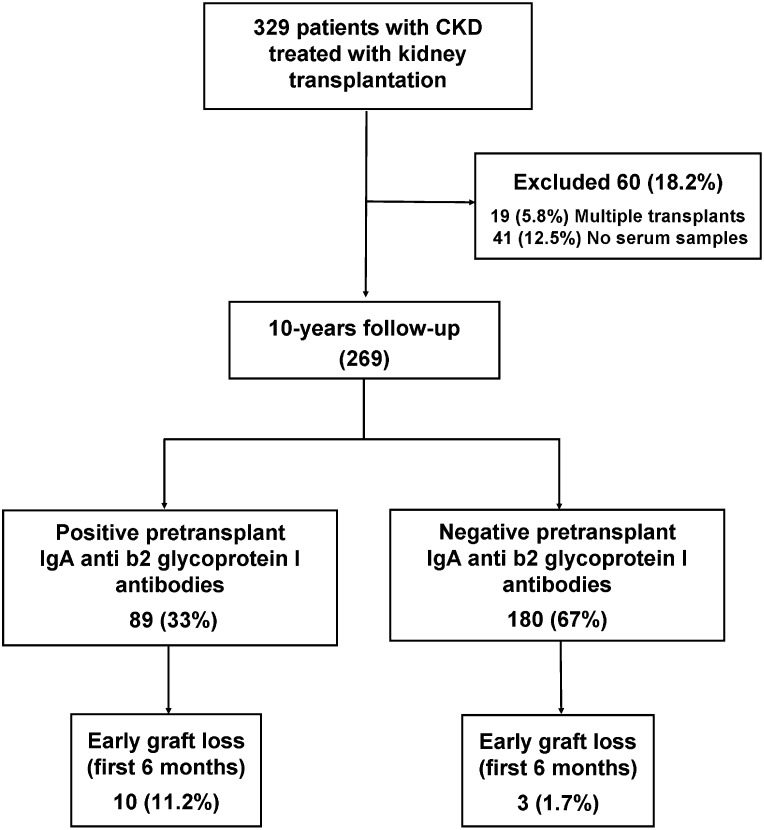
Eighty-nine patients (33%) were positive for IgA aB2GPI antibodies (group 1); 180 patients were negative (group 2). Demographic data of both groups are described in Table 1, algorithm is shown in Figure 1. Donor and recipient ages were higher in group 1 (P<0.001), and most of the patients with previous transplants were in group 2 (P=0.001) (Figure 1).
Table 1.
Clinical characteristics of patients in groups 1 and 2 before transplantation
| Condition | Group 1 (n=89; IgA aB2GPI-Positive) | Group 2 (n=180; IgA aB2GPI-Negative) | P Value | ||
|---|---|---|---|---|---|
| N or Mean | SEM or Percent | N or Mean | SEM or Percent | ||
| Sex (women) | 42 | 47.2% | 82 | 45.6% | 0.90 |
| Weight (kg) | 67.2 | ±1.5 | 66.8 | ±1.0 | 0.81 |
| Height (cm) | 163.5 | ±1.0 | 165.4 | ±0.7 | 0.16 |
| Age (yr) | 55.9 | ±1.3 | 47.9 | ±1.0 | <0.001 |
| Donor age (yr)a | 53.1 | ±2.0 | 43.5 | ±1.4 | <0.001 |
| Time on dialysis (mo) | 27.7 | ±3.1 | 37.1 | ±3.7 | 0.10 |
| Peritoneal dialysis | 14 | 15.7% | 24 | 13.3% | 0.73 |
| Causes of CKD | |||||
| Chronic GN | 18 | 20.2% | 51 | 28.3% | 0.20 |
| Interstitial kidney disease | 20 | 22.5% | 33 | 18.3% | 0.52 |
| Nephroangiosclerosis | 5 | 5.6% | 21 | 11.7% | 0.17 |
| Polycystic kidney disease | 15 | 16.9% | 29 | 16.1% | 0.98 |
| Diabetes | 11 | 12.4% | 13 | 7.2% | 0.25 |
| Unknown | 11 | 12.4% | 14 | 7.8% | 0.32 |
| Other | 9 | 10.1% | 19 | 10.6% | 0.92 |
| Pretransplant | |||||
| Severe thrombosis before transplantb | 10 | 11.2% | 7 | 3.9% | 0.04 |
| Stroke | 3 | 3.4% | 1 | 0.6% | 0.12 |
| Peritoneal dialysis | 14 | 15.7% | 24 | 13.3% | 0.73 |
| Dyslipidemia | 23 | 25.8% | 62 | 34.4% | 0.20 |
| Diabetes mellitus | 14 | 15.7% | 17 | 9.4% | 0.19 |
| Previous transplant | 3 | 3.4% | 34 | 18.9% | 0.001 |
| Double renal transplant | 9 | 10.1% | 13 | 7.2% | 0.56 |
| PRA>50% (at time of transplant) | 3 | 3.4% | 6 | 3.3% | 0.73 |
| Historical PRA>50% | 9 | 10.1% | 28 | 15.6% | 0.30 |
| Cold ischemia (h) | 20.7 | ±0.6 | 19.8 | ±0.4 | 0.18 |
| Warm ischemia in recipient (min) | 31.0 | ±0.7 | 31.1 | ±0.5 | 0.91 |
Donor age is a recipient age-dependent variable.
Excluding vascular access.
Figure 1.
The majority of patients with early graft loss were positive for IgA aB2GPI antibodies. Algorithm of disposition and outcomes.
Clinical Events and Course in the Early Post-Transplant Period (6 Months)
Thirteen patients (4.8%) lost their graft during the first 6 months (early graft loss).
Differences between patients with early graft loss and the remaining patients were age (60.4±2.7 versus 50.1±0.9 years; P<0.01), presence of delayed graft function (DGF; 53.8% versus 17.2%; P=0.003), and positivity for IgA aB2GPI antibodies (76.9% versus 30.8%; P=0.002) (Supplemental Table 2).
