Abstract
The planar cell polarity (PCP) signaling pathway is crucial for tissue morphogenesis. Van Gogh-like protein 2 (Vangl2) is central in the PCP pathway; in mice, Vangl2 loss is embryonically lethal because of neural tube defects, and mutations in Vangl2 are associated with human neural tube defects. In the kidney, PCP signaling may be important for tubular morphogenesis and organization of glomerular epithelial cells (podocytes) along the glomerular basement membrane. Podocyte cell protrusions (foot processes) are critical for glomerular permselectivity; loss of foot process architecture results in proteinuria and FSGS. Previously, we showed a profound effect of PCP signaling on podocyte shape, actin rearrangement, cell motility, and nephrin endocytosis. To test our hypothesis that the PCP pathway is involved in glomerular development and function and circumvent lethality of the ubiquitous Vangl2 mutation in the Looptail mouse, we generated a mouse model with a podocyte-specific ablation of the Vangl2 gene. We report here that podocyte-specific deletion of Vangl2 leads to glomerular maturation defects in fetal kidneys. In adult mice, we detected significantly smaller glomeruli, but it did not affect glomerular permselectivity in aging animals. However, in the context of glomerular injury induced by injection of antiglomerular basement membrane antibody, deletion of Vangl2 resulted in exacerbation of injury and accelerated progression to chronic segmental and global glomerular sclerosis. Our results indicate that Vangl2 function in podocytes is important for glomerular development and protects against glomerular injury in adult animals.
Keywords: glomerular disease, kidney development, genetics and development
Renal glomerular visceral epithelial cells (podocytes) are highly polarized cells with complex three-dimensional architecture characterized by the presence of unique actin-based projections (foot processes [FPs]).1 The FPs from neighboring podocytes form intercellular bridges, slit diaphragms, that are the only points of contact between adjacent cells and the base for the permselective filtration barrier, allowing small solutes to pass into the urinary space while retaining large proteins in the blood.1 Slit diaphragm protein complexes are connected with the podocyte cytoskeleton by various adaptor proteins and serve as a signaling nexus to relay information from the FP surface to control podocyte structure.2 The characteristic shape of podocytes is directly related to glomerular filtration. Mutations in proteins that link slit diaphragms and the cytoskeleton (e.g., α-actinin-4 and CD2-associated protein)3,4 or regulators of actin polymerization and organization (e.g., Inverted Formin-2, Myosin 1e, Rho GDP-Dissociation Inhibitor 1, and Cdc42)5–9 give rise to glomerular dysfunction, such as proteinuria, FP effacement, and progressive loss of GFR, which is often associated with FSGS.
The renal glomerulus arises at the most proximal part of the nephron through a complex sequence of molecular and morphologic transformations.10 The early nephron structures (comma- and S-shaped bodies) are made of closely packed cuboidal epithelial cells connected by tight junctions localized at the cell apex. Committed podocyte progenitors appear at the late S-shape body stage and are recognizable by the expression of Wilms’ Tumor 1 protein (WT1).11 As podocyte progenitors differentiate (late S-shape and early capillary loop stages), the tight junctions descend toward the basolateral aspect of the cell and are remodeled into a unique junction type (the slit diaphragm).12 Although slit diaphragms still express some classic tight junction markers (e.g., ZO-1 and Par3), they begin to express unique components, such as nephrin, podocin, and synaptopodin.12 The descent and remodeling of tight junctions coincide with the onset of primary and secondary FP extensions. Mature podocytes have a characteristically expanded apical surface,12,13 whereas FPs extending beneath the slit diaphragms comprise the basolateral surface.13 Interestingly, podocyte FPs interdigitate in a precisely alternating manner: an FP from one podocyte is linked only to FPs from a neighboring podocyte but never with the FPs originating from the same cell. The interdigitated FPs are arranged along the glomerular capillary and basement membrane in a direction orthogonal to their apicobasolateral polarity. The mechanisms that control such precise FP arrangement are presently unknown.
In many epithelial tissues, the axis of polarity orthogonal to apicobasolateral polarity is controlled by the planar cell polarity (PCP) pathway.14 The PCP pathway is known to govern various processes from collective cell migration to convergent extension and oriented cell division during vertebrate tissue morphogenesis.15 Mouse mutations in several PCP genes (including Van Gogh-like protein 2 [Vangl2]16 and others) cause severe congenital defects in many organs, including heart, lung, and ear; the most notable among them, a severe neural tube defect, craniorachischisis16–19; these mice die in utero around E17.5–E18.5 (E, embryonic day). Mutations in Vangl2 and other PCP genes have been associated with neural tube defects in humans.20,21
Detailed analysis of the Vangl2 mutant Looptail (Lp) mouse in our laboratory and by others revealed profound defects in kidney branching morphogenesis and glomerular maturation.22,23 With respect to the latter, glomeruli in Lp homozygous embryos display a significant developmental delay seen as a predominance of early capillary loop-stage glomeruli compared with the more mature glomerular stages seen in control embryos.22,23 We also observed significant changes in cell shape, cytoskeletal arrangement, motility, and nephrin endocytosis in cultured podocytes stimulated with a noncanonical PCP Wnt ligand, Wnt5a, or when endogenous Vangl2 was depleted by shRNAs.23,24 Together, these observations point to the importance of the PCP pathway in podocyte development. However, because of embryonic lethality of PCP mouse mutants, the question of whether PCP components are required for glomerular maintenance and function in adult animals remains unresolved.
In this study, we report the generation of a novel transgenic mouse in which Vangl2 expression is ablated in podocytes, leading to glomerular developmental defects. In adult mutant mice, glomeruli are substantially smaller and have fewer podocytes than in control animals, although basal kidney function remains normal. However, in the context of glomerular injury, animals lacking Vangl2 in podocytes have a heightened susceptibility to glomerular insult and a faster progression to the chronic segmental and global glomerular sclerosis reminiscent of FSGS. Taken together, our studies have uncovered a new role for the PCP pathway in glomerular development and the postinjury maintenance of glomerular function.
