Abstract
Nephrolithiasis is a prevalent condition with a high morbidity. Although dozens of monogenic causes have been identified, the fraction of single-gene disease has not been well studied. To determine the percentage of cases that can be molecularly explained by mutations in 1 of 30 known kidney stone genes, we conducted a high-throughput mutation analysis in a cohort of consecutively recruited patients from typical kidney stone clinics. The cohort comprised 272 genetically unresolved individuals (106 children and 166 adults) from 268 families with nephrolithiasis (n=256) or isolated nephrocalcinosis (n=16). We detected 50 likely causative mutations in 14 of 30 analyzed genes, leading to a molecular diagnosis in 14.9% (40 of 268) of all cases; 20 of 50 detected mutations were novel (40%). The cystinuria gene SLC7A9 (n=19) was most frequently mutated. The percentage of monogenic cases was notably high in both the adult (11.4%) and pediatric cohorts (20.8%). Recessive causes were more frequent among children, whereas dominant disease occurred more abundantly in adults. Our study provides an in-depth analysis of monogenic causes of kidney stone disease. We suggest that knowledge of the molecular cause of nephrolithiasis and nephrocalcinosis may have practical implications and might facilitate personalized treatment.
Keywords: kidney stones, hypercalciuria, human genetics, molecular genetics, renal tubular acidosis, Bartter syndrome
Nephrolithiasis (NL) is a highly prevalent condition affecting up to 10% of individuals in the Western world.1 It is associated with significant morbidity because of colicky pain, the necessity of surgical procedures, and progression to CKD.2 NL and related conditions, such as nephrocalcinosis (NC), share a well recognized heritability, emphasized by the fact that up to two thirds of hypercalciuric stone formers have relatives with NL.3–5 There are at least 30 genes that have been shown to cause single-gene forms of NL/NC by autosomal-dominant, autosomal-recessive, or X-linked transmission.5,6 The ability to detect the causative mutation(s) in a monogenic disease gene is of great diagnostic and potentially therapeutic importance, because there is an almost deterministic cause–effect relationship in monogenic disease.
However, the contribution of monogenic disorders to the overall prevalence of NL has never been studied comprehensively. Hence, data are lacking on the frequency of monogenic forms of NL in the overall population of stone formers. Furthermore, absence of a positive family history, which will be the rule in recessive genes and may be frequent in dominant genes with incomplete penetrance, often leads to the false assumption that a monogenic cause is unlikely. We, therefore, hypothesized that monogenic causes of NL/NC account for a high percentage of stone formers. For most individuals with NL/NC, mutation analysis for a causative genetic defect has not been accessible so far. With the advent of massively parallel sequencing techniques and high-throughput library preparation, mutation analysis of multiple genes in large cohorts has not only become technically feasible but also, cost-effective.7
To determine the percentage of monogenic causes in kidney stone disease, we analyzed, in a cohort of 272 genetically unresolved individuals (268 families) (Supplemental Table 1), all coding exons and splice sites of 30 known NL/NC-causing genes with a defined Online Mendelian Inheritance in Man (OMIM) phenotype (Supplemental Table 2 and Concise Methods). Participants were recruited consecutively and without prior preselection at three typical kidney stone clinics on the basis of diagnosed NL or NC as outlined in Concise Methods. We applied a high-throughput mutation analysis technique that we recently developed.7,8 As a result, we show that causative mutations in known NL/NC-causing genes are present in around 15% of these 268 families, and we suggest possible therapeutic and prophylactic implications for some of these genetic findings.
By targeted resequencing of 381 coding exons of 30 genes known to cause autosomal-dominant, autosomal-recessive, or X-linked NL/NC if mutated, we identified 12,589 single-nucleotide variants and 1246 deletion-insertion variants in 336 individuals (272 genetically unresolved individuals and 64 controls). To distinguish benign variants from disease-causing mutations, we evaluated each variant individually on the basis of strict criteria as described in Concise Methods. Overall, 162 (1.2%) variants met our criteria for being likely disease-causing variants, 139 of which were confirmed by Sanger sequencing (86%), whereas the others represented low-representation artifacts of multiplex PCR. After exclusion of single heterozygous variants in recessive genes and nonsegregating variants and subtraction of variants from duplicates and positive controls, a total of 50 most likely disease-causing variants remained (Supplemental Table 3).
As a result, we made a causal molecular genetic diagnosis that is likely to explain the disease phenotype in 40 unrelated individuals in our total cohort of 268 families with NL/NC (14.9%) (Tables 1 and 2). Pathogenic mutations were detected in the following six dominant disease genes: SLC7A9 (19 families), ADCY10 (3 families), SLC2A9 (2 families), SLC9A3R1 (2 families), SLC22A12 (2 families), and SLC4A1 (1 family) (Table 1, Supplemental Table 4). Furthermore, we identified pathogenic mutations in the following eight recessive disease genes: SLC3A1 (two families), CLCN5 (two families), CLDN16 (two families), ATP6V1B1 (one family), CYP24A1 (one family), AGXT (one family), SLC34A1 (one family), and SLC34A3 (one family) (Table 2, Supplemental Table 4).