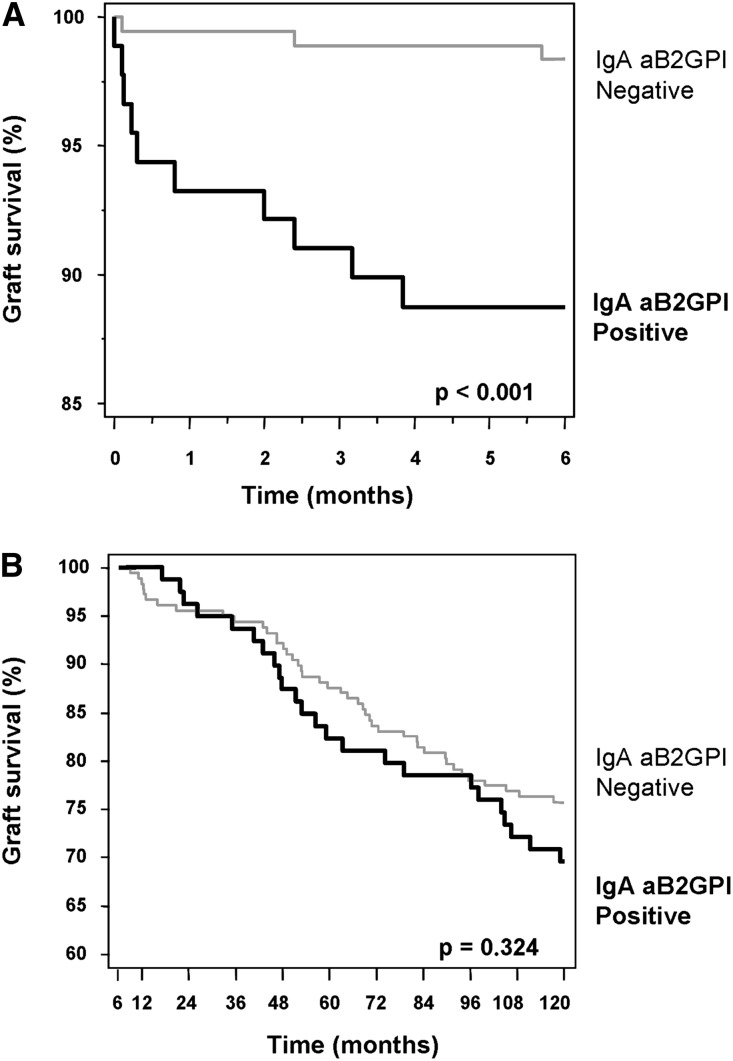
Graft loss was significantly higher in group 1 than group 2 (11.2% versus 1.7%; P=0.002) (Table 2). Kaplan–Meier survival analysis revealed significantly lower graft survival rates at 6 months in group 1 than in group 2 (P<0.001) (Figure 2A). The most striking finding was that arterial and/or venous thrombosis was the most frequent cause of graft loss and that it was only present in group 1 (8 of 10 versus 0 of 3 patients; P=0.04). DGF was also significantly higher in group 1 (28.1% versus 14.4%; P=0.01). There were no differences regarding AR. Mortality was low and similar in both groups (Table 2).
Table 2.
Clinical episodes early post-transplant (before 6 months)
| Condition | Group 1 (n=89; IgA aB2GPI-Positive) | Group 2 (n=180; IgA aB2GPI-Negative) | P Value | ||
|---|---|---|---|---|---|
| N | Percent | N | Percent | ||
| AR episodes | 7 | 7.9 | 9 | 5.0 | 0.51 |
| DGF | 25 | 28.1 | 26 | 14.4 | 0.01 |
| Patients with cardiovascular eventsa | 1 | 1.1 | 2 | 1.1 | >0.99 |
| Graft loss | 10 | 11.2 | 3 | 1.7 | 0.002 |
| Causes of graft loss | |||||
| Sudden death | 0 | 2 | 66 | >0.99 | |
| Vessel thrombosis | 8 | 80 | 0 | 0.04 | |
| Histologically confirmed AR | 0 | 1 | 33 | 0.23 | |
| Clinically suspected AR | 1 | 10 | 0 | >0.99 | |
| Primary nonfunction | 1 | 10 | 0 | >0.99 | |
| Mortality | 3 | 3.4 | 2 | 1.1 | 0.34 |
| Causes of death | |||||
| Shock | 2 | 66 | 0 | 0.40 | |
| Nephrectomy complications | 1 | 33 | 0 | >0.99 | |
| Sudden death | 0 | 2 | 100 | 0.10 | |
Nongraft loss or mortality-related.
Figure 2.
Survival curves at 120 months in patients positive and negative for IgA aB2GPI antibodies. (A) Graft survival during the first 6 months was significantly lower in patients positive for IgA aB2GPI antibodies (group 1) than patients negative for IgA aB2GPI antibodies (group 2). (B) Graft survival from the seventh month to the end of follow-up (not DCGS). No significant differences between groups for graft survival were observed.
Table 3 provides a detailed clinical description of the characteristics of 13 patients who lost their graft (11) or died (2). No significant associations were observed between pathologies or conditions listed on Tables 1 and 2 and other aPL antibodies (aCL any isotype or aB2GPI IgG and IgM isotypes; data not shown).
Table 3.