Results
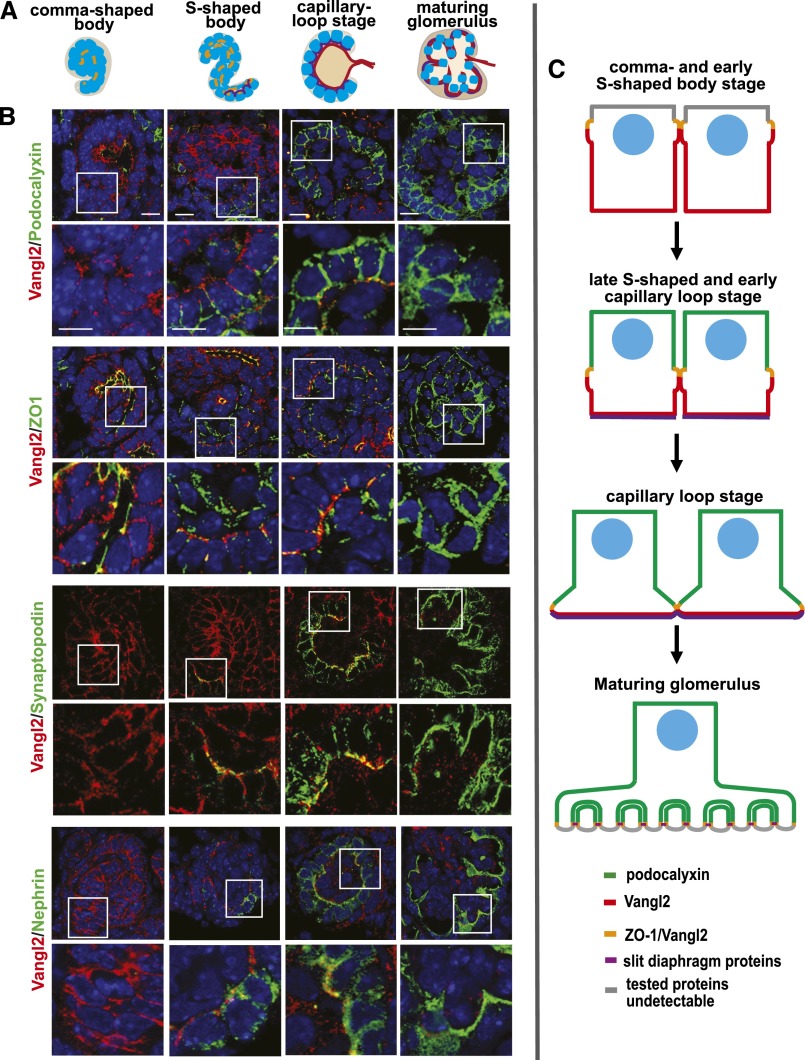
PCP Protein Vangl2 Is Dynamically Expressed during Glomerular Development
We previously reported that Vangl2 is expressed in developing podocytes.23 To further detail how its expression relates to the expression of other proteins during podocyte development, we performed double immunostaining with anti-Vangl2 antibody23,25 and antibodies against various podocyte markers. In E17.5 mouse embryonic kidneys, Vangl2 is highly expressed in the epithelial cells of comma- and S-shaped bodies at basolateral aspects of the cell membrane (Figure 1, Supplemental Figure 3). Podocalyxin (an apical plasma membrane marker) does not overlap with Vangl2, confirming basolateral expression of Vangl2. We detected a strong overlap between Vangl2 and tight junction marker ZO-1 (Figure 1B, yellow). This finding corroborates earlier observations that Vangl2 is tightly linked to the tight junction protein complex.26 As podocytes continue to develop, the apical surface expands, the basolateral surface contracts, and ZO-1–positive junctional complexes descend along the lateral surface as they are remodeled into slit diaphragms (Figure 1, S-shape and capillary loop stages). Accordingly, at the capillary loop stage, Vangl2 expression is confined to the basal aspect of developing podocytes; strong basal Vangl2 expression coincides with the onset of FP formation in the podocyte basal domain. Podocyte-specific markers synaptopodin and nephrin are expressed along the basolateral surface of podocyte progenitors in the late S-shaped body, where they overlap with Vangl2 (Figure 1B). In mature glomeruli, Vangl2 expression is significantly diminished and cannot be detected by immunofluorescence; Vangl2 mRNA is detectable by RT-PCR in glomerular lysates of adult animals.24
Figure 1.
Vangl2 is dynamically expressed in the developing glomerulus. (A) Schematic depiction of glomerular developmental stages. (B) Coimmunofluorescence staining of E17.5 C57/Bl6 wild-type kidney sections with anti-Vangl2 antibody (red) and antibodies against various podocyte markers: podocalyxin (marker of apical membrane; green), ZO-1 (marker of tight junctions; green), synaptopodin (marker of differentiating podocytes; green), and nephrin (slit diaphragm marker; green). Scale bars, 5 μm. (C) Schematic summary of Vangl2 expression during glomerular development.
Generation of a Podocyte-Specific Vangl2 Transgenic Mouse
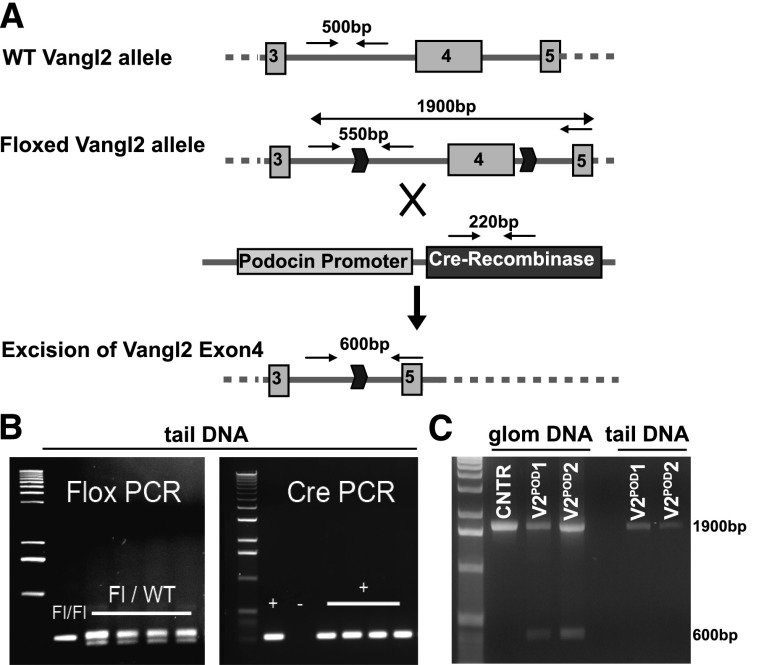
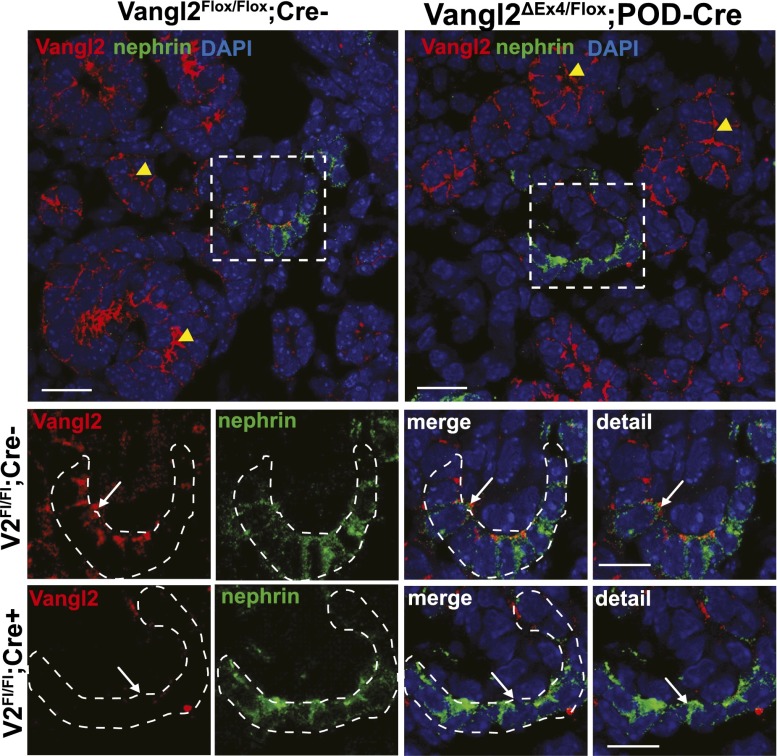
The kidneys in the Lp homozygous embryos are dysplastic (Supplemental Figure 1A)23 and display glomerular maturation defects,23 raising the possibility that abnormal tubules might have contributed to the glomerular defects seen in Lp mice. To study the role of Vangl2 specifically in the glomerulus and circumvent embryonic lethality of Lp mice, we used a novel conditional Vangl2-Floxed mouse model, in which the Floxed allele (Vangl2Flox/Flox) was either homozygous or combined with a null Vangl2 allele (ubiquitous Flox-mediated excision of Exon4, Vangl2ΔEx4/Flox) (E. Torban and P. Gros, unpublished data). These mice were mated with a heterozygous Podocin-Cre mouse27 to generate Vangl2ΔEx4/Flox;Pod-Cre (used in all functional and glomerular maturation studies) or Vangl2Flox/Flox;Pod-Cre (used for confirmation of Vangl2 excision) and Vangl2Flox/Flox;Cre− (used as controls). Both the Cre allele and the targeted Floxed allele (which has an insertion of an extra 50 bp compared with the wild-type allele) are detectable in tail-extracted DNA samples (Figure 2, A and B). Podocin promoter-driven Cre-mediated excision results in Vangl2 ablation in podocytes: excision of Exon4 in the Vangl2Flox/Flox;Pod-Cre mice was detected in the DNA isolated from sieved glomerular preparations (Figure 2C, lanes V2POD1 and V2POD2) but not in the control Vangl2Flox/Flox;Cre– animals or tail DNA from the same Vangl2Flox/Flox;Pod-Cre animals (Figure 2C, lane tail DNA). By immunofluorescence microscopy, we detected Vangl2 protein at the capillary loop-stage glomerulus in sections costained for nephrin (Figure 3, white arrows) in Vangl2Flox/Flox;Cre– animals. However, in Vangl2Flox/Flox;Pod-Cre tissues, Vangl2 protein expression could not be detected in developing podocytes (Figure 3, white arrows) but was clearly seen in the juxtaposed tubular structures (Figure 3, yellow arrowheads). Together, these results confirmed successful excision of Vangl2 specifically in developing podocytes.
