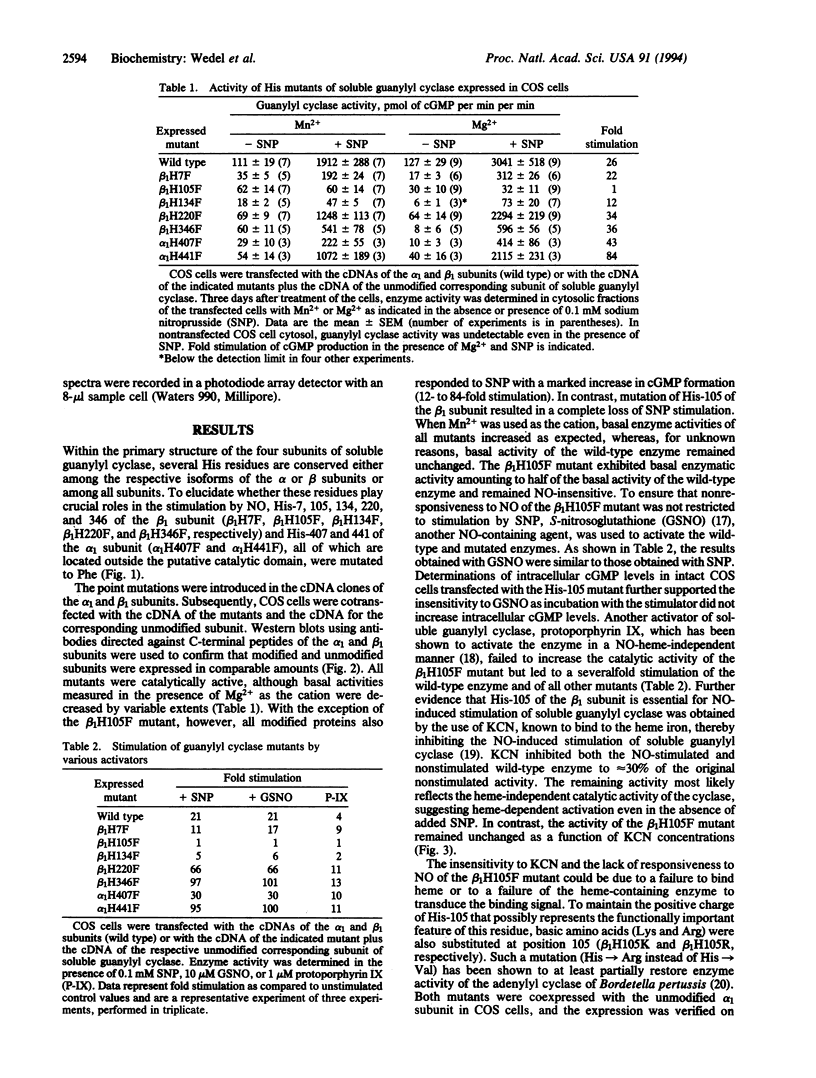
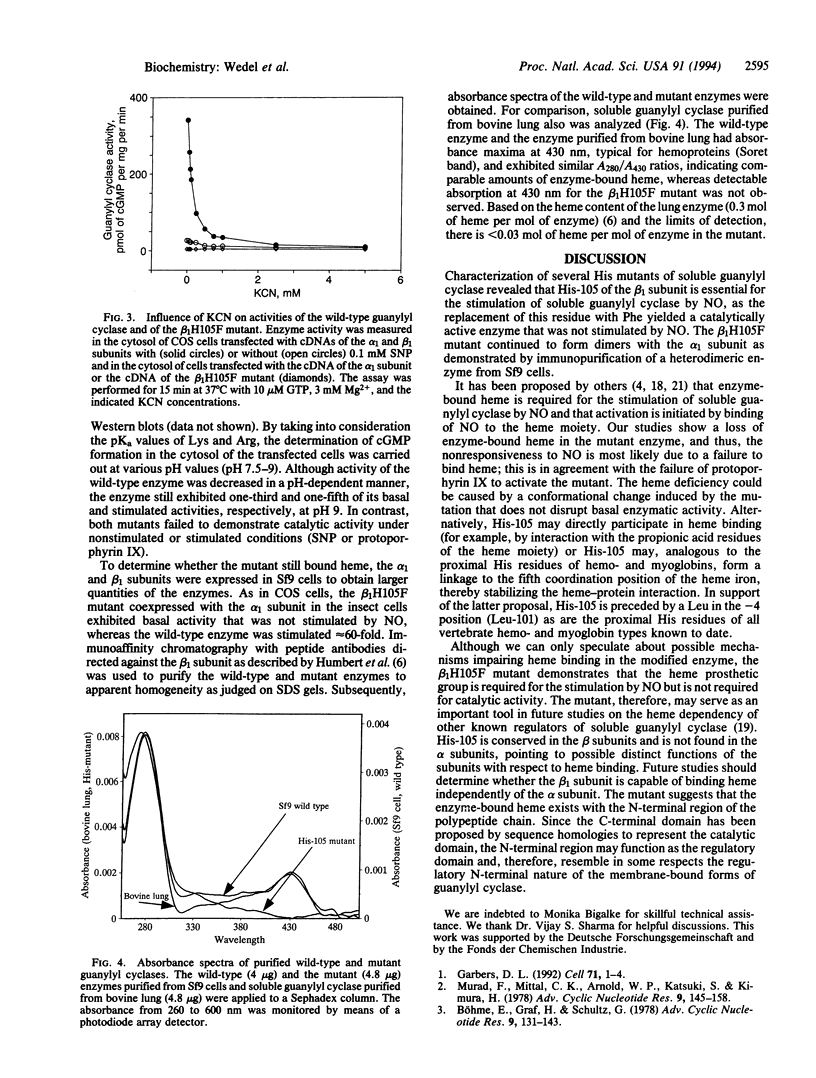
Abstract
Soluble guanylyl cyclase [GTP pyrophosphate-lyase (cyclizing); EC 4.6.1.2] is a hemoprotein that exists as a heterodimer; the heme moiety has been proposed to bind nitric oxide, resulting in a dramatic activation of the enzyme. Mutation of six conserved His residues reduced but did not abolish nitric oxide stimulation whereas a change of His-105 to Phe in the beta 1 subunit yielded a heterodimer that retained basal cyclase activity but failed to respond to nitric oxide. Heme was not detected as a component of the mutant heterodimer and protophorphyrin IX failed to stimulate enzyme activity. The activity of the His mutant was almost identical to that of the wild-type enzyme in the presence of KCN, suggesting that disruption of heme binding is the principal effect of the mutation. Thus, the mutation provides a means to inhibit the nitric oxide-sensitive guanylyl cyclase signaling pathway.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhme E., Graf H., Schultz G. Effects of sodium nitroprusside and other smooth muscle relaxants on cyclic GMP formation in smooth muscle and platelets. Adv Cyclic Nucleotide Res. 1978;9:131–143. [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Restoration of the responsiveness of purified guanylate cyclase to nitrosoguanidine, nitric oxide, and related activators by heme and hemeproteins. Evidence for involvement of the paramagnetic nitrosyl-heme complex in enzyme activation. J Biol Chem. 1978 Dec 10;253(23):8433–8443. [PubMed] [Google Scholar]
- Garbers D. L. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992 Oct 2;71(1):1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- Gerzer R., Radany E. W., Garbers D. L. The separation of the heme and apoheme forms of soluble guanylate cyclase. Biochem Biophys Res Commun. 1982 Sep 30;108(2):678–686. doi: 10.1016/0006-291x(82)90883-x. [DOI] [PubMed] [Google Scholar]
