Abstract
Background:
Helicobacter pylori infection is the most common chronic bacterial infection around the world and an important cause of gastrointestinal disorders, which might be involved in the pathogenesis of some extragastrointestinal disturbances as well as changes in serum lipid profile. Hypolipemic properties of omega-3 fatty acids have been studied in several studies.
Objectives:
The present study aimed to compare the effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) supplementation on the level of serum lipoproteins in H. pylori.
Patients and Methods:
In a randomized, double-blinded, placebo-controlled clinical trial in Iran, 105 Helicobacter pylori were randomly allocated to receive 2 g of daily EPA (35 patients), DHA (35 patients), or medium-chain triglyceride (MCT) oil as placebo (33 patients) along with conventional tetra-drug H. pylori eradication regimen for 12 weeks.
Results:
From 105 included patients, 97 (31 in EPA, 33 in DHA, and 33 in control groups) completed the study and were included in final analysis. The levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and the ratios of TG/HDL-C, TC/HDL-C, and LDL-C/HDL-C were not significantly different among the three groups, while the level of triglyceride (TG) was statistically different. DHA (-16.6 ± 30.34) and control (+ 15.32 ± 56.47) groups were statistically different with regard to changes in TG levels (P = 0.000).
Conclusions:
There was no difference between the effects of 2 g of EPA or DHA supplementation for 12 weeks on the levels of total cholesterol, LDL-C, HDL-C, TC/HDL-C, TG/HDL-C, and LDL-C/HDL-C; however, it had a desirable effect on the level of TG in a way that the effect of DHA was clearer.
Keywords: Helicobacter pylori, Eicosapentaenoic Acid, Docosahexaenoic Acid, Cholesterol, Triglyceride
1. Background
Helicobacter pylori, the most common cause of chronic bacterial infection worldwide (1), is the main etiologic factor of peptic ulcer and is responsible for several other gastrointestinal (GI) disorders (2). While about half of the world population are reported to be the carriers of this organism (3), the prevalence of the infection among the adult population in the Middle East and Iran have been reported to be 70% to 90% (2) and 69% (4), respectively. Complications of the infection depend on complex interactions between the bacteria and the host, e.g. infection virulence, genetics sequence, the age of the host, environmental factors, and dietary habits (5). Several studies have suggested that H. pylori infection might be involved in the pathogenesis of some extra-GI disturbances. Due to complications of H. pylori infection such as endothelial injury, smooth muscle proliferation, and local inflammation of blood vessel walls (1) and its effect on lipid metabolism (1, 2, 6), it is considered as a risk factor for cardiovascular diseases (7). The association of this bacteria with atherosclerotic diseases, gallbladder stone, dyspepsia, metabolic syndrome, type 1 diabetes mellitus, and insulin resistance have been reported in the studies conducted in Iran (8). Many studies have shown that infection with H. pylori increases the levels of total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), Apo B, Lp (a), TC/high-density lipoprotein cholesterol (HDL-C), LDL-C/HDL-C, and decreases in the levels of HDL-C and Apo A-I, and therefore, is risk factor of coronary heart disease (CHD), coronary artery disease (CAD), atherosclerosis, and stroke (1, 2, 6, 9, 10). Omega-3 fatty acids (N3FAs) have shown to be beneficial due to their anti-inflammatory, antithrombotic, antiarrhythmic, hypolipemic and vasodilatory properties (11). It has been noted that by suppressing the hepatic lipogenesis, N3FAs influence lipid metabolism (12), prevent TG synthesis, and decrease production and secretion of very-low density lipoprotein (VLDL) (13). Furthermore, various studies have shown that eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have different clinical effects on gene expression (14), blood pressure (15, 16), vascular function (15), serum lipid profile (17) such as TG (18) and HDL-C (15), size of lipoprotein particles such as HDL-C, LDL-C, and VLDL (14, 18), serum glucose and insulin in hyperlipidemic male adults (17), inflammatory mediators (19, 20), mood disorders such as depression (21, 22), visual disorders (23-26), and cognitive disorders such as dementia (27).
2. Objectives
Given the high prevalence of H. pylori infection, abnormal serum lipid profile in patients with this infection, and the potential beneficial effects of N3FAs supplementation on the improvement of the patients infected with this bacterium, this study aimed to compare the effects of EPA and DHA supplementation on the level of serum lipoproteins in those infected with H. pylori.