Figure 2.
Generation of podocyte-specific Vangl2 knockout mouse. (A) Strategy to excise Vangl2 in podocytes by using the Podocin-Cre deleter mouse. (B) Representative gel images of PCR reactions detecting (left panel) a floxed Vangl2 allele and (right panel) a Cre-positive allele. (C) Excision of Vangl2 Exon4 in podocytes generates a 600-bp band detectable only in glomerular lysates of podocyte-specific Vangl2Flox/Flox;Pod-Cre animals (V2-POD-1 and V2-POD-2) and not in Vangl2Flox/Flox;Cre– controls (CNTRs); only the Exon4-containing 1900-bp PCR product can be detected in tail DNA of the same podocyte-specific Vangl2Flox/Flox;Pod-Cre knockout animals. Please note that glomerular lysates may be contaminated by cells from adjacent proximal tubules (expressing high levels of Vangl2). Thus, the 1900-bp PCR product in glomerular lysates may be derived from the contaminants or caused by an incomplete excision in some glomeruli. Glom, glomerular; WT, wild type.
Figure 3.
Verification of Vangl2 excision in developing podocytes by immunofluorescence microscopy. (Upper panel) Low-magnification images of control (Vangl2flox/flox;Cre–) and knockout (Vangl2flox/flox;Pod-Cre) E17.5 kidney sections costained with anti-Vangl2 antibody (red) and antinephrin antibody (green). Yellow arrowheads point to the tubular structures positive for Vangl2 protein in both control Vangl2flox/flox;Cre– and knockout Vangl2flox/flox;Pod-Cre mice. DAPI, 4',6-diamidino-2-phenylindole. (Lower panel) High-magnification images of the capillary loop stage glomerulus (designated by an intermittent white line) in control Vangl2flox/flox;Cre– and knockout Vangl2flox/flox;Pod-Cre kidneys. Note the absence of a Vangl2-positive signal (white arrows) in the glomerulus of the Vangl2flox/flox;Pod-Cre sections, confirming successful excision of the Vangl2 gene. Scale bars,10 μm in upper panel; 5 μm in lower panel.
Effect of Podocyte-Specific Ablation of Vangl2 Function on Glomerular Development
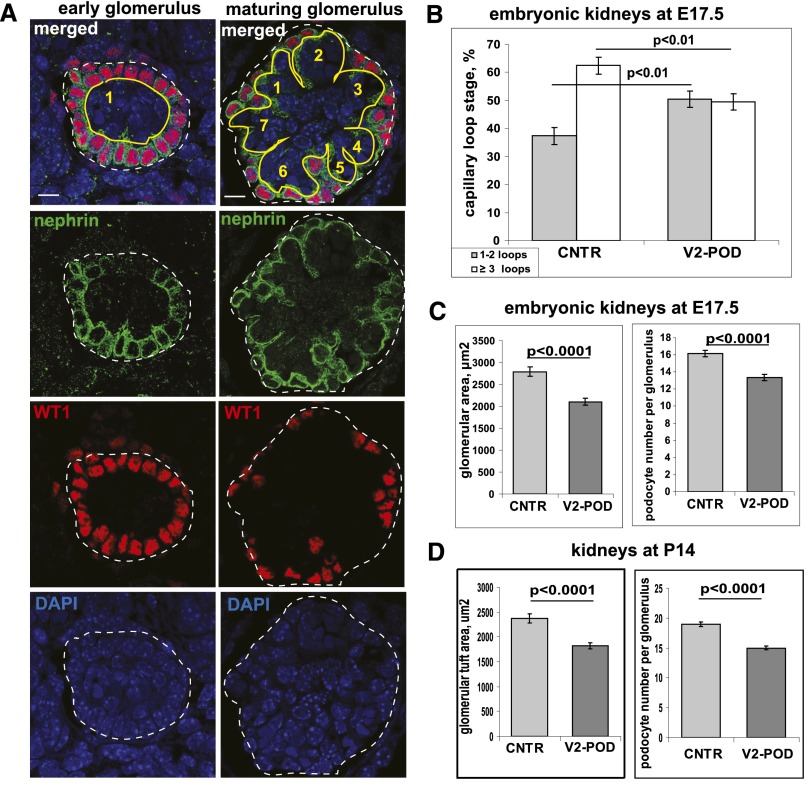
We quantified immature (one to two capillary loop stage) and more mature (three or more loops) glomeruli in Vangl2ΔEx4/Flox;Pod-Cre and Vangl2Flox/Flox;Cre– E17.5 embryos (Figure 4A). Although only 37.4%±3.0% of Vangl2Flox/Flox;Cre– glomeruli displayed early capillary loop morphology, we observed 50.5%±2.9% of immature glomeruli in mutant littermates (Figure 4B). This statistically significant shift in the immature versus more mature glomeruli ratio was similar to the glomerular phenotypes in E17.5 Lp mice studied by the same staining method23 or when hematoxylin and eosin-stained Lp tissues were examined by light microscopy (Supplemental Figure 1C); we detected no histologic abnormalities in the tubular interstitium of Vangl2ΔEx4/Flox;Pod-Cre mice (Supplemental Figure 4A). The Lp glomeruli were smaller than in wild-type animals (Supplemental Figure 1, B–D). Similarly, in Vangl2ΔEx4/Flox;Pod-Cre embryos, glomerular size was smaller (2106±76 μm2) compared with that in controls (2788±106 μm2; P<0.001) (Figure 4C). The average number of podocytes per glomerulus was also significantly less (13±0.4) in Vangl2ΔEx4/Flox;Pod-Cre glomeruli versus controls (16±0.4; P<0.001).
Figure 4.
Quantification of glomerular maturation. (A) Representative images of early and maturing glomeruli coimmunostained with anti-WT1 antibody (red), antinephrin antibody (green), and 4′,6-diamidino-2-phenylindole (DAPI; blue). Nephrin staining was used to count the number of capillary loops in each glomerulus. Scale bars, 5 μm. (B) Statistical analysis of glomerular maturation in control Vangl2flox/flox;Cre– (CNTR) and podocyte-specific Vangl2Flox/ΔEx4;Pod-Cre (V2-POD) transgenic mice; over 1000 glomeruli from three embryos per genotype were examined. A chi-squared statistical test was used. (C) Quantification of the glomerular tuft area and average number of podocytes per glomerulus in control and V2-POD E17.5 embryos. Three embryos per genotype were examined. In total, n=127 control and n=177 mutant glomeruli were examined; t test was used. (D) Quantification of the glomerular tuft area and average number of podocyte per glomerulus in control and V2-POD P14 animals. Three animals per genotype were examined. In total, n=100 control and n=114 mutant glomeruli were analyzed; t test was used. In all graphs, means and SEMs are shown.
In rodents, nephrogenesis continues for up to 2 weeks of postnatal life. Our viable conditional Vangl2ΔEx4/Flox;Pod-Cre mice allowed us to examine (as above) whether the developmental delay seen in Lp and Vangl2ΔEx4/Flox;Pod-Cre still persists at the end of kidney development. Our analysis revealed that all glomeruli in mutant and control P14 mouse kidneys were mature and that there was no significant difference in glomerular maturity between animals of both genotypes. However, the glomerular tuft area in the P14 (P, postnatal day) Vangl2ΔEx4/Flox;Pod-Cre littermates was significantly smaller (1819±62 μm2) compared with the control Vangl2Flox/Flox;Cre– animals (2375±92 μm2). The average podocyte number per glomerulus was also significantly different: 15±0.3 in Vangl2ΔEx4/Flox;Pod-Cre versus 19±0.4 in controls (P<0.001) (Figure 4D).