- Groebe D. R., Chung A. E., Ho C. Cationic lipid-mediated co-transfection of insect cells. Nucleic Acids Res. 1990 Jul 11;18(13):4033–4033. doi: 10.1093/nar/18.13.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harteneck C., Koesling D., Söling A., Schultz G., Böhme E. Expression of soluble guanylyl cyclase. Catalytic activity requires two enzyme subunits. FEBS Lett. 1990 Oct 15;272(1-2):221–223. doi: 10.1016/0014-5793(90)80489-6. [DOI] [PubMed] [Google Scholar]
- Harteneck C., Wedel B., Koesling D., Malkewitz J., Böhme E., Schultz G. Molecular cloning and expression of a new alpha-subunit of soluble guanylyl cyclase. Interchangeability of the alpha-subunits of the enzyme. FEBS Lett. 1991 Nov 4;292(1-2):217–222. doi: 10.1016/0014-5793(91)80871-y. [DOI] [PubMed] [Google Scholar]
- Humbert P., Niroomand F., Fischer G., Mayer B., Koesling D., Hinsch K. D., Gausepohl H., Frank R., Schultz G., Böhme E. Purification of soluble guanylyl cyclase from bovine lung by a new immunoaffinity chromatographic method. Eur J Biochem. 1990 Jun 20;190(2):273–278. doi: 10.1111/j.1432-1033.1990.tb15572.x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Degnan J. N., Baricos W. H., Kadowitz P. J., Wolin M. S. Activation of purified guanylate cyclase by nitric oxide requires heme. Comparison of heme-deficient, heme-reconstituted and heme-containing forms of soluble enzyme from bovine lung. Biochim Biophys Acta. 1982 Sep 17;718(1):49–59. doi: 10.1016/0304-4165(82)90008-3. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Wood K. S., Wolin M. S. Activation of purified soluble guanylate cyclase by protoporphyrin IX. Proc Natl Acad Sci U S A. 1982 May;79(9):2870–2873. doi: 10.1073/pnas.79.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koesling D., Böhme E., Schultz G. Guanylyl cyclases, a growing family of signal-transducing enzymes. FASEB J. 1991 Oct;5(13):2785–2791. doi: 10.1096/fasebj.5.13.1680765. [DOI] [PubMed] [Google Scholar]