3. Patients and Methods
This randomized, double-blinded, placebo-controlled clinical trial was performed in Tehran, Iran, during the winter of 2012. The population of the study included all adult patients, aged 20 to 60 years, who were admitted to the Gastrointestinal and Liver Disease Research Center of Iran University of Medical Sciences (Rasool Akram Hospital). The under study patients were diagnosed with H. pylori infection by a gastroenterologist based on the pathologic results of the stomach biopsy and met the eligibility criteria for participation in the study. These criteria included no history of treatment for H. pylori eradication, no gastric surgery, no history of peptic ulcer, no diseases causing inflammation or increased levels of inflammatory markers such as diabetes mellitus, no cardiovascular diseases, and no pulmonary inflammations such as asthma. Other criteria were taking no medications other than those prescribed during the study period, taking no antibiotics or bismuth during the previous two months, taking no antioxidant supplements such as selenium, zinc, or beta-carotene for at least three months prior to the study, taking no omega-3 supplements for at least three months prior to the study, no smoking, and having a body mass index (BMI) < 30 kg/m2. Exclusion criteria were changes in the type or dose of medication, changes in diet or daily physical activity, any use of antioxidant supplements, and consumption of < 80% of supplements during the study period. First, among those who were diagnosed with H. pylori infection via the tissue culture, patients who volunteered to participate in the study and met the inclusion criteria were selected and invited to participate in the study. After attending the orientation session and obtaining written informed consent, the participants were enrolled. To determine the sample size, preliminary data were obtained. The quantitative dependent variables were determined according to the study of Barbosa et al. (28) and Saifullah et al. (29). There was an attempt to reject the null hypothesis of no difference in serum levels of lipoproteins among the three groups, at type I error level (α) of 0.05 and test power (1-β) of 0.8. Therefore, a sample size of 30 patients per group was calculated but 35 patients in each group were selected, given that there would be 15% dropout during study. At the beginning of the study, information on socio-economic status of participants was collected by means of a questionnaire completed during an interview. For each participant, while wearing light clothes, body weight was measured, using a SECA scale with the precision of 0.5 kg. Each participant's height was measured in standing position without shoes, using a tape measure with precision of 0.5 cm. Finally, BMI was calculated (weight [kg]/ squared height [m2]). At the beginning of the study, 10 mL of blood samples were obtained from patients after 10 to 12 hours of fasting. Participants were randomly allocated to EPA, DHA, or control groups to receive 2 g of respectively morEPA, morDHA, or placebo daily for 12 weeks. Each morEPA and morDHA supplement had a purity of 75% containing 750 mg of N3FA (Minami-nutrition Co., Belgium). MorEPA contained 580 mg of EPA, 83 mg of DHA, and 87 mg of other N3FAs. MorDHA contained 63 mg of EPA, 465 mg of DHA, and 222 mg of other N3FAs. The placebo, used in the study, was medium-chain TG (MCT) oil, in 1-g capsules with a purity of 100%, designed by Canadian company of Viva to specifically resemble omega-3 capsules. Before the beginning of the study, all tins containing the capsules were coded by an independent person other than the researchers. Patients, in all three groups, received conventional tetra-drug eradication regimen of H. pylori including metronidazole (500 mg, bid), amoxicillin (1 g, bid), bismuth sub-citrate (240 mg, bid), and omeprazole (20 mg, bid) for 12 weeks. The report of daily dietary intake was collected by a 24-hour diet recall questionnaire once before the beginning of the study, then at fourth week, eighth week, and at the end of the study. The data were then analyzed by Nutritionist IV software. The reports of the level of physical activity were obtained through face-to-face interview using the international physical activity questionnaire (IPAQ) at the beginning and the end of the study. Patients were asked not to change their usual dietary intake and physical activity during the study. During the 12-week intervention, the patients were called to resolve possible problems and ensure that the participants had consumed the drugs and supplements. At the end of week 12, the patients were asked to refer for H. pylori eradication check and related blood tests. All initial checks were repeated except height measurement. Serum lipoproteins were measured with calibrated instruments and in the following ways: TC and TG by enzymatic-colorimetric (photometric) method using Parsazmun company kits (Iran); and LDL-C and HDL-C by enzymatic selective protection/endpoint (turbidimetry) method using Zist-Shimi company kit (Iran). The intra-assay and inter-assay precision (CV%) were respectively as follows: CHOD kit (for TC measurement), 0.61 and 1.22; GPO-PAP kit (for TG measurement), 1.82 and 1.04; LDL-C measurement kit, 1.50 and 1.97; and HDL-C measurement kit, 2.12 and 2.73. Data entry and statistical analysis were performed using SPSS 16 (SPSS Inc., Chicago, IL, USA). Quantitative variables were reported with mean and standard deviation (mean ± SD) and their normal distribution was determined by Kolmogorov-Smirnov test. The difference in blood biochemical variables was compared in each group before and after the intervention. For variables with normal distribution, paired-samples t-test and for variables other than those, Wilcoxon test were used. To compare quantitative variables between the three groups, analysis of variance (ANOVA) and Kruskal-Wallis tests were used for normally distributed variables or otherwise, respectively. Differences with P < 0.05 were considered statistically significant. The protocol of study complied with the Helsinki declaration and informed consent was obtained from each patient. The study was approved by the Ethics Committee of Public Health School of Iran University of Medical Sciences and was registered in Iranian Center of Clinical Trial Registration with the ID number of IRCT 201101122709N16.