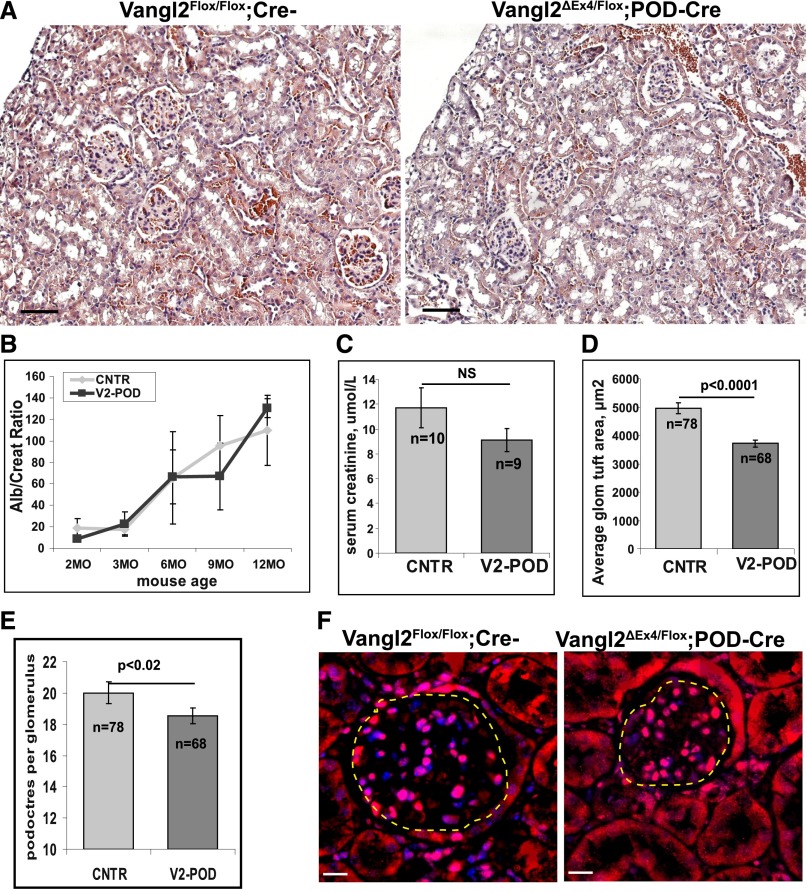
Deletion of Vangl2 function in adult mice does not affect glomerular permselective function during the first 12 months: neither urine albumin-to-creatinine ratio (UACR) (Figure 5B) nor serum creatinine level was significantly different between the control and mutant 1-year-old animals (Figure 5C). However, histologic analysis revealed a significant difference in the average glomerular tuft area in Vangl2ΔEx4/Flox;Pod-Cre mice (3718±129 μm2) versus controls (4956±187 μm2; P<0.001) (Figure 5, A and D); the average number of podocytes per glomerulus in 1-year-old mice was also modestly but significantly different: 18±0.5 in Vangl2ΔEx4/Flox;Pod-Cre and 20±0.7 in control Vangl2Flox/Flox;Cre– animals (P=0.02) (Figure 5, E and F).
Figure 5.
Kidney morphology and glomerular function in adult Vangl2 conditional knockout mice. (A) Representative images of 1-year-old (left panel) Vangl2flox/flox;Cre–and (right panel) Vangl2ΔEx4/Flox;Pod-Cre kidneys stained with hematoxylin and eosin. Scale bar, 30 μm. (B) UACR (Alb/Creat; micrograms per gram) in adult male control (CNTR) versus Vangl2ΔEx4/Flox;Pod-Cre (V2-POD) animals at 2 months (n=4 and n=6, respectively), 3 months (n=4 and n=6, respectively), 6 months (n=4 and n=6, respectively), 9 months (n=18 and n=12, respectively), and 1 year of age (n=14 and n=16, respectively). No significant differences were observed at any time point. (C) Serum creatinine measurements in 1-year-old mice (n = the number of animals per genotype). (D and E) Quantification of the glomerular tuft area and average number of podocytes per glomerulus in the 1-year-old control (n=78 glomeruli from three animals) and V2-POD (n=66 glomeruli from three animals); t test was used. Means and SEMs are presented. (F) Representative images of control and mutant glomeruli of 1-year-old mice immunostained with anti-WT1 antibody (red) and 4′,6-diamidino-2-phenylindole (blue). Tuft areas designated by dotted lines are measured by ImageJ software. Scale bars, 5 μm.
Deletion of Vangl2 Increases Susceptibility to Glomerular Injury
To ascertain whether deletion of Vangl2 exacerbates glomerular injury and/or affects recovery of glomerular permselectivity, we injected 8- to 10-week-old Vangl2ΔEx4/Flox;Pod-Cre and Vangl2Flox/Flox;Cre– males with sheep antiglomerular basement membrane (anti-GBM) antiserum.28 This well established mouse model of glomerular injury is characterized by severe proteinuria that peaks in the first 24–48 hours postinjury with subsequent remission29; at 4 weeks postinjection, these animals may develop sclerotic glomerular lesions characteristic of FSGS.28,30 We collected urine at 24 and 48 hours postinjection and one time per week thereafter. At 4 weeks postinjury, animals were euthanized; 12- to 14-week-old uninjected animals of both genotypes were used for histologic evaluation at baseline. To ensure that animals of both genotypes were subjected to a similar glomerular insult, we monitored glomerular deposition of heterologous (sheep) anti-GBM antibodies, production and deposition of autologous (mouse) antibodies, and activation of complement. At 4 weeks postinjections, tissues were stained with antibodies against sheep IgG, mouse IgG, and mouse C3 similarly to the procedure described previously.31,32 We did not detect any differences in deposition of these antibodies or C3 (Supplemental Figure 2).
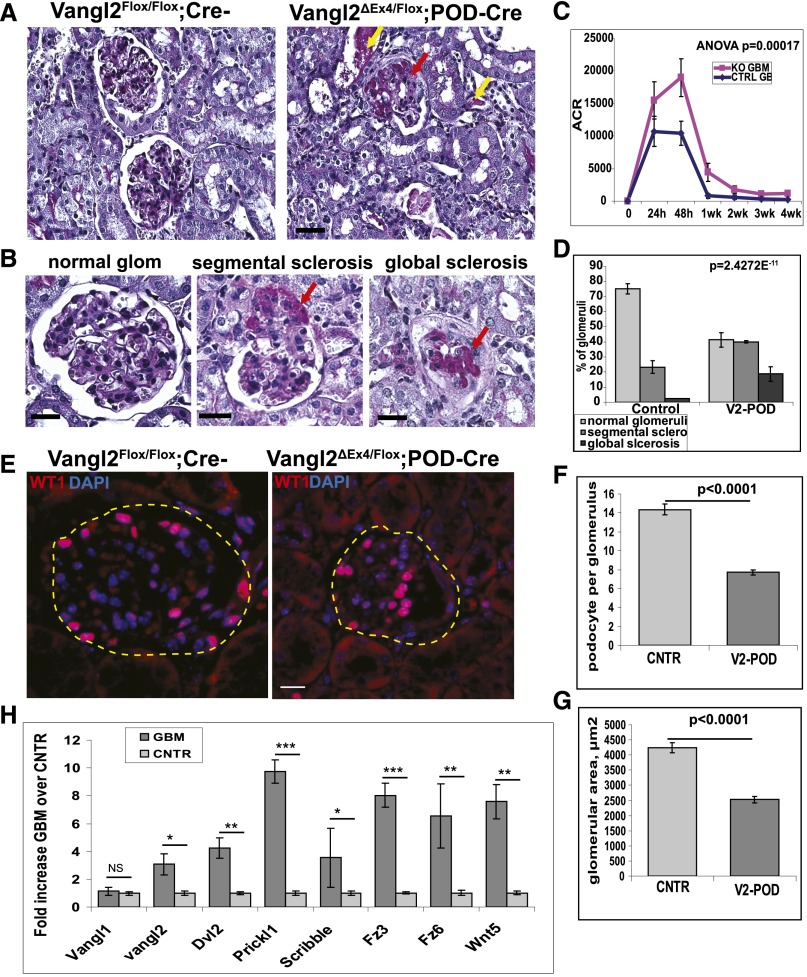
Measurements of the UACR revealed significantly greater albuminuria in Vangl2ΔEx4/Flox;Pod-Cre compared with Vangl2Flox/Flox;Cre– control animals 4 weeks after anti-GBM antibody administration (Figure 6C). The increase in the UACR in mutant mice was paralleled by a significantly higher percentage of glomeruli with various morphologic abnormalities (Figure 6, A, B, and D) in mutant versus control animals at 4 weeks. In control mice, normal glomeruli constituted 75.1%±3.4% of the total, whereas 23.2%±4.2% of glomeruli were segmentally sclerosed. The latter included glomeruli with segmental expansion of the extracellular matrix, denuded capillary walls adherent to Bowman’s capsule, and glomeruli with hypercellularity. In the control cohort, we found globally sclerosed glomeruli only occasionally (2.4%±0.5%) (Figure 6D). In contrast, kidneys of the Vangl2ΔEx4/Flox;Pod-Cre animals displayed substantially more advanced disease: 41.3%±4.8% of glomeruli were deemed normal and 39.95%±0.72% of glomeruli displayed segmental sclerotic lesions, matrix expansion, and/or adhesions to Bowman’s capsule. Interestingly, very few of the mutant glomeruli showed hypercellularity; rather, there was substantial global sclerosis (18.7%±4.7%), indicating that mutant mice had progressed to a more advanced disease stage (Figure 6D). There were no glomerular abnormalities in any animals at baseline (before injections) by light or transmission electron microscopy (Supplemental Figure 4).