Table 1.
Twenty-nine molecular diagnoses established in six dominant genes in a cohort of 272 individuals (268 families) with NL/NC
| Gene/ Individual | Nucleotide Change | Amino Acid Change | State | PPh2 | Evolutionary Conservation | Ref. | Age of Onset (yr) | Stone Analysis | Clinical Diagnosis (Prescreening) | Genetic Diagnosis (Postscreening) | Practical Implication (Because of Genetic Diagnosis) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLC7A9 | ||||||||||||
| B208–21 | c.313G>A | p.Gly105Arg | Het | 0.998 | D. melanog. | 9 | 1 | Cystine | CU+NL | ► | CU, type B | — |
| JAS-C8a | c.313G>A | p.Gly105Arg | Het | 0.998 | D. melanog. | 9 | 17 | CaOx | Idiopathic NL | ► | CU, type B | Quantify urinary cystine |
| JAS-E5 | c.313G>A | p.Gly105Arg | Het | 0.998 | D. melanog. | 9 | 17 | Ca | CU+NL/NC | ► | CU, type B | Hydration and urinary alkalinization |
| F1029–21a,b | c.313G>A | p.Gly105Arg | Het | 0.998 | D. melanog. | 9 | 6 | ND | Idiopathic NC | ► | CU, type B | Quantify urinary cystine, hydration, and urinary alkalinization |
| c.544G>A | p.Ala182Thr | Het | 0.056 | D. melanog. | 10 | |||||||
| JAS-D30 | c.313G>A | p.Gly105Arg | Het | 0.998 | D. melanog. | 9 | 25 | Cystine | CU+NL | ► | CU, type B | — |
| c.614del | p.Lys205Argfs*59 | Het | — | — | Novel | |||||||
| JAS-D31 | c.411_412del | p.Pro139Leufs*69 | Het | — | — | 10 | 28 | Cystine | CU+NL | ► | CU, type B | — |
| JAS-D47 | c.411_412del | p.Pro139Leufs*69 | Het | — | — | 10 | 45 | Cystine | CU+NL | ► | CU, type B | — |
| JAS-D34 | c.411_412del | p.Pro139Leufs*69 | Het | — | — | 10 | 35 | Cystine | CU+NL | ► | CU, type B | — |
| JAS-D28 | c.544G>A | p.Ala182Thr | Hom | 0.056 | D. melanog. | 10 | 30 | Cystine | CU+NL | ► | CU, type B | — |
| JAS-F41a | c.544G>A | p.Ala182Thr | Het | 0.056 | D. melanog. | 10 | 60 | ND | HC+NL | ► | CU, type B | Quantify urinary cystine |
| JAS-F50a | c.544G>A | p.Ala182Thr | Het | 0.056 | D. melanog. | 10 | 50 | ND | Idiopathic NL | ► | CU, type B | Quantify urinary cystine |
| JAS-D57 | c.544G>A | p.Ala182Thr | Het | 0.056 | D. melanog. | 10 | 7 | Cystine | CU+NL | ► | CU, type B | — |
| c.614dup | p.Asn206Glufs*3 | Het | — | — | 11 | |||||||
| JAS-F87a | c.614dup | p.Asn206Glufs*3 | Het | — | — | 11 | 19 | CaPO | HC+NL | ► | CU, type B | Quantify urinary cystine |
| JAS-G10 | c.614dup | p.Asn206Glufs*3 | Het | — | — | 11 | 43 | Cystine | CU+NL | ► | CU, type B | — |
| JAS-D29 | c.671C>T | p.Ala224Val | Hom | 0.999 | S. cerevisae | 12 | 15 | Cystine | CU+NL | ► | CU, type B | — |
| JAS-D55 | c.671C>T | p.Ala224Val | Het | 0.999 | S. cerevisae | 12 | 26 | Cystine | CU+NL | ► | CU, type B | — |
| JAS-F19a | c.671C>T | p.Ala224Val | Het | 0.999 | S. cerevisae | 12 | 28 | ND | HC+NL | ► | CU, type B | Quantify urinary cystine |
| c.1369T>C | p.Tyr457His | Het | 0.059 | D. rerio | Novel | |||||||
| B114–21 | c.814del | p.Val272Cysfs*6 | Het | — | — | 13 | 6 | Cystine | CU+NL | ► | CU, type B | — |
| c.997C>T | p.Arg333Trp | Het | 0.999 | D. melanog. | 14 | |||||||
| JAS-E9 | c.1353C>A | p.Tyr451* | Het | — | — | Novel | 26 | Cystine | CU+NL | ► | CU, type B | — |
| c.1400–2A>G | 3′ splice | Het | — | — | Novel | |||||||
| ADCY10 | ||||||||||||
| JAS-F8 | c.1263C>A | p.Tyr421* | Het | — | — | Novel | 10 | ND | Idiopathic HC | ► | Idiopathic HC | Monitor urinary Ca |
| JAS-F68 | c.1282G>A | p.Asp428Asn | Het | 0.996 | C. intestinalis | Novel | 50 | CaOx | Idiopathic HC+NC | ► | Idiopathic HC | Monitor urinary Ca |