Description of early graft loss episodes
| Receptor Age (yr) | Donor Age (yr) | Single/Double Transplant | PRA (%) | IgA aB2GPI | Immunosuppression | DGF | Clinical Diagnosis | Time from Transplant (d) | Histopathologic Diagnostic | Evolution |
|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 49 | Single | 0 | + | TAC+MMF | − | Renal thrombosis | 1 | Thrombosis in venules | Nephrectomy.; hemodialysis |
| 40 | 52 | Single | 0 | + | TAC+MMF | + | Renal thrombosis | 2 | Venous thrombosis | Nephrectomy.; hemodialysis |
| 71 | 74 | Double | 3 | + | CsA+MMF | + | Renal thrombosis | 4 | Venous and arterial thrombosis | Nephrectomy.; hemodialysis |
| 72 | 69 | Single | 0 | + | CsA+MMF | − | Renal thrombosis | 6 | Venous and arterial thrombosis | Nephrectomy.; hemodialysis |
| 61 | 68 | Single | 0 | + | CsA+MMF | + | Renal thrombosis | 9 | Venous thrombosis | Nephrectomy.; hemodialysis |
| 60 | 45 | Single | 81 | + | CsA+MMF | − | Renal thrombosis | 24 | Venous thrombosis | Nephrectomy.; died in the early postoperative period |
| 66 | 73 | Single | 0 | + | TAC+MMF | + | Primary nonfunction | 60 | No histologic evidence of AR | Hemodialysis |
| 72 | 71 | Single | 0 | + | CsA+MMF | + | Renal thrombosis | 72 | Thrombosis in venules | Nephrectomy.; hemodialysis; died 2 mo postextraction with clinically compatible Asherson syndrome |
| 63 | 77 | Double | 0 | + | CsA+MMF | − | Renal thrombosis | 95 | Venous and arterial thrombosis | Nephrectomy.; hemodialysis |
| 65 | 66 | Single | 0 | + | CsA+MMF | − | Suspected | 115 | No evidence of AR | Immediately after graft loss, small vessel venous thrombosis of the skin appeared; this continued to spread until becoming systemic and then evolved to shock, and the patient died 1 mo after the graft loss |
| 63 | 79 | Single | 57 | − | CsA+MMF | + | Sudden death | 3 | No biopsy | |
| 51 | 44 | Single | 73 | − | TAC+MMF | − | Sudden death | 69 | No biopsy | |
| 51 | 66 | Double | 0 | − | CsA+MMF | + | AR | 171 | AR | Nephrectomy.; hemodialysis |
IgA aB2GPI Antibodies Are an Independent Risk Factor for Early Graft Loss
Early graft loss factors that were significant in the univariate analysis (Supplemental Table 2) were included in the Cox multivariate analysis (Table 4). Donor age and treatment with cyclosporin A (CsA) variables present high correlation with receptor age. The policy with older donors was to transplant these kidneys into older recipients (old-for-old transplantation), and donor age was similar to recipient age (Pearson correlation coefficient=0.743; P<0.001). Older patients were treated with CsA (Spearman rank correlation coefficient=0.641; P<0.001). Of three variables, only recipient age was included in the Cox multivariable analysis. Hazard ratio (HR) of IgA aB2GPI antibodies for graft loss without adjusting for other risk factors was 7.09 (95% confidence interval, 1.97 to 25.61; P=0.003). It stands out that the HR continued to be clearly significant (HR, 4.07; 95% confidence interval, 1.06 to 15.58; P=0.04) in the Cox regression model.
Table 4.
Cox multivariate analysis (P<0.001) of risk factors associated with early graft loss statistically significant in univariate analysis
| Covariate | Univariate HR | 95% CI | P Value | Multivariate HR | 95% CI | P Value |
|---|---|---|---|---|---|---|
| IgA aB2GPI-positive | 7.09 | 1.97 to 25.61 | 0.003 | 4.07 | 1.06 to 15.58 | 0.04 |
| Receptor age (yr) | 1.06 | 1.01 to 1.11 | 0.01 | 1.04 | 0.98 to 1.10 | 0.19 |
| DGF | 5.29 | 1.79 to 15.64 | 0.003 | 3.58 | 1.18 to 10.81 | 0.03 |
| Cold ischemia | 1.15 | 1.01 to 1.31 | 0.04 | 1.07 | 0.94 to 1.21 | 0.30 |
95% CI, 95% confidence interval.
IgA aB2GPI Antibodies Are an Independent Risk Factor for DGF
DGF was more frequent in patients with IgA aB2GPI antibodies (odds ratio [OR], 2.31; 95% CI, 1.24 to 4.31; P<0.01). In a multivariate analysis with other well known DGF risk factors,26 such as donor age, previous transplants, and prolonged cold ischemia time, IgA aB2GPI antibodies were identified as independent risk factors for DGF (OR, 1.97; 95% CI, 1.02 to 3.81; P=0.04) (Table 5).
Table 5.
Logistic regression multivariate analysis (P=0.01) of risk factors associated with DGF
| Covariate | Univariate OR | 95% CI | P Value | Multivariate OR | 95% CI | P Value |
|---|---|---|---|---|---|---|
| IgA aB2GPI-positive | 2.31 | 1.24 to 4.31 | 0.008 | 1.97 | 1.02 to 3.81 | 0.04 |
| Donor age (yr) | 1.03 | 1.01 to 1.04 | 0.004 | 1.02 | 1.00 to 1.04 | 0.02 |
| Cold ischemia>24 h | 1.04 | 0.98 to 1.11 | 0.24 | 0.98 | 0.44 to 2.16 | 0.96 |
| Previous transplant | 0.68 | 0.25 to 1.85 | 0.45 | 1.21 | 0.41 to 3.55 | 0.73 |
95% CI, 95% confidence interval.
Late Post-Transplant Period (from 7 Months to 10 Years)
There were no significant differences between both groups regarding graft survival from 7 months to 10 years (Figure 2B). Morbidity and causes of graft loss and mortality in this period were similar in both groups (Supplemental Table 3).
Complete Follow-Up (0–120 Months)
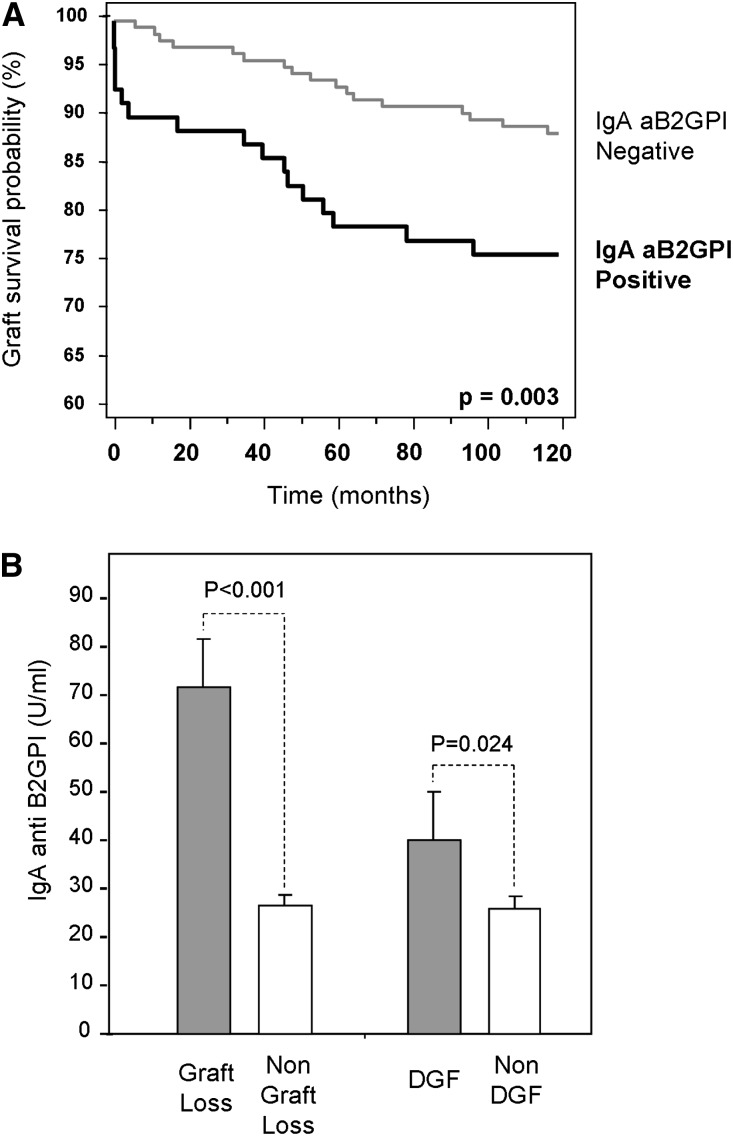
Considering all of the patients, noncensored graft survival and death-censored graft survival (DCGS) at 120 months were 70.3% and 82.9%, respectively. Noncensored graft survival was significantly lower in group 1: 61.8% versus 74.4% (P=0.02). However, these differences were more significant when analyzed for DCGS: 72% versus 88.4% (P=0.003) (Figure 3A). Pretransplant levels of IgA aB2GPI antibodies were higher in those who had lost their graft or developed DGF versus those who had not (Figure 3B).