4. Results
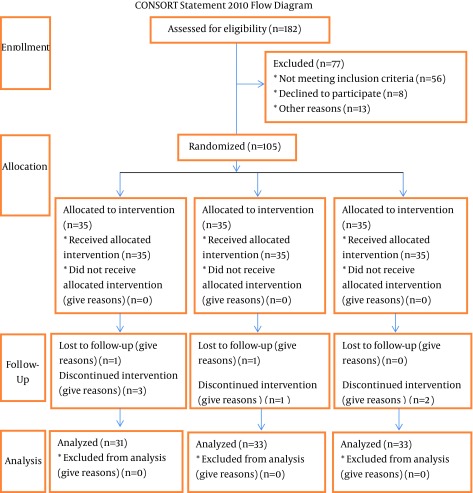
From 105 enrolled patients, 97 (31 in the EPA, 33 in the DHA, and 33 in the control groups) completed the study. Eight patients were excluded from the study because of migration, adverse effects of drugs and supplements, or refusing to continue (Figure 1). The sex and age distribution among study groups were as follows: EPA group, 12 males and 19 females (mean age, 35.54 ± 9.15 years); DHA group, 14 males and 19 females (mean age, 37.09 ± 10.28 years); and control group, 12 males and 21 females (mean age, 38.60 ± 10.92 years) (Tables 1 and 2). There were no significant differences among the intervention and control groups regarding the distribution of sex, age, weight, daily dietary intake, and the level of physical activity at the beginning of the study; however, the BMI was significantly different (P = 0.04). Moreover, no significant change was observed in all three groups in the mean weight, BMI, daily dietary intake, and physical activity level during the study period (Tables 1 and 2). In addition, there were no significant differences among the three groups in the levels of biochemical variables at the baseline (Table 3). According to the findings of the study, there was no significant difference among the three groups in the average levels of TC, LDL-C, HDL-C, TC/HDL-C, TG/HDL-C, and LDL-C/HDL-C, while the level of TG was statistically different (P = 0.000). The post hoc analysis (Tukey's test) showed that DHA and control groups were different with regard to TG levels (Table 3). In the EPA group, there was a significant reduction in the mean serum levels of TC, TC/HDL-C, and TG/HDL-C at the end of the study in comparison to the initial values (P = 0.035, P = 0.014, and P = 0.019, respectively), while the change in the mean levels of LDL-C, HDL-C, TG, and LDL-C/HDL-C were not significant. In the DHA group, there was a significant reduction in the mean serum levels of TG, TC/HDL-C, and TG/HDL-C at the end of the study in comparison to the initial values (P = 0.003, P = 0.014, and P = 0.000, respectively), whereas the levels of TC, LDL-C, HDL-C, and LDL-C/HDL-C did not change significantly. There was a significant increase in the serum level of TG (P = 0.024) in the control group (Tables 3 and 4).
Figure 1. CONSORT Statement 2010 Flow Diagram.