Figure 6.
Deletion of Vangl2 in podocytes leads to exacerbation of glomerular injury. (A) Histologic analysis of kidneys from (left panel) Vangl2flox/flox;Cre– (control) and (right panel) Vangl2Flox/ΔEx4;Pod-Cre (V2-POD) animals at 4 weeks after injections with sheep anti-GBM antibody. In A and B, paraffin-embedded sections were stained with periodic acid–Schiff (PAS). The red arrows point to areas of extracellular matrix expansion (sclerotic lesions) and adhesions to Bowman’s capsule; yellow arrows point to proteinacious cast in tubular lumens. Scale bars, 20 μm. (B) Representative images of PAS-stained glomeruli from animals at 4 weeks postinjury. Scale bar, 15 μm. (C) UACR (ACR; micrograms per gram) at 0, 24, and 48 hours and 1, 2, 3, and 4 weeks after anti-GBM antibody administration in control (CTRL GE) and Vangl2Flox/ΔEx4;Pod-Cre (KO GBM) animals (n=10 for each genotype at each time point); statistical analysis was by two-way ANOVA. (D) Quantification of sclerotic glomerular lesions seen in PAS-stained sections of control (n=125 glomeruli from three animals) and Vangl2Flox/ΔEx4;Pod-Cre (V2-POD; n=182 glomeruli from three animals); chi-squared test was used. In all experiments, means and SEMs are presented. (E) Representative images of paraffin sections from (left panel) Vangl2flox/flox;Cre– (control) and (right panel) Vangl2Flox/ΔEx4;Pod-Cre (V2-POD) animals at 4 weeks after sheep anti-GBM antibody administration. Sections were stained with anti-WT1 antibody (red) and 4′,6-diamidino-2-phenylindole (DAPI; blue) to visualize podocytes. Scale bars, 5 μm. (F) Quantification of the average number of podocytes per glomerulus in control Vangl2Flox/Flox;Cre– (CNTR) and Vangl2Flox/ΔEx4;Pod-Cre mice at 4 weeks after anti-GBM antibody administration (P<0.001). (G) Quantification of average glomerular tuft area in control (CNTR) and mutant mice at 4 weeks (P<0.001). In F and G, podocytes and glomerular area were examined in >100 glomeruli per genotype (n=3 animals per genotype). Means and SEMs are presented; (H) Quantitative PCR analysis of PCP gene expression in glomerular mRNA isolated from control 3-month-old mice (CNTR) before and 48 hours after injections with anti-GBM antibody. Three animals were analyzed per condition, and all experiments were repeated two times in triplicate for each animal. In all experiments (unless indicated otherwise), a two-way unequal variance t test was used. Means and SEMs are presented in all experiments. *P≤0.02; **P≤0.002; ***P≤0.001.
The average number of podocytes per glomerulus 4 weeks postinjury differed almost 2-fold: 7.7±0.3 in Vangl2ΔEx4/Flox;Pod-Cre animals versus 14.4±0.5 podocytes in Vangl2Flox/Flox;Cre– animals (Figure 6, E and F). Although the number of podocytes per glomerulus in uninjected 3-month-old animals also differed between Vangl2ΔEx4/Flox;Pod-Cre (17±0.3) and Vangl2Flox/Flox;Cre− (19±0.5), the decrease in mutant animals amounted to only 11%, indicating that there was a preferential loss of mutant versus control podocytes in the context of injury. Postinjury, the average area of mutant glomeruli also decreased significantly and was at approximately 60% of that in control animals: 2525±100 μm2 in Vangl2ΔEx4/Flox;Pod-Cre versus 4245±174 μm2 in control GBM-treated animals, which is consistent with a more advanced stage of glomerular injury in mutant mice (Figure 6G). Finally, we detected a significant increase in transcription of several PCP genes at 48 hours postinjection (Figure 6H), supporting the hypothesis that molecules of the PCP pathway are re-expressed in response to podocyte injury.
Discussion
In this study, we report that the core PCP gene Vangl2 is essential for timely podocyte development and modulates the severity of glomerular injury in adult mice. These results identify, for the first time, a role for Vangl2 in the maintenance of glomerular structure and permselectivity in the adult kidney.
PCP Pathway and Glomerular Development
Most epithelial cells display two distinct forms of polarity: apicobasolateral polarity and PCP. Hartleben et al.33 recently established that nephrin interacts with the key apicobasolateral Par3-Par6–atypical protein kinase C functional cassette, and disruption of this cassette leads to FP effacement, FSGS, and death within 6 weeks of birth.33,34 The PCP pathway also contributes to podocyte biology: Lp−/− mice display defects in glomerular morphology and maturation.22,23 Glomerular defects observed in our new mouse with podocyte-specific Vangl2 ablation are reminiscent to those seen in the Lp−/− mouse.
From the published literature and our observations, we propose that podocyte-specific deletion of Vangl2 may affect podocyte development through several mechanisms. (1) Loss of Vangl2 affects cytoskeletal rearrangement underlying collective cell movement during podocyte differentiation. Both formation of FPs and their extension toward the glomerular capillary have been suggested to represent an example of collective cell migration.35 Coincidently, Vangl2 is expressed in podocytes at the highest level before and at the time when actin-based FPs start to form. The knockdown of Vangl2 in cultured mouse podocytes affects filamentous F-actin arrangement and decreases the number of cell protrusions and cell motility24; mild abnormalities in actin distribution in the Lp−/− glomerulus were also reported.22 We, thus, propose that glomerular maturation defects seen in conditional Vangl2 and Lp embryos arise from suboptimal cytoskeletal arrangements within FPs. (2) We recently reported that loss of Vangl2 in cultured cells led to a decrease in nephrin internalization.23 A similar role of PCP signaling in endocytosis of E-cadherin was reported in Drosophila, where it is associated with abnormal development of wing cells and elongation of the trachea.36,37 Likewise, in mouse hippocampal neurons, Vangl2 controls internalization of adhesion molecule N-cadherin in the postsynaptic dendritic spines, affecting synaptogenesis.38 We, therefore, suggest that loss of Vangl2 causes suboptimal slit diaphragm remodeling, leading to a delayed podocyte differentiation and glomerular maturation in Vangl2ΔEx4/Flox;Pod-Cre mice.