| JAS-F29 | c.4477del | p.Leu1493Serfs*24 | Het | — | — | Novel | 40 | ND | Idiopathic NL | ► | Idiopathic HC | Monitor urinary Ca |
| SLC2A9 | ||||||||||||
| B179–21a | c.1343C>T | p.Pro448Leu | Het | 0.997 | D. melanog. | Novel | 6 | ND | Idiopathic NL | ► | RHUC2 | Recheck serum/urinary uric acid |
| B230–21a | c.1419+1G>A | 5′ splice | Het | — | — | Novel | 7 | ND | Idiopathic HC+NL | ► | RHUC2 | Recheck serum/urinary uric acid |
| SLC9A3R1 | ||||||||||||
| B224–21a | c.673G>A | p.Glu225Lys | Het | 0.816 | D. rerio | 15 | 5 | ND | Idiopathic HC+NL | ► | NPHLOP2 | Screen family members at risk, prevention of bone disease (osteoporosis/fractures) |
| B109–21a | c.888+2T>C | 5′ splice site | Het | — | — | Novel | 17 | ND | Idiopathic NL | ► | NPHLOP2 | Screen family members at risk, prevention of bone disease (osteoporosis/fractures) |
| SLC22A12 | ||||||||||||
| JAS-F98 | c.431T>C | p.Leu144Pro | Het | 0.999 | S. cerevisae | Novel | 25 | CaOx | HU+NL | ► | RHUC1 | — |
| B155–12a | c.1300C>T | p.Arg434Cys | Het | 0.235 | D. melanog. | 16 | 17 | Ca | Idiopathic NL | ► | RHUC1 | Recheck serum/urinary uric acid |
| SLC4A1 | ||||||||||||
| JAS-E8 | c.2716G>C | p.Glu906Gln | Het | 0.597 | D. rerio | Novel | 29 | ND | HC+NC | ► | Primary dRTA | Correct acidosis |
PPh2, Polyphen2–HumVar (http://genetics.bwh.harvard.edu/pph2/); Het, heterozygous; D. melanog., Drosophila melanogaster; CU, cystinuria; CaOx, calcium oxalate; CaPO, calcium phosphate; Hom, homozygous; ND, no data; HC, hypercalciuria; S. cerevisae, Saccharomyces cerevisae; D. rerio, Danio rerio; C. intestinalis, Ciona intestinalis; RHUC2, renal hypouricemia type 2; NPHLOP2, hypophosphatemic nephrolithiasis/osteoporosis-2; HU, hypouricemia; RHUC1, renal hypouricemia type 1; dRTA, distal renal tubular acidosis.
Molecular genetic diagnosis added new aspects to the previously established clinical diagnosis (11 of 29=37.9%).
For individual F1029–21, both heterozygous mutations are on the same allele.
Table 2.
Twelve molecular diagnoses established in eight recessive genes in a cohort of 272 individuals (268 families) with NL/NC
| Gene/ Individual | Nucleotide Change | Amino Acid Change | State | PPh2 | Evolutionary Conservation | Ref. | Age of Onset (yr) | Stone Analysis | Clinical Diagnosis (Prescreening) | Genetic Diagnosis (Postscreening) | Practical Implication (Because of Genetic Diagnosis) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLC3A1 | ||||||||||||
| JAS-B21a | c.1354C>T | p.Arg452Trp | Hom | 1 | S. cerevisae | 17 | 16 | Cystine | CU+NL | ► | CU, type A | — |
| JAS-B22a | c.1354C>T | p.Arg452Trp | Hom | 1 | S. cerevisae | 17 | 16 | Cystine | CU+NL | ► | CU, type A | — |
| JAS-G7 | c.1400T>C | p.Met467Thr | Hom | 0.289 | D. melanog. | 18 | 55 | Cystine | CU+NL | ► | CU, type A | — |
| ATP6V1B1 | ||||||||||||
| B214–21b | c.481G>A | p.Glu161Lys | Hom | 0.005 | S. cerevisae | 19 | 6 | ND | NL | ► | dRTA, deafness | Prompt audiometry, screen family members at risk |
| CLCN5c | ||||||||||||
| B111–21 | c.344G>A | p.Trp115* | Hem | — | — | Novel | 8 | ND | Dent disease | ► | Dent disease/NL type 1 | — |
| B167–21b,d | c.1009G>A | p.Glu337Lys | Hem | 0.987 | S. cerevisae | Novel | 11 | ND | FHHNC/PH | ► | Dent disease/NL type 1 | ESRD, no recurrence of disease in RTX, screen family members at risk |
| CLDN16 | ||||||||||||
| JAS-C1d | c.445C>T | p.Arg149* | Hom | — | — | 20 | 20 | ND | FHHNC+NL | ► | FHHNC | ESRD, no recurrence of disease in RTX, screen family members at risk |