Table 1. Distribution of Age, Weight, and Body Mass Index among Study Groups a.
| Variable | EPA (n = 31) | DHA (n = 33) | Control (n = 33) | P Value c |
|---|---|---|---|---|
| Age, y | 54.35 ± 9.15 | 37.09 ± 10.28 | 38.60 ± 10.92 | 0.45 |
| Weight, kg | ||||
| Before intervention | 68.11 ± 12.35 | 70.19 ± 9.92 | 73.81 ± 11.00 | 0.10 |
| After intervention | 69.23 ± 11.24 | 72.09 ± 10.80 | 71.72 ± 11.42 | 0.21 |
| P Value d | 0.13 | 0.20 | 0.23 | - |
| BMI, kg/m 2 | ||||
| Before intervention | 24.17 ± 3.52 | 25.59 ± 3.23 | 26.22 ± 3.42 | 0.04 |
| After intervention | 24.35 ± 3.72 | 25.85 ± 3.73 | 26.20 ± 3.09 | 0.05 |
| P Value d | 0.18 | 0.21 | 0.19 | - |
a Abbreviations: EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; BMI, body mass index.
c ANOVA test.
d Paired-samples t-test.
Table 2. Distribution of Sex and Physical Activity Variables in Three Groups a, b.
| Variable | EPA (n = 31) | DHA (n = 33) | Control (n = 33) | P Value c |
|---|---|---|---|---|
| Sex | ||||
| Male | 13 (37.1) | 14 (40) | 14 (40) | 0.96 |
| Female | 22 (62.9) | 21 (60) | 21 (60) | |
| Physical activity | ||||
| Mild | 24 (77.4) | 30 (90.9) | 27 (81.8) | 0.30 |
| Moderate | 7 (22.6) | 3 (9.1) | 6 (18.2) |
a Abbreviations: EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
b Data are presented as No. (%).
c Chi-square test.
Table 3. Distribution of Blood Biochemical Variables at the Beginning and the End of the Study a, b.
| Variable | EPA (n = 31) | DHA (n = 33) | Control (n = 33) | P Value |
|---|---|---|---|---|
| TC, mg/dL | ||||
| Baseline | 164.09 ± 34.20 | 178.58 ± 48.25 | 170.62 ± 42.89 | 0.397 |
| At the end | 156.06 ± 32.17 | 172.39 ± 39.42 | 172.94 ± 36.25 | 0.108 |
| Difference | -8.03 ± 20.62 | -6.19 ± 41.99 | 2.32 ± 26.40 | 0.348 |
| P Value | 0.035 | 0.418 | 0.611 | - |
| TG, mg/dL | ||||
| Baseline | 95.66 ± 27.14 | 111.16 ± 31.60 | 97.79 ± 29.04 | 0.080 |
| At the end | 91.94 ± 20.41 | 95.10 ± 26.89 | 113.12 ± 59.72 | 0.141 |
| Difference | -3.72 ± 19.13 | -16.6 ± 30.34 | 15.32 ± 56.47 | 0.000 |
| P Value | 0.438 | 0.003 | 0.024 | - |
| LDL-C, mg/dL | ||||
| Baseline | 92.50 ± 23.14 | 100.10 ± 23.48 | 92.97 ± 24.56 | 0.367 |
| At the end | 88.81 ± 18.49 | 98.39 ± 22.05 | 99.47 ± 24.58 | 0.104 |
| Difference | -3.69 ± 18.99 | -1.71 ± 26.40 | 6.50 ± 20.93 | 0.130 |
| P Value | 0.281 | 0.729 | 0.079 | - |
| HDL-C, mg/dL | ||||
| Baseline | 43.75 ± 10.46 | 45.71 ± 10.64 | 43.65 ± 11.78 | 0.703 |
| At the end | 45.84 ± 10.68 | 49.26 ± 12.42 | 46.38 ± 11.30 | 0.450 |
| Difference | 2.09 ± 10.06 | 3.55 ± 12.02 | 2.73 ± 8.76 | 0.855 |
| P Value | 0.248 | 0.111 | 0.078 | - |
| TC/HDL, ratio | ||||
| Baseline | 3.80 ± 0.50 | 3.92 ± 0.85 | 3.89 ± 0.70 | 0.943 |
| At the end | 3.46 ± 0.52 | 3.54 ± 0.49 | 3.78 ± 0.41 | 0.022 |
| Difference | -0.34 ± 0.69 | -0.37 ± 0.98 | -0.11 ± 0.15 | 0.393 |
| P Value | 0.014 | 0.014 | 0.362 | - |
a Abbreviations: EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; BMI, body mass index; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
b Data are presented as Mean ± SD.