- Koesling D., Harteneck C., Humbert P., Bosserhoff A., Frank R., Schultz G., Böhme E. The primary structure of the larger subunit of soluble guanylyl cyclase from bovine lung. Homology between the two subunits of the enzyme. FEBS Lett. 1990 Jun 18;266(1-2):128–132. doi: 10.1016/0014-5793(90)81523-q. [DOI] [PubMed] [Google Scholar]
- Koesling D., Herz J., Gausepohl H., Niroomand F., Hinsch K. D., Mülsch A., Böhme E., Schultz G., Frank R. The primary structure of the 70 kDa subunit of bovine soluble guanylate cyclase. FEBS Lett. 1988 Oct 24;239(1):29–34. doi: 10.1016/0014-5793(88)80539-8. [DOI] [PubMed] [Google Scholar]
- Mayer B., Brunner F., Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacol. 1993 Jan 26;45(2):367–374. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- Munier H., Bouhss A., Krin E., Danchin A., Gilles A. M., Glaser P., Bârzu O. The role of histidine 63 in the catalytic mechanism of Bordetella pertussis adenylate cyclase. J Biol Chem. 1992 May 15;267(14):9816–9820. [PubMed] [Google Scholar]
- Murad F., Mittal C. K., Arnold W. P., Katsuki S., Kimura H. Guanylate cyclase: activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv Cyclic Nucleotide Res. 1978;9:145–158. [PubMed] [Google Scholar]
- Nakane M., Arai K., Saheki S., Kuno T., Buechler W., Murad F. Molecular cloning and expression of cDNAs coding for soluble guanylate cyclase from rat lung. J Biol Chem. 1990 Oct 5;265(28):16841–16845. [PubMed] [Google Scholar]
- Nakane M., Saheki S., Kuno T., Ishii K., Murad F. Molecular cloning of a cDNA coding for 70 kilodalton subunit of soluble guanylate cyclase from rat lung. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1139–1147. doi: 10.1016/s0006-291x(88)80992-6. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Gilman A. G. Adenylyl cyclases. Cell. 1992 Sep 18;70(6):869–872. doi: 10.1016/0092-8674(92)90236-6. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Yuen P. S., Potter L. R., Garbers D. L. A new form of guanylyl cyclase is preferentially expressed in rat kidney. Biochemistry. 1990 Dec 11;29(49):10872–10878. doi: 10.1021/bi00501a002. [DOI] [PubMed] [Google Scholar]