Figure 3.
Death-censored graft survival curve in 10-years follow-up, and pretransplant levels of IgA aB2GPI antibodies in patients with allograft failure. (A) Graft survival (DCGS) in the follow-up was significantly lower in patients in group 1. (B) Pretransplant mean levels of IgA aB2GPI in patients who had early allograft failure (graft loss or DGF) were higher than in patients without these events.
Patient survival, AR, malignancies, and cardiovascular disease were similar between the two groups. Immunosuppression was different according initial CsA-based immunosuppression in older patients (Table 6).
Table 6.
Episodes in complete follow-up in both groups
| Condition | Group 1 (n=89; IgA aB2GPI-Positive) | Group 2 (n=180; IgA aB2GPI-Negative) | P Value | ||
|---|---|---|---|---|---|
| N | Percent | N | Percent | ||
| DGF | 25 | 28.1 | 26 | 14.4 | 0.01 |
| Episodes of AR | 12 | 13.5 | 17 | 9.4 | 0.43 |
| Graft loss | 34 | 38.2 | 46 | 25.6 | 0.05 |
| Causes of graft loss | |||||
| AR | 2 | 5.9 | 1 | 2.2 | 0.57 |
| Uropathy | 0 | 0 | 1 | 2.2 | >0.99 |
| Death | 13 | 38.2 | 28 | 60.9 | 0.08 |
| Chronic allograft nephropathy | 8 | 23.5 | 12 | 26.1 | >0.99 |
| Graft thrombosis | 8 | 23.5 | 0 | 0 | <0.001 |
| Recurrence of disease. | 1 | 2.9 | 2 | 4.3 | >0.99 |
| Others | 1 | 2.9 | 1 | 2.2 | >0.99 |
| Death | 17 | 19.1 | 30 | 16.7 | 0.75 |
| Causes of death | |||||
| Stroke | 2 | 11.8 | 4 | 13.3 | >0.99 |
| Heart failure | 1 | 5.9 | 0 | 0 | 0.36 |
| Other cardiovascular disease | 2 | 11.8 | 2 | 6.7 | 0.61 |
| Infection | 4 | 23.5 | 6 | 20.0 | >0.99 |
| Cancer | 1 | 5.9 | 4 | 13.3 | 0.64 |
| Sudden death | 1 | 5.9 | 3 | 10.0 | >0.99 |
| Others | 6 | 35.3 | 9 | 30.0 | 0.75 |
| Unknown | 0 | 0 | 2 | 6.7 | 0.53 |
| Other complications | |||||
| Patients with cancer | 12 | 13.5 | 22 | 12.2 | 0.92 |
| Patients with cardiovascular events | 14 | 15.7 | 28 | 15.6 | 0.89 |
| Immunosuppressant treatment | |||||
| Calcineurin inhibitors | 87 | 97.8 | 178 | 98.8 | 0.85 |
| CsAa | 40 | 44.9 | 39 | 21.7 | <0.001 |
| TAC | 47 | 52.8 | 139 | 77.2 | <0.001 |
| MMF | 84 | 94.4 | 166 | 92.2 | 0.69 |
| Corticosteroids | 87 | 97.8 | 179 | 99.4 | 0.53 |
| Sirolimus | 4 | 4.5 | 15 | 8.3 | 0.37 |
Treatment with CsA is a recipient age-dependent variable.
The most striking difference between both groups was observed in the first months after transplantation when thrombotic episodes occurred. On the basis of the analysis of the temporal distribution of overall and death-censored graft losses, it stands out that the majority of the group 1 graft losses is concentrated in the first quarter after transplantation, particularly in the first 1 month.
Discussion
We have found that the presence of pretransplant IgA aB2GPI antibodies is an independent risk factor for graft loss, especially because of thrombosis, in the first period after transplantation. Consequently, graft survival at 6 months was significantly lower in IgA aB2GPI-positive recipients than patients without these antibodies. To the best of our knowledge, this study is the first time that IgA aB2GPI antibodies have been directly and independently linked to early graft loss and DGF in patients after renal transplant.
More efficient immunosuppression has dramatically reduced AR incidence and improved graft survival (90%–95% 1 year after transplantation). However, early graft loss still remains a major cause of concern.27 Although AR is associated with a 1.43-fold increase in graft loss (1.6-fold in DCGS),28 at present, AR is not the most frequent cause of early graft loss.29 In a series of 2381 consecutive deceased donor transplants, 4.6% of allografts (109) failed within 30 days of transplantation. Vessel thrombosis was the most common cause of early graft loss (44% of total early failures).27 The findings in the Spanish cohort study that included 2600 patients transplanted in the 2000–2002 period were similar to the findings of our study. The former study found that renal vessel thrombosis, particularly venous thrombosis, was the most frequent cause of graft loss in the first year.5
The graft loss described in this work (4.8%) is similar to other previously reported graft loss.27,29 It is important to note that graft loss was significantly higher in patients with IgA aB2GPI antibodies and that thrombotic events were the primary cause of graft loss. Interestingly, the thrombotic complications (diagnosed using Sidney criteria) were only observed in the group 1 patients. Two patients in group 2 died from sudden death of an unknown cause. The absence of imaging techniques or a necropsy study makes it difficult to establish whether a thrombotic event, according to Sidney criteria, ultimately caused these deaths.