| B178–21 | c.453G>T | p.Leu151Phe | Hom | 0.985 | C. intestinalis | 21 | 1 | ND | FHHNC | ► | FHHNC | — |
| CYP24A1 | ||||||||||||
| B223–21b | c.428_430del | p.Glu143del | Hom | — | D. rerio | 22 | 12 | ND | FHHNC | ► | (Infantile) HC/NL | Infantile HC: not restricted to infancy (12 yr), monitor vitamin D levels and serum Ca, avoid vitamin D |
| AGXT | ||||||||||||
| B106–21 | c.416_418del | p.Val139del | Het | — | X. tropicalis | Novel | 12 | CaOx | PH1 | ► | PH1 | Definite clinical diagnosis was obtained by liver biopsy; invasive biopsy could have been avoided |
| c.846+1G>T | 5′ splice site | Het | — | — | Novel | |||||||
| SLC34A1 | ||||||||||||
| B168–21b,e | c.271_291del | p.Val91_Ala97del | Het | — | — | Novel | 0.4 | ND | NC+PH | ► | NPHLOP1/FS | Recheck serum/urinary phosphate, screen family members at risk, prevention of bone disease (osteoporosis/fractures) |
| c.1534C>T | p.Arg512Cys | Het | 1 | C. elegans | Novel | |||||||
| SLC34A3 | ||||||||||||
| JAS-F43b | c.1454G>A | p.Arg485His | Het | 0.981 | C. elegans | Novel | 18 | ND | Idiopathic NL | ► | HHRH | Monitor serum/urinary phosphate and vitamin D, prevention of bone disease (osteoporosis/fractures) |
| c.1585A>T | p.Ile529Phe | Het | 0.12 | C. savigny | Novel |
PPh2, Polyphen2–HumVar (http://genetics.bwh.harvard.edu/pph2/); Hom, homozygous; S. cerevisae, Saccharomyces cerevisae; CU, cystinuria; D. melanog., Drosophila melanogaster; ND, no data; dRTA, distal renal tubular acidosis; Hem, hemizygous; FHHNC, familial hypomagnesemia with hypercalciuria and nephrocalcinosis; PH, primary hyperoxaluria; RTX, renal transplantation; C. intestinalis, Ciona intestinalis; D. rerio, Danio rerio; HC, hypercalciuria; Het, heterozygous; X. tropicalis, Xenopus tropicalis; C. elegans, Caenorhabditis elegans; NPHLOP1, hypophosphatemic nephrolithiasis/osteoporosis-1; FS, Fanconi syndrome; C. savigny, Caenorhabditis savigny; HHRH, hereditary hypophosophatemic rickets with hypercalciuria.
Note that JAS-B21 and JAS-B22 are siblings (same family); all other listed individuals are unrelated.
Molecular genetic diagnosis added new aspects to the previously established clinical diagnosis (5 of 12=41.6%).
CLCN5 is located on the X chromosome (X-linked recessive mode of inheritance).
Individuals B167–21 and JAS-C1 both reached ESRD in their 20s and 30s.
For B168–21, segregation analysis shows compound heterozygosity.
No pathogenic mutations were identified in the genes APRT, ATP6V0A4, CA2, CASR, CLCNKB, CLDN19, FAM20A, GRHPR, HNF4A, HOGA1, HPRT1, KCNJ1, OCRL, SLC12A1, VDR, and XDH. In total, 20 of 50 mutations (40%) detected were novel pathogenic variants, which have not been previously reported (Human Gene Mutation Database; http://www.hgmd.cf.ac.uk/ac/index.php).9–22
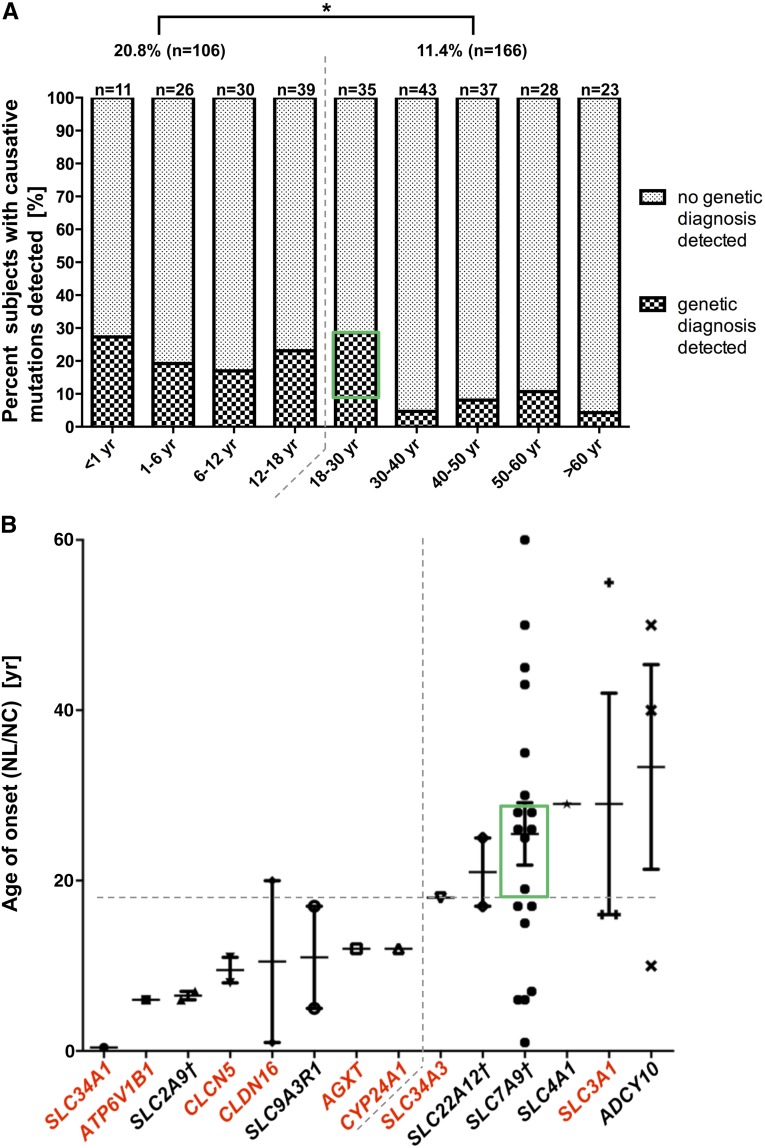
In the pediatric subgroup of individuals with age of onset before 18 years (defined by age at first stone or age at diagnosis of NC), we identified a causative mutation in 20.8% (22 of 106) of individuals (Figure 1A). In contrast, in the adult cohort (defined by age of onset ≥18 years), deleterious variants were detected in 11.4% (19 of 166) of individuals (Figure 1A). The distribution of median age of onset across 14 genes, in which causative mutations were found, correlated with the mode of inheritance as follows: although four of six dominant genes were associated with adult manifestation (≥18 years), mutations in recessive genes were mainly identified in probands with an age of onset before 18 years (Figure 1B).