Deletion of Vangl2 gene in podocytes did not affect glomerular functions in adult mice. In concordance with the lack of phenotype in adult mice, we observed that 2-week-old mutant mice did not exhibit differences in glomerular maturity versus control animals, indicating a postnatal developmental catch up. Because Vangl2 is highly expressed when podocytes start generating FPs but only at low levels in differentiated podocytes, we propose that the earliest steps of podocyte differentiation (and glomerular maturation) are Vangl2-dependent, whereas Vangl2 function is dispensable at the later stages. Interestingly, tissue-specific loss of Vangl2 in the inner ear affects PCP of the stereociliary bundles on the sensory hair cells only during embryonic development. In 10-day-old neonates, refinement of inner ear hair cell PCP occurs independent of Vangl2, leading to a much less severe PCP phenotype.39
PCP Pathway and Podocyte Maintenance and Function in Adult Mice
At 1 year of age, Vangl2ΔEx4/Flox;Pod-Cre mutant mice display urinary albumin excretion and serum creatinine comparable with those of age-matched controls. Therefore, under normal physiologic conditions, the function of Vangl2 is dispensable for the maintenance of glomerular permselectivity. However, in the context of glomerular injury, we observed that loss of Vangl2 affects severity of the acute phase immediately after GBM antibody administration and exacerbates subsequent progression to FSGS. Importantly, we also detected a substantial increase in the PCP gene transcription within 48 hours postinjury, suggesting a likely requirement for the PCP signaling in podocyte recovery (e.g., re-establishing FP actin organization). The suboptimal nephron endowment caused by defects in branching morphogenesis may affect filtration function.40 Podocyte-specific excision of Vangl2 led to a temporary glomerular developmental delay, and no tubular defects were detected, making it unlikely that it would have affected branching morphogenesis and thus, contributed to the observed enhanced response to the injury. However, throughout life, mice with loss of Vangl2 in podocytes displayed a modest but significant reduction in podocyte number per glomerulus. However, postinjury, mutant mice displayed only half as many podocytes per glomerulus compared with control animals, suggesting that loss of Vangl2 (and the normal PCP signaling) affects podocyte recovery and/or turnover. Our results indicate that the initial reduced number of podocytes per glomerulus together with inadequate recovery and accelerated loss of podocytes after anti-GBM antibody administration contributed to the exacerbated glomerular injury in Vangl2ΔEx4/Flox;Pod-Cre animals.
In summary, by generating a transgenic mouse with a podocyte-specific excision of the core PCP gene Vangl2, we avoided embryonic death characteristic of PCP mouse mutants. We show that the function of the PCP protein, Vangl2, in podocytes is important for timely glomerular development, defines the number of podocytes per glomerulus, and positively modulates responses to glomerular injury in adult animals.
Concise Methods
Mouse Strains and Animal Husbandry
Generation of the conditional Vangl2Flox strain will be described in detail elsewhere (P. Gros, unpublished data). Briefly, the 129Sv-derived stem cells positive for the targeting vector containing LoxP sites flanking Exon4 of Vangl2 gene were injected into blastocysts of the C57Bl6/J mice. Chimeric males were mated with C57Bl6/J females, and F1 offspring with a germ-line transmission were identified by PCR as carrying the Flox allele (see below). The Vangl2Flox/+ mice were backcrossed to the C57Bl6/J mice to expand the colony and then intercrossed to obtain Vangl2Flox/Flox mice. Some Vangl2Flox/+ mice were also crossed to the More-Cre mouse to generate a constitutively excised Exon4 in all tissues (Vangl2ΔEx4/+ allele); More-Cre deleter mouse (C57Bl6/J background) enables an efficient LoxP-mediated recombination in the E5 embryo.41 The Vangl2Flox/Flox and Vangl2ΔEx4/+ animals were intercrossed to obtain Vangl2ΔEx4/Flox animals. To ablate Vangl2 expression in podocytes, we used a Podocin-Cre mouse line that expresses Cre-recombinase under regulation of the podocin promoter.27 Vangl2 Flox/Flox and Vangl2ΔEx4/Flox mice were crossed to a heterozygous Pod-Cre mouse to generate Vangl2Flox/Flox;Pod-Cre (we refer to them as V2-POD where applicable). Vangl2Flox/Flox negative for Cre allele were used as controls.
Heterozygous Vangl2 mutant Lp+/− mice were brother–sister mated; Lp−/− and matching wild-type littermates were recovered at E17.5. All adult and embryonic tissues were briefly washed in PBS (pH 7.4) and fixed in 4% paraformaldehyde. Embryos were either cryopreserved in Tissue-Tek OCT Compound (Sakura Finetek) for immunofluorescence staining or dehydrated in ethanol and embedded in paraffin for morphologic studies using standard procedures; the paraffin embedding was done at the Goodman Cancer Research Center (GCRC) Histology Core Facility, McGill University. Husbandry and all animal procedures were according to the Canadian Animal Act and approved by the McGill University Institutional Animal Care Committee (Protocol 4197 [to P.G.] and Protocol 5423 [to E.T.]).
Genotyping
All genotyping procedures were done with DNA isolated from mouse tail biopsies (except where indicated) using the Fast-Lysis PCR Genotyping Kit (ZmTech Scientifique, Montreal, Canada) as described by the manufacturer. In some experiments, we isolated DNA from glomeruli prepared by differential sieving as previously described in the work by Babayeva et al.24 with minor modifications. Briefly, cortices of Vangl2Flox/Flox;Pod-Cre and Vangl2Flox/Flox;Cre– kidneys dissected from 2-month-old animals (n=3 per genotype) were manually minced using sharp blades in PBS (pH 7.4) and gently pushed through 150-μm sieves. The isolated glomeruli were extensively washed in PBS and collected into tubes. The glomerular fractions contained approximately 70%–75% glomeruli by visual inspection under light microscope; the remaining 25%–30% were tubular fraction adjoined to the glomerulus. Glomerular DNA was isolated using the Fast-Lysis PCR Genotyping Kit. The following primers were used: to detect Vangl2Flox allele, forward: Vangl2 5-TCTTGATTTGTGGCCCAGGCTGAT-3′ and reverse: 5′-TGCTCAGCCAAGATTGGGAACTCT-3′; to detect excision of Exon4 (Vangl2ΔEx4 allele), forward: 5′-GACATGTATCACCTCACTTGGCTGGA-3′ and reverse: 5′-CTTACTATGTGCAAACCACCTTC-3′; to detect Cre allele, forward: 5′-AGGTTCGTGCACTCATGGA-3′ and reverse: 5′-TCGACCAGTTTAGTTACCC-3′.
Mouse Models of Glomerular Injury
Sheep anti-rat GBM antiserum was used to induce anti-GBM nephritis as described previously.28 Briefly, the anti-GBM serum was diluted 1:10 in filter-sterilized 1× PBS; 100 μl diluted antiserum was injected two times at 0 and 24 hours through the lateral tail vein (equal to two 10-μl treatments) to the 8- to 10-week-old males. Control animals were injected with 100 μl sterile PBS. Urine was collected at 0, 24, and 48 hours and 1, 2, 3, and 4 weeks after administration of the anti-GBM antibody. At 4 weeks postinjury, mice were euthanized, and the extent of glomerular injury was examined by histologic and immunofluorescence methods (below).31,32
Urine/Blood Collection and Analyses
For urine collection, each mouse was placed in an individual simplified metabolic cage. The metabolic apparatus was a normal mouse cage with no bedding and a small plastic grid placed at the bottom. Before collection, cages were autoclaved and dried, and mice were placed in the cage until urine was produced (approximately 2 hours). Urine that was not contaminated with fecal waste was collected into autoclaved Eppendorf tubes and frozen at −20°C (short-term storage) or −80°C (long-term storage). UACR was determined using the Mouse Albumin ELISA Urinary Assay Kit (Bethyl Labs) and the Creatinine Urinary Assay Kit (Cayman Chemical) according to the manufacturers’ instructions. Blood was collected by puncture of the saphenous vein into 200-µl serum gel tubes. Blood was allowed to clot at room temperature and centrifuged such that the clot was separated from serum by the gel layer. Serum was transferred to fresh tubes and brought to the Clinical Laboratories of the Montreal Children’s Hospital for serum creatinine analysis by the standard enzymatic method.