Time to the thrombotic event is an important factor. Five patients developed arterial and/or vein thrombosis within the first 9 days after deceased donor renal transplantation. Although all of them had IgA aB2GPI antibodies, these phenomena might be surgery-related. It is well known that surgery can be a risk factor for thrombosis, mainly in uremic patients with severe atherosclerosis, and post-transplant vessel thrombosis is related to surgeon experience and technique. However, transplants in both groups of patients were performed by the same team of senior surgeons with homogeneous expertise during the 2000–2002 period analyzed in the study. Furthermore, surgery may also be a second trigger for thrombosis (see below) in patients with aPL.30
In old-for-old transplantation,31 prevalence of thrombotic events is higher than in standard deceased donor renal transplantation.32 Among patients who lost grafts, three were older than 60 years and had received kidneys from older than 60 years deceased donors.
An interest finding is that four of eight thrombosis patients had DGF. It is well known that allograft thrombosis is more frequent in patients with DGF. However, three patients developed thrombosis at 24, 72, and 95 post-transplantation days without any predisposing condition, such as perirenal fluid collections, trauma, late postoperative complications, or coagulation problems. These patients were also positive for IgA aB2GPI antibodies. Although the presence of arterial/venous thrombosis could be explained by several well known factors, IgA aB2GPI antibodies were invariably present in all patients with vascular events. We could not identify pretransplant IgA aB2GPI antibodies as an independent risk factor for thrombosis in a multivariate analysis, because the number of patients was small (only eight). However, these results strongly suggest that these autoantibodies could trigger episodes of early post-transplant thrombosis.
Ten-year graft survival was significantly lower in group 1 patients, and graft losses in this group mainly occurred within the first post-transplantation months. Therefore, graft survival from the 6-month to 10-year post-transplantation period was not significantly different between the two groups. These data strongly suggest that the negative effect of pretransplant IgA aB2GPI antibodies after renal transplantation is related to the first period after transplantation.
Although the main immune response to transplanted kidney is MHC-mediated alloimmunity, autoreactivity directed against non-MHC antigens also contributes to post-transplant graft injury.33,34 Autoantibodies that have been associated with rejection in animal models may be important in allograft loss. However, their real role in humans is still insufficiently known.35,36 Dinavahi et al.33 searched for autoantibodies (IgG isotype) against graft-expressed, non-HLA antigens, including B2GPI, and did not find aB2GPI antibodies. No IgA antibodies were included in their analysis.
Pretransplant antiendothelial cell antibodies have been associated with endothelial injury that can be manifested as early rejection episodes that usually lead to graft loss.37–40 B2GPI could act as an antiendothelial cell antibody-related antigen. It is localized on the endothelial cell surfaces associated with lipids or membrane proteins as receptors.41 aB2GPI antibodies can activate endothelial cells,42 modulating the expression of adhesion molecules and cytokine production.43 Cell activation seems to be a major pathogenic cause in the pathogenesis of APS.44
The prevalence of pretransplant IgA aB2GPI antibodies is found in one third of transplanted patients, which is a number similar to that previously reported in patients on hemodialysis.24,25 This finding raises the question of why only a few patients positive for IgA aB2GPI antibodies developed this thrombotic complication in the first weeks after renal transplantation. This situation is well known in patients with aPL, in whom the presence of antibodies is not sufficient to induce thrombosis formation. These patients need a trigger factor to develop thrombotic episodes, usually infection, inflammation, or surgery.45 Several possible trigger factors, mainly surgical, are present in the context of renal transplantation. However, the potential role of infectious agents, inflammatory state, conditioning immunosuppressive treatments, and other unknown factors needs additional investisgation.46 Antibodies of IgA isotype directed against B2GPI have been previously associated with a higher risk for vascular disease, including peripheral vascular disease, cerebral ischemia, stroke, and myocardial infarction.47,48
The exact mechanism of aB2GPI antibodies causing thrombosis and vascular disease remains unknown; however, inhibition of B2GPI anticoagulant activity has been suggested as a pathogenic mechanism.45 Furthermore, it is unknown how the immune response of IgA antibodies against B2GPI is generated. Some infections have been considered to trigger an immune response, with a bias toward the production of antibodies by molecular mimicry between pathogens and B2GPI epitopes.48,49 Proposed models of hapten carriers complexes generated by B2GPI interaction with dialysis membranes24 could be discarded, because the prevalence of IgA aB2GPI antibodies in patients with CKD undergoing peritoneal dialysis or not dialyzed is similar to the observed prevalence in patients on hemodialysis.50 Other hypotheses, such as the production of misfolded B2GPI in the diseased kidney associated with molecular mimicry in the context of mucosal infections, should be studied.50
The association between DGF and the presence of IgA aB2GPI antibodies is another point of interest. A higher incidence of primary graft nonfunction in patients with pretransplant IgG aCL antibodies has been previously described,51 although subsequent studies on the basis of detection of aB2GPI and aCL antibodies of IgG and IgM isotypes were unable to confirm this association.52,53 In the multivariate analysis, the presence of IgA aB2GPI was an independent risk factor for DGF. We can hypothesize that some patients with DGF may have a less severe form of post-transplant vascular involvement that is also related to IgA aB2GPI antibodies. This situation could be equivalent to reversible mild thrombotic microangiopathy.54 In this regard, future research needs to be carried out.
It is noteworthy that most of the patients who received a retransplant were in group 2 (34, 92%). We have found that patients with pretransplant antibodies become negative immediately after transplant and that most patients remain negative, even if the graft is lost and they return to dialysis.50 This observation is concomitant with the fact that most of the patients positive for IgA aB2GPI are first transplants.
The most important limitation of our study is the small number of patients included; also, their enrolment was limited to a single center. Therefore, the resulting number of post-transplant thrombosis is small (this is the real situation in every center), and therefore, it was not possible to perform a multivariate analysis for this complication. Also, by the nature of this study, we did not routinely perform a pretransplant study of hypercoagulability including prothrombin mutations, activated protein C resistance testing, antithrombin activity testing, and protein C and protein S activity testing. Despite this result, for the first time, we have shown that the presence of pretransplant IgA aB2GPI antibodies is an independent risk factor for early graft loss, mainly caused by thrombosis, and DGF after renal transplantation. Identification of risk factors for graft thrombosis may reduce early renal graft loss.55 If our findings are corroborated, anticoagulation early after transplantation might be recommended in patients positive for IgA aB2GPI antibodies. Prospective and multicenter studies with more patients to show the usefulness of IgA aB2GPI antibodies as an early transplant failure biomarker are mandatory.