Figure 1.
Distribution of established molecular genetic causes of NL/NC. (A) Percentage of subjects with identified molecular diagnoses across grouped ages of onset. This percentage is significantly higher in the pediatric cohort (age of onset<18 years) compared with the adult cohort (age of onset≥18 years): 20.8% versus 11.4%. *P≤0.05. In A and B, the green rectangles indicate six individuals with heterozygous SLC7A9 mutations enriched within the age group ≥18–30 years (6 of 19=31.6%). (B) Distribution of age of onset across mutated causative genes. Genes with a dominant mode are annotated in black, whereas genes with a recessive mode are annotated in red. †For SLC2A9, SLC22A12, and SLC7A9, detected mutations were primarily dominant, although both modes of inheritance have been reported. Detected mutations in four of six genes with a median onset ≥18 years are in dominant genes (upper right quadrant as indicated by dashed lines), whereas six of eight genes with a median onset of disease <18 years are in recessive genes (lower left quadrant as indicated by dashed lines).
For the gene SLC7A9, which was found most commonly mutated in this cohort, there was no correlation between age of onset and allelic strength (truncating versus missense variant) (Supplemental Figure 1A). Almost one third of individuals with SLC7A9 mutations manifested within the age range of ≥18–30 years, with a median at 26 years (Figure 1). However, there was a genotype–phenotype correlation in that individuals with two pathogenic variants of SLC7A9 (8 of 19 unrelated individuals) showed a significantly earlier manifestation compared with individuals with only one pathogenic variant (mean: 18 versus 30 years) (Supplemental Figure 1B). For approximately 60% of individuals, the identified genetic diagnosis confirmed the previously obtained clinical diagnosis. In approximately 40% of cases, however, the genetic diagnosis contributed additional etiologic and diagnostic information to what was clinically suspected, suggesting practical implications (Tables 1 and 2).
We here examined an international cohort of 272 typical kidney stone formers for the presence of mutations in 30 genes that cause NL/NC if mutated. We identified 50 (20 novel) pathogenic alleles in 14 different genes in 40 of 268 families (14.9%).
This work, to the best of our knowledge, is the most extensive genetic screening of known NL/NC-causing genes in a combined cohort of pediatric and adult individuals with kidney stones and/or NC. The overall percentage of families with pathogenic mutations exceeds the general assumption of a relatively small contribution of monogenic causes to the general population of stone formers. Remarkably, in almost 21% of the pediatric cohort and 11% of the adult cohort, we identified causative mutations in 1 of 14 genes. The fact that we did not find mutations in the remaining 16 genes suggests that mutations in those genes are less prevalent. Although it is generally assumed that around 85% of causative mutations in monogenic disorders reside within coding regions and adjacent splice sites,23 copy number variations and deleterious deep intronic variants are undetectable with the screening approach that we applied to this study. These limitations as well as population genetic factors may have led to false negatives and a selection bias in regards to the distribution of molecular diagnoses in this study cohort.