Immunofluorescence Microscopy
E17.5 embryos of various genotypes were cryopreserved in OCT using standard protocol, and 5-μm sections were prepared. The sections were treated with 0.5% Triton X-100 in 1× PBS and blocked at room temperature for 1 hour in 10% normal goat serum (Jackson Immuno Research Laboratories, West Grove, PA) in the presence of 0.1% Triton. Labeling with specific mAbs or polyclonal antibodies was performed at room temperature for 1 hour followed by detection with secondary anti-rabbit, anti-guinea pig, or anti-mouse AlexaFluor488- or AlexaFluor568-conjugated antibodies (Molecular Probes) where applicable. Nuclei were stained with 4′,6-diamidino-2-phenylindole (Invitrogen). The stained sections were mounted in Prolong Gold Antifade (Molecular Probes). In some experiments, E17.5 and dissected kidneys from 14-day-old mice were paraffin-embedded at the GCRC Histology Core Facility, McGill University, and 4-μm sections were prepared. The sections were deparaffinized by standard procedure and thereafter processed as cryosections. To visualize podocytes in the kidneys from 3-month-old and 1-year-old animals, paraffin-embedded 4-μm sections were deparaffinized and incubated sequentially with 50 mM ammonium chloride in PBS (pH 7.4) for 15 minutes at room temperature and 150 mM glycine in PBS (pH 7.4) for 1 hour at 4°C. Epitope retrieval was achieved by incubating sections in 10 mM sodium citrate buffer (pH 6.0) in a microwave oven for 4–6 minutes at the high-power setting. The sections were washed in PBS and immunostained as above. The following primary antibodies were used: rabbit antinephrin antibody (provided by Dr. Takano), guinea pig antinephrin antibody (1:150; Acris, Herford, Germany), rabbit WT1 antibody (1:400; Santa Cruz, CA), rabbit Vangl2 antibody42 (1:20), mouse antisynaptopodin (1:200; ProGen Biotechnik), mouse antipodocalyxin (1:300; BD Biosciences, Mississauga, ON, Canada), and mouse anti–ZO-1 (1:150; Invitrogen, Burlington, ON, Canada). Images of developing glomeruli were taken on the LSM 780 Laser Scanning Confocal Microscope (Carl Zeiss) using the Plan-Apochromat ×63 objective or by acquiring Z projections on AxioObservor-100 (Carl Zeiss) using AxioVision 4.8 software. All images were processed using Adobe Photoshop CS5 and assembled in Adobe Illustrator CS5.
Histology
Whole E17.5 embryos or dissected kidneys from 1-year-old animals were paraffin-embedded at the GCRC Histology Core Facility, McGill University; 4-μm sections were prepared from control and mutant embryos/kidneys, and the sections were deparaffinized and stained with Mayer hematoxylin and eosin (Sigma-Aldrich) using standard protocol. Glomerular tufts in animals of all ages were measured by tracing the perimeter of the glomeruli on the kidney sections stained with anti-WT1 antibody and 4′,6-diamidino-2-phenylindole. For this procedure, we manually enhanced image exposure to clearly visualize the tuft edges. AxioVision4.8 software was used. To visualize glomerular lesions postinjury, kidneys from the 3-month-old animals (collected 4 weeks after injections with anti-GBM antibodies or PBS [mock injury]) were paraffin-embedded at the GCRC, sectioned at 4 μm, and stained with periodic acid–Schiff at the GCRC Histology Core. Among anti-GBM–injured mice, for each genotype, we used three animals: two animals with the highest proteinuria and one animal with the proteinuria corresponding to the mean value in each of the Vangl2ΔEx4/Flox;Pod-Cre or Vangl2Flox/Flox;Cre– cohorts. The glomeruli were classified as segmental glomerulosclerosis if a portion of the glomerulus appeared normal together with an abnormal segmental adhesion of the glomerular capillary to Bowman’s capsule (similar to the tip type of FSGS in humans)43 or the glomerulus showed an expanded extracellular matrix (seen as pink staining in periodic acid–Schiff-treated sections) or extended areas of hypercellularity. Glomeruli entirely occupied by extracellular matrix (pink color) or shrunken glomerular tufts without visible glomerular capillaries were classified as global sclerosis. All light microscopy images were acquired on an AxioFoth microscope. All fluorescence microscopy images were acquired on an AxioObservor 100 microscope using AxioVision 4.8 software as above. For statistical analysis, kidneys of three animals per genotype for each staining were examined.
Quantitative PCR
Mouse glomerular fractions from 3-month-old mice 48 hours postinjection with anti-GBM antibody were prepared by differential sieving as described for genotyping. RNA was prepared by the Trizol method (Invitrogen) according to the manufacturer. RNA was reverse-transcribed using the iScript cDNA Synthesis Kit (Bio-Rad). cDNA was used for quantitative PCR analysis using the LightCycler 480 SYBR Green I Master (Roche) according to the manufacturer’s instructions, and data were collected using the LightCycler 480 Software, version 1.5.0.39 (Roche). Thermal cycling parameters were as follows: (1) preincubation: 95°C for 5 minutes; (2) amplification: 95°C for 10 seconds, 60°C for 30 seconds, and 72°C for 15 seconds for 40 cycles; (3) melting curve generation: 95°C for 5 seconds and 62°C for 60 seconds with 97°C acquisition. The following mouse-specific primer sequences were used: Vangl1 forward: 5′-CAGCAGCACTACCACAGCAT-3′, Vangl1 reverse: 5′-GAGCCATCGATCCTT GTCAT-3′, Vangl2 forward: 5′-TGCTCATGGTGCTTGTCTTC-3′, Vangl2 reverse: 5′-GGAGCTCCAGCAGAACTACG-3′, Dvl2 forward: 5′-CAAAGTAACGAGCGTGGTGA-3′, Dvl2 reverse: 5′-TCGTCGTTGCTCATGTTCTC-3′, Prickle1 forward: 5′-TGAACTCTTCCATGCGCAC-3′, Prickle1 reverse: 5′-CTGGGACTTGGATCTCCTGA-3′, Scrib1 forward: 5′-GACACCCTTACCGTGCTTGT-3′, Scrib1 reverse: 5′-GGTCAATGGACGAAATGCTT-3′, Fzd3 forward: 5′-GAAGCAAAGCAGGGAGTGTC-3′, Fzd3 reverse: 5′-ATGCTGCCGTGAGGTAGTCT-3′, Fzd6 forward: 5′-CTCTTAGCCGGCATCATCTC-3′, Fzd6 reverse: 5′-TCT CCCAGGTGATCCTGTTC-3′, Wnt5a forward: 5′-CAGAATTCGTGGTGTGAATGA-3′, and Wnt5a reverse: 5′-CGGTAATTAGGGCTTTCCAAC-3′. Expression of the housekeeping mouse β2-microglobulin gene (B2M) was used for normalization of the PCP gene expression: B2M forward: 5′-TGCAGAGTTAAGCATGCCAGTATGG-3′, B2M reverse: 5′-TGATGCTTGATCACATGTCTCG-3′. The relative gene expression for each PCP gene was normalized by the value calculated as a mean of the B2M triplicate from the same sample run in the same experiment. The relative expression of each gene was calculated by using formula relative expression=2−ΔCp. Three of each GBM-treated and untreated control mice were used, all samples were run in triplicate, and experiments for the complete set of samples for each gene were repeated two times.
Statistical Analyses
Data were analyzed by using two-way ANOVA and unpaired unequal variance t test for most experiments. For statistical analysis of glomeruli at various developmental stages, we used the chi-squared test. A P value<0.05 was considered significant. Kidneys from a minimum of three adult animals or three embryos for each genotype were used for all immunofluorescence and histologic analyses. In all experiments, means and SEMs are presented.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Tomoko Takano (McGill University) for sharing the Podocin-Cre mouse line with us (originally from Dr. Susan Quaggin) and rabbit antinephrin antibody, Joan Papillon for technical assistance with characterization of the glomerular basement membrane injury model, and Susan Gauthier for breeding Looptail and Vangl2ΔEx4/Flox mice.