Concise Methods
Study Design
We performed a historical cohort study that included all patients who had received a kidney transplant in a 3-year period (from January 1, 2000, to December 31, 2002) in the Hospital 12 de Octubre Hospital (Madrid, Spain) with a 10-year prospective follow-up. These patients were also included in the Spanish Forum Renal Group cohort with 2600 patients transplanted in 14 hospitals in Spain.3,5 In 2013, IgA aB2GPI antibodies were retrospectively examined in pretransplant serum samples.
The first aim was to investigate the possible influence of IgA aB2GPI antibodies on early outcomes after renal transplantation (6 months). Main end points were graft loss, causes of graft loss, and graft survival at 6 months.
The secondary aim was to investigate the long-term clinical outcomes in patients positive versus negative for IgA aB2GPI antibodies (7–120 months). Secondary end points were graft and patient survival at 10 years, causes of graft loss, and death.
The study was approved by the Hospital 12 de Octubre Institutional Review Board.
Patients
All of the 329 patients who received a kidney transplant from January 1, 2000 to December 31, 2002 were recruited for the study. All donors were heart-beating (brain death). Sixty patients were excluded. Forty-one patients were excluded, because pretransplant serum samples (1–15 days before transplantation) were unavailable; 19 patients were excluded, because they had also received a second organ (combined liver–kidney, pancreas–kidney, or heart–kidney). All patients were negative for Factor V Leiden (disposition algorithm and outcomes) (Figure 1). There were no significant differences between the clinical characteristics of 60 excluded patients and 269 patients who were finally analyzed (Supplemental Table 1). Of note is that no patient was diagnosed with hemolytic uremic syndrome as an original disease. Demographic data of 269 patients studied were 266 Caucasian, 1 Asiatic, and 2 East African. All of the 269 patients included were prospectively followed up for a period of up to 10 years or until graft failure or death.
Database
The donor and recipient characteristics in our database include age, original disease, time on dialysis, serology, immunologic data, and pretransplant cardiovascular condition. In this way, body mass index, arterial hypertension, hyperlipidemia, diabetes, smoking, and pretransplant cardiovascular disease were specifically recorded. Immunosuppressive treatment at the time of transplantation was also recorded.
The following data were recorded after surgery: incidence of thrombotic and cardiovascular events, neoplasia, ARs, and DGF. Patient and graft survival and causes of graft loss and mortality were also recorded.
Immunosuppressive Treatment
The most frequently used immunosuppressive protocol was on the basis of TAC associated with steroids and MMF with or without induction. In patients older than 60 years who received kidneys from older than 60 years donors, immunosuppression regimen was on the basis of CsA, steroids, and MMF with or without induction.3,5,32
Induction Treatment
In hyperimmunized patients, Thymoglobulin, (rabbit antithymocyte globulin, 1.5 mg/kg for 4–7 days), steroids, TAC (0.2 mg/kg per day), and MMF (1 g/d) were used. In elderly people (over 60 years of age), Basiliximab (mAbs anti–IL-2R, 20 mg days 0 and 4) with steroids, CsA (10 mg/kg per day), and MMF (2 g/d) were administered.
Definitions
Thrombotic events are defined as venous thrombosis, arterial thrombosis, graft thrombosis, acute stroke, transient ischemic attack, pulmonary thromboembolism, deep venous thrombosis, or acute arterial thrombotic episodes in lower limbs diagnosed by images techniques or histologic study.17
Primary nonfunction is considered to be a graft having good perfusion that never functioned in which a biopsy was performed to exclude other causes, such as rejection.
DGF is defined as initial graft nonfunction that requires hemodialysis during the first 1 week after surgery after accelerated or hyper-AR, vascular complications, and urinary tract obstruction were ruled out.
Cardiovascular event is considered as existing if at least one of the following events was present: myocardial infarction, angina, coronary revascularization, stroke, heart failure, or peripheral bypass.
AR is defined as acute deterioration in allograft function associated with specific histopathologic changes in the graft. Clinically suspected AR was applied to patients with clinical criteria that suggested AR but without histologic data that confirmed AR.
Laboratory Determinations
aCL and aB2GPI antibodies were measured in the pretransplant serum used for crossmatch or a serum sample obtained up to 15 days before transplantation.
Autoantibodies were quantified by ELISAs. The QUANTA Lite Kit (INOVA Diagnostics Inc., San Diego, CA) was used to determine IgG aCL, IgM aCL, IgA aCL, IgG aB2GPI, IgM aB2GPI, and IgA aB2GPI antibodies. Using the manufacturer's guidelines, antibody levels >20 units/ml were considered positive. The manufacturer’s cutoff was perfectly comparable with that observed in the Spanish population.19
Crossmatching between recipient (serum) and donor (lymphocyte suspension) was performed by cytolysis mediated by a specific antibody in the presence of complement.56 Patients were transplanted only if the crossmatch was negative.
Panel reactive antibody (PRA) score represents the proportion of the population to which the person being tested will react through preexisting antibodies. PRA was performed with a pooled lymphocyte panel prepared from at least 35 unrelated, nonselected genotypes and studied by complement-dependent cytotoxicity. Individuals with PRA value≥50% were considered as sensitized.
Statistical Methods
Results are expressed as mean±SEM or absolute frequency and percentage. Association between qualitative variables was determined with Pearson chi-squared test or Fisher exact test when appropriate. Comparisons were performed using the t test in scaled variables with two categories.
Survival was calculated using the Kaplan–Meier method, and differences between the distributions of survival were assessed with the log-rank test. Graft loss risk was also estimated by multivariate analysis using the Cox regression model. HRs with probabilities <0.05 were considered significant. Multivariate analysis of dichotomous outcome was performed using logistic regression model. ORs with probabilities <0.05 were considered significant.
Data were processed and analyzed using the STATA 11 statistical program (StataCorp., College Station, TX) and MedCalc for Windows, version 13.1 (MedCalc Software, Ostend, Belgium).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Margarita Sevilla and Pilar Suarez for excellent technical assistance and Barbara Shapiro for excellent work of translation and English revision of the paper.
This study was funded by Fundación Mutua Madrileña Grant 2008-090 and Fondo de Investigaciones Sanitarias Grants PS09-02023 and PI12-00108.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014030228/-/DCSupplemental.