Table 4. Distribution of Blood Biochemical Variables at the Beginning and the End of the Study (con't) a, b.
| Variable | EPA (n = 31) | DHA (n = 33) | Control (n = 33) | P Value |
|---|---|---|---|---|
| TG/HDL, ratio | ||||
| Baseline | 2.23 ± 0.57 | 2.50 ± 0.82 | 2.34 ± 0.93 | 0.402 |
| At the end | 2.07 ± 0.50 | 2.00 ± 0.58 | 2.64 ± 2.42 | 0.024 |
| Difference | -0.17 ± 0.40 | -0.51 ± 0.77 | -0.30 ± 2.09 | 0.053 |
| P value | 0.019 | 0.000 | 0.224 | - |
| LDL/HDL, ratio | ||||
| Baseline | 2.14 ± 0.41 | 2.22 ± 0.44 | 2.17 ± 0.43 | 0.719 |
| At the end | 1.97 ± 0.35 | 2.03 ± 0.29 | 2.11 ± 0.32 | 0.208 |
| Difference | -0.16 ± 0.56 | -0.20 ± 0.54 | -0.05 ± 0.43 | 0.486 |
| P Value | 0.111 | 0.052 | 0.484 | - |
a Abbreviations: EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; TG, triglyceride; LDL, low-density lipoprotein; and HDL, high-density lipoprotein.
b Data are presented as Mean ± SD.
5. Discussion
Infection with H. pylori can be treated using standard therapy in more than 80% of infected patients (30). The treatment regimen, examined in the present study, is based on a combination of two antibiotics and a proton pump inhibitor. The three-drugs regimen with a proton pump inhibitor, clarithromycin, and either amoxicillin or metronidazole, is the most commonly administered treatment regimen for the eradication of the infection; however, the major reasons for the failure in eradication therapy are antibiotic resistance and intolerance due to secondary effects. There are several ways to reduce the chances of failure in the treatment: detection of newer and more effective drugs to kill the bacteria, immunization method (vaccine) to stimulate host immune defenses, or extension of nutritional practices for infection control (5). Statistical analysis of Cochran’s and Mantel-Haenszel was applied, as there was the likelihood that the significant difference among the three groups, in BMI, would have an influence on the variables. The results of this analysis suggested that the mean difference in the BMI among three groups could not be a confounding factor in the interpretation of the findings. This study demonstrated that EPA or DHA supplementation significantly differed regarding their effect on the level of serum TG in patients with H. pylori infection; this difference was observed between DHA and control groups. Different effect of EPA and DHA on serum lipid profile had been demonstrated in some studies (17, 18). There was a significant reduction in TG level in comparison to the control value following the DHA treatment, while the decrease values in the EPA group failed to reach significance (18). Reduction in TG level in DHA supplemented group was more than in EPA group (15, 31, 32). Therefore, DHA might be more efficacious in improving the plasma lipid profile than EPA might be. Nonetheless, the findings of this study showed that the mean level of serum TG increased significantly in the control group. Some studies have shown that MCTs significantly increased serum TGs (33, 34). Parinyasiri et al. showed that six-months administration of daily dose of 4-g N FAs (containing EPA and DHA) in patients with IgA nephropathy resulted in a significant reduction in serum TG level, significant increase in HDL-C, and insignificant reduction in TC. In that study, the dose and duration of supplementation were two times of those in our study, and the basic concentration of the serum levels of TG and TC of the patients were higher than that of our study. Therefore, the mean difference between the beginning and the end of the study was higher and clearer than that of our study was (35). Maki et al. noticed that receiving 4 g/d of N3FAs for six weeks in patients with essential hypercholesterolemia caused a significant reduction in serum TG and significant increase in HDL-C and LDL-C levels while it had no effect on the serum TC level. In that study, the supplement dose was twice as much as our dosage while the duration of supplementation was half of that in our study and the basic concentration of the serum levels of TC and LDL-C of the patients in that study were higher than those in our study were, which underlay the inconsistency between their findings with regard to TC and LDL-C with ours (36). The mechanism by which DHA might increase serum level of HDL-C is not known; however, it can be related to change in activity of lipid transfer protein that is decreased after supplementation with N3FAs (17). Damsgaard et al. showed that addition of 5 mL of fish oil to diet of nine-month-old infants, for three months led to a significant elevation in serum TC and LDL-C and a significant reduction in serum TG while it had no effect on the serum HDL-C. Some results of that study were inconsistent with ours. It is difficult to compare these