SLC7A9 was, by far, the most prevalent disease-causing gene in our cohort, with a median age of first stone at 26 years (Figure 1B). This finding is in line with retrospective data derived from stone composition analysis showing the predominance of cystinuria as the major monogenic cause of stone disease in the adult population.24 Presence of two pathogenic variants in SLC7A9 led to earlier manifestation in our cohort (Supplemental Figure 1B). Whether this finding is because of recessive inheritance or the presence of two mutations on the same allele could not be fully investigated for reasons of incomplete allelic segregation data. Interestingly, in six individuals with mutated SLC7A9, clinical data did not raise the suspicion of cystinuria, and three of them exhibited calcium-containing kidney stones (JAS-C8, JAS-E5, and JAS-F87) (Tables 1 and 2). There are recent reports showing similar findings of calcium-based stones in individuals with heterozygous SLC7A9 mutations.25,26 Additionally, Martins et al.27 showed that cystine promotes calcium oxalate crystal formation in vitro, implying that cystinuria may be a risk factor for calcium oxalate calculi. In conclusion, these data emphasize the need to screen urine for the presence of excess dibasic amino acids, including cystine, in all stone formers, even if the molecular genetic diagnosis has been established.28 Although cystinuria is the most common clinically known genetic diagnosis, in some of these individuals, only broad genetic screening revealed the etiology, affecting their future treatment and the preventative measures that could then be instituted.
Admittedly, for ADCY10, in which we found three putatively deleterious alleles, the evidence for single-gene causation is controversial. The OMIM phenotype implies susceptibility to absorptive hypercalciuria. This notion is on the basis of a single case control study by Reed et al.29 and has never been confirmed in larger-sized cohorts.
Nevertheless, our study supports the conception that NL/NC is genetically broadly heterogeneous. We provide evidence that the role of monogenic causes accounts for a significantly higher percentage of kidney stone formers than generally suspected, especially in the adult population. Most importantly, identification of the monogenic causes of NL/NC may not only have important prognostic implications but also lead to therapeutic consequences (Tables 1 and 2): in >40% of the cases in our study, the molecular genetic diagnoses contributed a new aspect to the previously established clinical diagnosis, suggesting practical implications, such as avoiding vitamin D (CYP24A1), initiating audiometry (ATP6V1B1), or excluding the risk of recurrence in renal transplants (CLCN5 or CLDN16) (Tables 1 and 2).
An excellent example of individualized therapy on the basis of molecular genetic diagnostics was previously shown for individuals with primary hyperoxaluria type 1 caused by AGXT mutations, where pyridoxine sensitivity is associated with the presence of a distinct allele (Gly170Arg).30 Furthermore, invasive and potentially harmful procedures, such as a diagnostic liver biopsy in individuals with suspected primary hyperoxaluria type 1, can be circumvented after diagnostic gene panels become part of the clinical repertoire.31 The use of such a broad genetic screening device may also help raise awareness of extremely rare disorders and be beneficial in cases of atypical clinical presentation or hindered standard diagnostics because of advanced CKD.
Concise Methods
Human Participants
We obtained blood samples and pedigrees after receiving informed consent from individuals with reported NL/NC. The study was approved by the Institutional Review Boards of the Boston Children’s Hospital (BCH) and the Newcastle and North Tyneside Research Ethics Committee. Participants were included from typical kidney stone clinics in a consecutive manner over a time period of 2 years at the University of Newcastle and University Clinic Skopje and 5 months at the BCH. Enrollment in the study was on the basis of the following clinical diagnoses by an investigator nephrologist: NL of any kind (n=256) or isolated NC (n=16) (Supplemental Table 1). Of 256 individuals with NL, 11 individuals also showed NC on renal ultrasound and/or computed tomography scan. Excluded from the study were subjects with a kidney stone-related disorder known to be secondary to drugs (i.e., vitamin D) or primary systemic disease (primary hyperparathyroidism). In total, mutation analysis was conducted in 336 DNA samples (7×48 samples), 272 of which represented genetically unresolved individuals from 268 different families with NL/NC. The remaining 64 samples comprised 33 unaffected controls (negative controls), 7 duplicate samples, 9 samples from individuals who, in retrospect, did not match any of the inclusion criteria after thorough clinical re-evaluation, and 15 samples from individuals with established molecular diagnoses in any 1 of 30 known NL/NC genes (positive controls). Study participants’ ethnicities were as follows: western and northern Europe (n=174), eastern Europe (n=69), the United States (European American; n=18), Asia (n=6), the Middle East (n=3), southern Europe (n=1), and South America (n=1) (Supplemental Table 1). There were 171 men (62.9%) and 101 women (37.1%) in the study; 106 individuals were diagnosed before 18 years of age (pediatric cohort; 39.0%), and 166 individuals were diagnosed as adults (adult cohort; 61.0%).
Mutation Analysis of Known NL/NC-Causing Genes
Mutation analysis was carried out on DNA extracted according to standard methods from peripheral blood or saliva obtained from the study participants. Targeted amplification of 428 amplicons at a time was performed by multiplexed PCR using Fluidigm Access-Array technology followed by barcoding and next generation resequencing on an Illumina MiSeq platform as previously established by our group.7,8 Sanger DNA sequencing was further conducted for single-mutation confirmation. All coding exons and adjacent splice sites of the following 30 genes that are known to cause monogenic forms of NL/NC if mutated (defined by an OMIM phenotype) were screened: ADCY10, AGXT, APRT, ATP6V0A4, ATP6V1B1, CA2, CASR, CLCN5, CLCNKB, CLDN16, CLDN19, CYP24A1, FAM20A, GRHPR, HNF4A, HOGA1, HPRT1, KCNJ1, OCRL, SLC12A1, SLC22A12, SLC2A9, SLC34A1, SLC34A3, SLC3A1, SLC4A1, SLC7A9, SLC9A3R1, VDR, and XDH (Supplemental Table 2).