References
- 1.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF, ELITE-Symphony Study : Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, Green MD, Jha V, Josephson MA, Kiberd BA, Kreis HA, McDonald RA, Newmann JM, Obrador GT, Vincenti FG, Cheung M, Earley A, Raman G, Abariga S, Wagner M, Balk EM, Kidney Disease: Improving Global Outcomes : KDIGO clinical practice guideline for the care of kidney transplant recipients: A summary. Kidney Int 77: 299–311, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Morales JM, Marcén R, del Castillo D, Andres A, Gonzalez-Molina M, Oppenheimer F, Serón D, Gil-Vernet S, Lampreave I, Gainza FJ, Valdés F, Cabello M, Anaya F, Escuin F, Arias M, Pallardó L, Bustamante J: Risk factors for graft loss and mortality after renal transplantation according to recipient age: A prospective multicentre study. Nephrol Dial Transplant 27[Suppl 4]: iv39–iv46, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponticelli C, Moia M, Montagnino G: Renal allograft thrombosis. Nephrol Dial Transplant 24: 1388–1393, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Morales JM, Marcén R, Andrés A, Molina MG, Castillo DD, Cabello M, Capdevila L, Campistol JM, Oppenheimer F, Serón D, Vernet SG, Lampreave I, Valdés F, Anaya F, Escuín F, Arias M, Pallardó L, Bustamante J: Renal transplantation in the modern immunosuppressive era in Spain: Four-year results from a multicenter database focus on post-transplant cardiovascular disease. Kidney Int Suppl 11: S94–S99, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Terasaki PI, Kreisler M, Mickey RM: Presensitization and kidney transplant failures. Postgrad Med J 47: 89–100, 1971 [PMC free article] [PubMed] [Google Scholar]
- 7.Dragun D, Philippe A, Catar R: Role of non-HLA antibodies in organ transplantation. Curr Opin Organ Transplant 17: 440–445, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Wood KJ, Goto R: Mechanisms of rejection: Current perspectives. Transplantation 93: 1–10, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Nankivell BJ, Alexander SI: Rejection of the kidney allograft. N Engl J Med 363: 1451–1462, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Triplett DA: Antiphospholipid antibodies. Arch Pathol Lab Med 126: 1424–1429, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Fischer MJ, Rauch J, Levine JS: The antiphospholipid syndrome. Semin Nephrol 27: 35–46, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Groot PG, Urbanus RT: The significance of autoantibodies against β2-glycoprotein I. Blood 120: 266–274, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Ragusa MA, Costa S, Cefalù AB, Noto D, Fayer F, Travali S, Averna MR, Gianguzza F: RT-PCR and in situ hybridization analysis of apolipoprotein H expression in rat normal tissues. Int J Mol Med 18: 449–455, 2006 [PubMed] [Google Scholar]
- 14.Pierangeli SS, Chen PP, Raschi E, Scurati S, Grossi C, Borghi MO, Palomo I, Harris EN, Meroni PL: Antiphospholipid antibodies and the antiphospholipid syndrome: Pathogenic mechanisms. Semin Thromb Hemost 34: 236–250, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Moestrup SK, Schousboe I, Jacobsen C, Leheste JR, Christensen EI, Willnow TE: Beta2-glycoprotein-I (apolipoprotein H) and beta2-glycoprotein-I-phospholipid complex harbor a recognition site for the endocytic receptor megalin. J Clin Invest 102: 902–909, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klaerke DA, Røjkjaer R, Christensen L, Schousboe I: Identification of beta2-glycoprotein I as a membrane-associated protein in kidney: Purification by calmodulin affinity chromatography. Biochim Biophys Acta 1339: 203–216, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA: International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 4: 295–306, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Murthy V, Willis R, Romay-Penabad Z, Ruiz-Limon P, Martinez-Martinez LA, Jatwani S, Jajoria P, Seif A, Alarcon GS, Papalardo E, Liu J, Vila LM, McGwin G, Jr., McNearney TA, Maganti R, Sunkureddi P, Parekh T, Tarantino M, Akhter E, Fang H, Gonzalez EB, Binder W, Norman G, Teodorescu M, Reveille JD, Petri M, Pierangeli SS: Value of isolated IgA anti-β-glycoprotein I positivity in the diagnosis of the antiphospholipid syndrome. Arthritis Rheum 65: 3186–3193, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-García R, Serrano M, Martínez-Flores JÁ, Mora S, Morillas L, Martín-Mola MA, Morales JM, Paz-Artal E, Serrano A: Isolated IgA anti-β2 glycoprotein I antibodies in patients with clinical criteria for antiphospholipid syndrome. J Immunol Res 2014: 704395, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Flores JA, Serrano M, Alfaro J, Mora S, Paz-Artal E, Morales JM, Serrano A: Heterogeneity between diagnostic tests for IgA anti-beta2 glycoprotein I: Explaining the controversy in studies of association with vascular pathology. Anal Chem 85: 12093–12098, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Uthman I, Khamashta M: Antiphospholipid syndrome and the kidneys. Semin Arthritis Rheum 35: 360–367, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Grönhagen-Riska C, Teppo AM, Helanterä A, Honkanen E, Julkunen H: Raised concentrations of antibodies to cardiolipin in patients receiving dialysis. BMJ 300: 1696–1697, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunet P, Aillaud MF, San Marco M, Philip-Joet C, Dussol B, Bernard D, Juhan-Vague I, Berland Y: Antiphospholipids in hemodialysis patients: relationship between lupus anticoagulant and thrombosis. Kidney Int 48: 794–800, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Serrano A, García F, Serrano M, Ramírez E, Alfaro FJ, Lora D, de la Cámara AG, Paz-Artal E, Praga M, Morales JM: IgA antibodies against β2 glycoprotein I in hemodialysis patients are an independent risk factor for mortality. Kidney Int 81: 1239–1244, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Hadhri S, Rejeb MB, Belarbia A, Achour A, Skouri H: Hemodialysis duration, human platelet antigen HPA-3 and IgA isotype of anti-β2glycoprotein I antibodies are associated with native arteriovenous fistula failure in Tunisian hemodialysis patients. Thromb Res 131: e202–e209, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Perico N, Cattaneo D, Sayegh MH, Remuzzi G: Delayed graft function in kidney transplantation. Lancet 364: 1814–1827, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Phelan PJ, O’Kelly P, Tarazi M, Tarazi N, Salehmohamed MR, Little DM, Magee C, Conlon PJ: Renal allograft loss in the first post-operative month: Causes and consequences. Clin Transplant 26: 544–549, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Cole