This work was supported by Canadian Institute of Health Research Operating Grants MOP 53264 (to A.V.C.), MOP 114873 (to P.G.), and MOP 102646 (to E.T.) and a Halpin Foundation–American Society of Nephrology Early Carrier Grant (to E.T.). B.R. is supported by the McGill University Health Center Research Institute and McGill University Physiology Department Graduate Student Awards. A.V.C. holds the Catherine McLaughlin Hakim Chair at McGill University. P.G. is the James McGill Chair at McGill University.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014040340/-/DCSupplemental.
References
- 1.Quaggin SE, Kreidberg JA: Development of the renal glomerulus: Good neighbors and good fences. Development 135: 609–620, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Simons M, Hartleben B, Huber TB: Podocyte polarity signalling. Curr Opin Nephrol Hypertens 18: 324–330, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Michaud JL, Lemieux LI, Dubé M, Vanderhyden BC, Robertson SJ, Kennedy CR: Focal and segmental glomerulosclerosis in mice with podocyte-specific expression of mutant alpha-actinin-4. J Am Soc Nephrol 14: 1200–1211, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS: CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300: 1298–1300, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Krendel M, Kim SV, Willinger T, Wang T, Kashgarian M, Flavell RA, Mooseker MS: Disruption of Myosin 1e promotes podocyte injury. J Am Soc Nephrol 20: 86–94, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mele C, Iatropoulos P, Donadelli R, Calabria A, Maranta R, Cassis P, Buelli S, Tomasoni S, Piras R, Krendel M, Bettoni S, Morigi M, Delledonne M, Pecoraro C, Abbate I, Capobianchi MR, Hildebrandt F, Otto E, Schaefer F, Macciardi F, Ozaltin F, Emre S, Ibsirlioglu T, Benigni A, Remuzzi G, Noris M, PodoNet Consortium : MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med 365: 295–306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta IR, Baldwin C, Auguste D, Ha KC, El Andalousi J, Fahiminiya S, Bitzan M, Bernard C, Akbari MR, Narod SA, Rosenblatt DS, Majewski J, Takano T: ARHGDIA: A novel gene implicated in nephrotic syndrome. J Med Genet 50: 330–338, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott RP, Hawley SP, Ruston J, Du J, Brakebusch C, Jones N, Pawson T: Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J Am Soc Nephrol 23: 1149–1154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown EJ, Schlöndorff JS, Becker DJ, Tsukaguchi H, Tonna SJ, Uscinski AL, Higgs HN, Henderson JM, Pollak MR: Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet 42: 72–76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costantini F: Genetic controls and cellular behaviors in branching morphogenesis of the renal collecting system. Wiley Interdiscip Rev Dev Biol 1: 693–713, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreidberg JA: Gene targeting in kidney development. Med Pediatr Oncol 27: 445–452, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Grahammer F, Schell C, Huber TB: The podocyte slit diaphragm—from a thin grey line to a complex signalling hub. Nat Rev Nephrol 9: 587–598, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Hartleben B, Widmeier E, Wanner N, Schmidts M, Kim ST, Schneider L, Mayer B, Kerjaschki D, Miner JH, Walz G, Huber TB: Role of the polarity protein Scribble for podocyte differentiation and maintenance. PLoS ONE 7: e36705, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallingford JB: Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol 28: 627–653, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Goodrich LV, Strutt D: Principles of planar polarity in animal development. Development 138: 1877–1892, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P: Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet 28: 251–255, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Guo N, Nathans J: The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci 26: 2147–2156, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yates LL, Schnatwinkel C, Murdoch JN, Bogani D, Formstone CJ, Townsend S, Greenfield A, Niswander LA, Dean CH: The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum Mol Genet 19: 2251–2267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, Economides AN, Yang Y: Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell 20: 163–176, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torban E, Iliescu A, Gros P: An expanding role of Vangl proteins in embryonic development. Curr Top Dev Biol 101: 237–261, 2012 [DOI] [PubMed] [Google Scholar]
- 21.De Marco P, Merello E, Cama A, Kibar Z, Capra V: Human neural tube defects: Genetic causes and prevention. Biofactors 37: 261–268, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Yates LL, Papakrivopoulou J, Long DA, Goggolidou P, Connolly JO, Woolf AS, Dean CH: The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Hum Mol Genet 19: 4663–4676, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babayeva S, Rocque B, Aoudjit L, Zilber Y, Li J, Baldwin C, Kawachi H, Takano T, Torban E: Planar cell polarity pathway regulates nephrin endocytosis in developing podocytes. J Biol Chem 288: 24035–24048, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babayeva S, Zilber Y, Torban E: Planar cell polarity pathway regulates actin rearrangement, cell shape, motility, and nephrin distribution in podocytes. Am J Physiol Renal Physiol 300: F549–F560, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Torban E, Wang HJ, Groulx N, Gros P: Independent mutations in mouse Vangl2 that cause neural tube defects in looptail mice impair interaction with members of the Dishevelled family. J Biol Chem 279: 52703–52713, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, Murdoch J, Warchol ME, Wenthold RJ, Kelley MW: Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. J Neurosci 26: 5265–5275, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eremina V, Wong MA, Cui S, Schwartz L, Quaggin SE: Glomerular-specific gene excision in vivo. J Am Soc Nephrol 13: 788–793, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Pippin JW, Krofft RD, Naito S, Liu ZH, Shankland SJ: Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. Am J Physiol Renal Physiol 304: F1375–F1389, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Ohse T, Vaughan MR, Kopp JB, Krofft RD, Marshall CB, Chang AM, Hudkins KL, Alpers CE, Pippin JW, Shankland SJ: De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease. Am J Physiol Renal Physiol 298: F702–F711, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salant DJ, Cybulsky AV: Experimental glomerulonephritis. Methods Enzymol 162: 421–461, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Cybulsky AV, Takano T, Papillon J, Khadir A, Liu J, Peng H: Complement C5b-9 membrane attack complex increases expression of endoplasmic reticulum stress proteins in glomerular epithelial cells. J Biol Chem 277: 41342–41351, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Hartleben B, Schweizer H, Lübben P, Bartram MP, Möller CC, Herr R, Wei C, Neumann-Haefelin E, Schermer B, Zentgraf H, Kerjaschki D, Reiser J, Walz G, Benzing T, Huber TB: Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem 283: 23033–23038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber TB, Hartleben B, Winkelmann K, Schneider L, Becker JU, Leitges M, Walz G, Haller H, Schiffer M: Loss of podocyte aPKClambda/iota causes polarity defects and nephrotic syndrome. J Am Soc Nephrol 20: 798–806, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: Regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Classen AK, Anderson KI, Marois E, Eaton S: Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell 9: 805–817, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Warrington SJ, Strutt H, Strutt D: The Frizzled-dependent planar polarity pathway locally promotes E-cadherin turnover via recruitment of RhoGEF2. Development 140: 1045–1054, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagaoka T, Ohashi R, Inutsuka A, Sakai S, Fujisawa N, Yokoyama M, Huang YH, Igarashi M, Kishi M: The Wnt/Planar cell polarity pathway component Vangl2 induces synapse formation through direct control of N-cadherin. Cell Rep 6: 916–927, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Copley CO, Duncan JS, Liu C, Cheng H, Deans MR: Postnatal refinement of auditory hair cell planar polarity deficits occurs in the absence of Vangl2. J Neurosci 33: 14001–14016, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah MM, Sampogna RV, Sakurai H, Bush KT, Nigam SK: Branching morphogenesis and kidney disease. Development 131: 1449–1462, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Tallquist MD, Soriano P: Epiblast-restricted Cre expression in MORE mice: A tool to distinguish embryonic vs. extra-embryonic gene function. Genesis 26: 113–115, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Torban E, Wang HJ, Patenaude AM, Riccomagno M, Daniels E, Epstein D, Gros P: Tissue, cellular and sub-cellular localization of the Vangl2 protein during embryonic development: Effect of the Lp mutation. Gene Expr Patterns 7: 346–354, 2007 [DOI] [PubMed] [Google Scholar]
- 43.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.