Sensitivity of Mutation Detection
To calculate the sensitivity of mutation detection, we included 15 DNA samples with 18 known mutations in single-gene cases of NL/NC as positive controls. Overall, 17 of 18 alleles were redetected (sensitivity: 94.4%). The reason for missing one mutation was an amplicon failure of AGXT exon1 caused by target sequence location within a GC-rich region.
Mutation Calling of Genetic Variants as Likely Disease-Causing
Read alignment and variant detection were performed using CLC Genomics Workbench software as previously described.7,8 We considered variants as likely disease-causing according to the following inclusion and exclusion criteria.
Inclusion criteria: (1) truncating mutation (stop gained, abrogation of start or stop codon, abrogation of obligatory splice site, or frame shift) or (2) missense mutation if one of the following is applied: (1) continuous evolutionary conservation to at least Danio rerio; (2) in silico prediction by Polyphen2-HumVar with a score>0.90, suggesting a probably damaging effect on the protein level; or (3) the given disease-causing allele is supported by functional data.
Exclusion criteria: (1) allele is present in healthy controls of the Exome Variant Server database with a minor allele frequency of >2.0% (i.e., >240 in 12,000 control chromosomes) or (2) lack of segregation of a mutant allele according to the affected status of the family members whenever DNA of family members is available.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the physicians Dr. M. Somers, Dr. W. Harmon, Dr. L. Weber, and Dr. T. Irzyniec and the participating patients and their families. We thank P. Hogg for technical support.
S.J.R., D.T.T., and J.A.S. are supported by the Northern Counties Kidney Research Fund. F.H. is an Investigator of the Howard Hughes Medical Institute, a Doris Duke Distinguished Clinical Scientist, and the Warren E. Grupe Professor of Pediatrics. This research was supported by National Institutes of Health Grant R01-DK088767 (to F.H.) and March of Dimes Foundation Grant 6FY11-241.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Search for Monogenic Causes of Kidney Stones,” on pages 507–510.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014040388/-/DCSupplemental.
References
- 1.Scales CD, Jr., Smith AC, Hanley JM, Saigal CS, Urologic Diseases in America Project : Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rule AD, Bergstralh EJ, Melton LJ, 3rd, Li X, Weaver AL, Lieske JC: Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 4: 804–811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieske JC, Turner ST, Edeh SN, Smith JA, Kardia SLR: Heritability of urinary traits that contribute to nephrolithiasis. Clin J Am Soc Nephrol 9: 943–950, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldfarb DS, Fischer ME, Keich Y, Goldberg J: A twin study of genetic and dietary influences on nephrolithiasis: A report from the Vietnam Era Twin (VET) Registry. Kidney Int 67: 1053–1061, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Stechman MJ, Loh NY, Thakker RV: Genetics of hypercalciuric nephrolithiasis: Renal stone disease. Ann N Y Acad Sci 1116: 461–484, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Edvardsson VO, Goldfarb DS, Lieske JC, Beara-Lasic L, Anglani F, Milliner DS, Palsson R: Hereditary causes of kidney stones and chronic kidney disease. Pediatr Nephrol 28: 1923–1942, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halbritter J, Diaz K, Chaki M, Porath JD, Tarrier B, Fu C, Innis JL, Allen SJ, Lyons RH, Stefanidis CJ, Omran H, Soliman NA, Otto EA: High-throughput mutation analysis in patients with a nephronophthisis-associated ciliopathy applying multiplexed barcoded array-based PCR amplification and next-generation sequencing. J Med Genet 49: 756–767, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Halbritter J, Porath JD, Diaz KA, Braun DA, Kohl S, Chaki M, Allen SJ, Soliman NA, Hildebrandt F, Otto EA, GPN Study Group : Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum Genet 132: 865–884, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisceglia L, Fischetti L, Bonis PD, Palumbo O, Augello B, Stanziale P, Carella M, Zelante L: Large rearrangements detected by MLPA, point mutations, and survey of the frequency of mutations within the SLC3A1 and SLC7A9 genes in a cohort of 172 cystinuric Italian patients. Mol Genet Metab 99: 42–52, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Feliubadaló L, Font M, Purroy J, Rousaud F, Estivill X, Nunes V, Golomb E, Centola M, Aksentijevich I, Kreiss Y, Goldman B, Pras M, Kastner DL, Pras E, Gasparini P, Bisceglia L, Beccia E, Gallucci M, de Sanctis L, Ponzone A, Rizzoni GF, Zelante L, Bassi MT, George AL, Jr., Manzoni M, De Grandi A, Riboni M, Endsley JK, Ballabio A, Borsani G, Reig N, Fernández E, Estévez R, Pineda M, Torrents D, Camps M, Lloberas J, Zorzano A, Palacín M, International Cystinuria Consortium : Non-type I cystinuria caused by mutations in SLC7A9, encoding a subunit (bo,+AT) of rBAT. Nat Genet 23: 52–57, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Leclerc D, Boutros M, Suh D, Wu Q, Palacin M, Ellis JR, Goodyer P, Rozen R: SLC7A9 mutations in all three cystinuria subtypes. Kidney Int 62: 1550–1559, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Botzenhart E, Vester U, Schmidt C, Hesse A, Halber M, Wagner C, Lang F, Hoyer P, Zerres K, Eggermann T, Arbeitsgemeinschaft für Pädiatrische Nephrologie (APN) : Cystinuria in children: Distribution and frequencies of mutations in the SLC3A1 and SLC7A9 genes. Kidney Int 62: 1136–1142, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Bisceglia L, Reznik-Wolf H, Di Perna M, Pras E: Human gene mutations. Gene symbol: SLC3A1. Disease: Cystinuria. Hum Genet 122: 215, 2007 [PubMed] [Google Scholar]