EH, Johnston O, Rose CL, Gill JS: Impact of acute rejection and new-onset diabetes on long-term transplant graft and patient survival. Clin J Am Soc Nephrol 3: 814–821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, Cosio FG: Identifying specific causes of kidney allograft loss. Am J Transplant 9: 527–535, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Erkan D, Yazici Y, Peterson MG, Sammaritano L, Lockshin MD: A cross-sectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatology (Oxford) 41: 924–929, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Giessing M, Budde K, Fritsche L, Slowinski T, Tuerk I, Schoenberger B, Neumayer HH, Loening SA: “Old-for-old” cadaveric renal transplantation: Surgical findings, perioperative complications and outcome. Eur Urol 44: 701–708, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Andrés A, Morales JM, Herrero JC, Praga M, Morales E, Hernández E, Ortuño T, Rodício JL, Martínez MA, Usera G, Díaz R, Polo G, Aguirre F, Leiva O: Double versus single renal allografts from aged donors. Transplantation 69: 2060–2066, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Dinavahi R, George A, Tretin A, Akalin E, Ames S, Bromberg JS, Deboccardo G, Dipaola N, Lerner SM, Mehrotra A, Murphy BT, Nadasdy T, Paz-Artal E, Salomon DR, Schröppel B, Sehgal V, Sachidanandam R, Heeger PS: Antibodies reactive to non-HLA antigens in transplant glomerulopathy. J Am Soc Nephrol 22: 1168–1178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahdi BM: A glow of HLA typing in organ transplantation. Clin Transl Med 2: 6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tinckam KJ, Chandraker A: Mechanisms and role of HLA and non-HLA alloantibodies. Clin J Am Soc Nephrol 1: 404–414, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Win TS, Pettigrew GJ: Humoral autoimmunity and transplant vasculopathy: When allo is not enough. Transplantation 90: 113–120, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa Y, Saito K, Morioka T, Tomita Y, Takahashi K, Oite T: The clinical significance of antibody to vascular endothelial cells after renal transplantation. Clin Transplant 16[Suppl 8]: 51–57, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Pober JS, Orosz CG, Rose ML, Savage CO: Can graft endothelial cells initiate a host anti-graft immune response? Transplantation 61: 343–349, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Sumitran-Karuppan S, Tyden G, Reinholt F, Berg U, Moller E: Hyperacute rejections of two consecutive renal allografts and early loss of the third transplant caused by non-HLA antibodies specific for endothelial cells. Transpl Immunol 5: 321–327, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Sun Q, Liu Z, Chen J, Chen H, Wen J, Cheng D, Li L: Circulating anti-endothelial cell antibodies are associated with poor outcome in renal allograft recipients with acute rejection. Clin J Am Soc Nephrol 3: 1479–1486, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunoyer-Geindre S, Kruithof EK, Galve-de Rochemonteix B, Rosnoblet C, Gruenberg J, Reber G, de Moerloose P: Localization of beta2-glycoprotein 1 in late endosomes of human endothelial cells. Thromb Haemost 85: 903–907, 2001 [PubMed] [Google Scholar]
- 42.Kornberg A, Renaudineau Y, Blank M, Youinou P, Shoenfeld Y: Anti-beta 2-glycoprotein I antibodies and anti-endothelial cell antibodies induce tissue factor in endothelial cells. Isr Med Assoc J 2[Suppl]: 27–31, 2000 [PubMed] [Google Scholar]
- 43.Pierangeli SS, Harris EN: Probing antiphospholipid-mediated thrombosis: The interplay between anticardiolipin antibodies and endothelial cells. Lupus 12: 539–545, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Brandt KJ, Kruithof EK, de Moerloose P: Receptors involved in cell activation by antiphospholipid antibodies. Thromb Res 132: 408–413, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Meroni PL, Borghi MO, Raschi E, Tedesco F: Pathogenesis of antiphospholipid syndrome: Understanding the antibodies. Nat Rev Rheumatol 7: 330–339, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Shoenfeld Y, Blank M, Cervera R, Font J, Raschi E, Meroni PL: Infectious origin of the antiphospholipid syndrome. Ann Rheum Dis 65: 2–6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.George J, Harats D, Gilburd B, Afek A, Levy Y, Schneiderman J, Barshack I, Kopolovic J, Shoenfeld Y: Immunolocalization of beta2-glycoprotein I (apolipoprotein H) to human atherosclerotic plaques: Potential implications for lesion progression. Circulation 99: 2227–2230, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Willis R, Harris EN, Pierangeli SS: Pathogenesis of the antiphospholipid syndrome. Semin Thromb Hemost 38: 305–321, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Cruz-Tapias P, Blank M, Anaya JM, Shoenfeld Y: Infections and vaccines in the etiology of antiphospholipid syndrome. Curr Opin Rheumatol 24: 389–393, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Serrano M, Martínez-Flores JA, Castro MJ, García F, Lora D, Perez D, Gonzalez E, Paz-Artal E, Morales JM, Serrano A: Renal transplantation dramatically reduces IgA anti-beta-2-glycoprotein I antibodies in patients with endstage renal disease. J Immunol Res 2014: 641962, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagenknecht DR, Becker DG, LeFor WM, McIntyre JA: Antiphospholipid antibodies are a risk factor for early renal allograft failure. Transplantation 68: 241–246, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Forman JP, Lin J, Pascual M, Denton MD, Tolkoff-Rubin N: Significance of anticardiolipin antibodies on short and long term allograft survival and function following kidney transplantation. Am J Transplant 4: 1786–1791, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Fernández-Fresnedo G, López-Hoyos M, Segundo DS, Crespo J, Ruiz JC, De Francisco AL, Arias M: Clinical significance of antiphospholipid antibodies on allograft and patient outcome after kidney transplantation. Transplant Proc 37: 3710–3711, 2005 [DOI] [PubMed] [Google Scholar]
- 54.De Serres SA, Isenring P: Renal thrombotic microangiopathy revisited: When a lesion is not a clinical finding. Saudi J Kidney Dis Transpl 21: 411–416, 2010 [PubMed] [Google Scholar]
- 55.Keller AK, Jorgensen TM, Jespersen B: Identification of risk factors for vascular thrombosis may reduce early renal graft loss: A review of recent literature. J Transplant 2012: 793461, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel R, Terasaki PI: Significance of the positive crossmatch test in kidney transplantation. N Engl J Med 280: 735–739, 1969 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.