- 14.Font MA, Feliubadaló L, Estivill X, Nunes V, Golomb E, Kreiss Y, Pras E, Bisceglia L, d’Adamo AP, Zelante L, Gasparini P, Bassi MT, George AL, Jr., Manzoni M, Riboni M, Ballabio A, Borsani G, Reig N, Fernández E, Zorzano A, Bertran J, Palacín M, International Cystinuria Consortium : Functional analysis of mutations in SLC7A9, and genotype-phenotype correlation in non-Type I cystinuria. Hum Mol Genet 10: 305–316, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Karim Z, Gérard B, Bakouh N, Alili R, Leroy C, Beck L, Silve C, Planelles G, Urena-Torres P, Grandchamp B, Friedlander G, Prié D: NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med 359: 1128–1135, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Tasic V, Hynes AM, Kitamura K, Cheong HI, Lozanovski VJ, Gucev Z, Jutabha P, Anzai N, Sayer JA: Clinical and functional characterization of URAT1 variants. PLoS ONE 6: e28641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endsley JK, Phillips JA, 3rd, Hruska KA, Denneberg T, Carlson J, George AL, Jr.: Genomic organization of a human cystine transporter gene (SLC3A1) and identification of novel mutations causing cystinuria. Kidney Int 51: 1893–1899, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Calonge MJ, Gasparini P, Chillarón J, Chillón M, Gallucci M, Rousaud F, Zelante L, Testar X, Dallapiccola B, Di Silverio F, Barcelo P, Estivill X, Zorzano A, Nunes V, Palacín M: Cystinuria caused by mutations in rBAT, a gene involved in the transport of cystine. Nat Genet 6: 420–425, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Mohebbi N, Vargas-Poussou R, Hegemann SC, Schuknecht B, Kistler AD, Wüthrich RP, Wagner CA: Homozygous and compound heterozygous mutations in the ATP6V1B1 gene in patients with renal tubular acidosis and sensorineural hearing loss. Clin Genet 83: 274–278, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP: Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285: 103–106, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Weber S, Hoffmann K, Jeck N, Saar K, Boeswald M, Kuwertz-Broeking E, Meij II, Knoers NV, Cochat P, Suláková T, Bonzel KE, Soergel M, Manz F, Schaerer K, Seyberth HW, Reis A, Konrad M: Familial hypomagnesaemia with hypercalciuria and nephrocalcinosis maps to chromosome 3q27 and is associated with mutations in the PCLN-1 gene. Eur J Hum Genet 8: 414–422, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Bröking E, Fehrenbach H, Wingen AM, Güran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M: Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 365: 410–421, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, Nayir A, Bakkaloğlu A, Ozen S, Sanjad S, Nelson-Williams C, Farhi A, Mane S, Lifton RP: Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci U S A 106: 19096–19101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jungers P, Joly D, Blanchard A, Courbebaisse M, Knebelmann B, Daudon M: Lithiases rénales héréditaires monogéniques: Récents acquis diagnostiques et thérapeutiques. Nephrol Ther 4: 231–255, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Cupisti A, Farnesi I, Armillotta N, Francesca F: Staghorn cystine stone in a 72-year-old recurrent calcium stone former. Clin Nephrol 78: 76–80, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Elkoushy MA, Andonian S: Characterization of patients with heterozygous cystinuria. Urology 80: 795–799, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Martins MC, Meyers AA, Whalley NA, Rodgers AL: Cystine: A promoter of the growth and aggregation of calcium oxalate crystals in normal undiluted human urine. J Urol 167: 317–321, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Sayer JA, Moochhala SH, Thomas DJ: The medical management of urolithiasis. Br J Med Surg Urol 3: 87–95, 2010 [Google Scholar]
- 29.Reed BY, Heller HJ, Gitomer WL, Pak CY: Mapping a gene defect in absorptive hypercalciuria to chromosome 1q23.3-q24. J Clin Endocrinol Metab 84: 3907–3913, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Monico CG, Rossetti S, Olson JB, Milliner DS: Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int 67: 1704–1709, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Rumsby G, Williams E, Coulter-Mackie M: Evaluation of mutation screening as a first line test for the diagnosis of the primary hyperoxalurias. Kidney Int 66: 959–963, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.