results with our results because the regulation of lipid metabolism might be different in infants. The observed increased cholesterol might not be caused by omega-3 long-chain polyunsaturated fatty acids (LCPUFAs), but might be due to an increased intake of fat and cholesterol delivered by the fish oil (37). Clarke suggested that ingestion of N3LCPUFA would affect lipid metabolism by suppressing hepatic lipogenesis, reducing hepatic TG output, and increasing fatty acid (FA) oxidation in the liver and muscle tissue, possibly via interaction with transcription factors that regulate the expression of genes encoding key regulatory proteins of lipid metabolism. Lowering TG concentrations might decrease transfer of TG to LDL-C and removal of cholesterol from LDL-C, resulting in larger, more cholesterol-rich LDL-C particles (12). The mechanism of lowering effect of long-chain N3FAs on serum TG concentrations has been attributed to a suppressed hepatic lipogenesis with a concomitant reduction in the supply of FAs for TG synthesis and VLDL secretion, and to an enhanced clearance of VLDL in the liver or peripheral tissues (38-40). The mechanism that could illustrate the TG-lowering effects of marine N3FAs has not been described well. An interpreting model can be at the level of gene transcription, which include several metabolic nuclear receptors such as hepatic X receptor, hepatic nuclear factor 4-α (HNF4-α), farnesoid X receptor (FXR), and peroxisome proliferator-activated receptors (PPARs). Each of these receptors is regulated by sterol regulatory element-binding protein-1c (SREBP-1c), the main genetic factor controlling the lipogenesis. The N3FAs show TG-lowering effects via hepatic lipogenesis suppression, decreasing the SREBP-1c level, upregulation fat oxidation in the liver and skeletal muscle by PPAR activation, and increasing the flow of glucose to glycogen by downregulation of HNF 4-α. The N3FAs have more TG-lowering effects than omega-6 FAs have, through downregulation of proteins coding the genes that stimulate lipid synthesis and upregulation of proteins coding the genes that stimulate FAs oxidation. Moreover, these FAs can act via increasing the uptake of postprandial chylomicron by decreasing VLDL secretion and direct stimulation of lipoprotein lipase (LPL) activity. Sum of these effects supports the consumption of N3FAs as an effective clinical factor in treatment of hypertriglyceridemia (41). Taziki et al. (42) showed that in patients on hemodialysis, daily intake of 2 g of N3FAs, containing EPA and DHA, for 12 weeks caused a significant reduction in serum TG and increase in HDL-C, while it had no significant effect on the serum levels of TC and LDL-C. In that study, the dose of supplement and intervention duration were similar to those in our study; the cause of ineffectiveness of supplementation on some serum lipid levels was mentioned to be the administration of rather low dose of N3FAs (2 g/d) (42). Most evidence suggests that N3FAs prevent TG synthesis, reduce the production and secretion of VLDL particles and increases the conversion of VLDL to LDL-C via decreasing lipogenesis by decreasing the enzymatic conversion of acetyl-CoA to FAs, increasing β-oxidation of FAs, inhibiting both phosphatidic acid phosphatase/phosphohydrolase (PAP) (an enzyme that catalyzes the reaction of converting phosphatidic acid to diacylglycerol) and diacylglycerol acyltransferase (DGAT) (an enzyme that catalyzes the final step in TG synthesis), increasing the degradation of apolipoprotein B, and increasing LPL activity (13). Trials in humans, using mixtures enriched in EPA and DHA, have suggested that these two FAs have different effects on serum lipids. More precisely, EPA selectively lowers TGs whereas DHA selectively lowers cholesterol (43, 44); however, very high doses of N3FAs (equal to 12-24 g/d) were consumed in these studies. These findings were in contrast with our findings. In a study, Mori and Woodman stated that human studies show that DHA might be more effective in increasing HDL-C and fasting and postprandial TG (15) while in our study, these N3FAs on serum had no difference in affecting level of HDL-C; however, there was favorable effect on serum TG, in a way that the effect of DHA was clearer. In another study, Mori et al. suggested that daily intake of 4 g of EPA, DHA, or placebo for six weeks, had no differential effect on serum TC and HDL-C while both of them decreased serum TG and serum level and particle size of LDL-C increased in DHA receiving group (17). The results of that study had more similarity with ours. The difference of the results of above studies could be result of the difference in amount of consumed N3FA, the model of omega-3 presentation (fish, fish oil, or purified oil), or patient's lipoprotein phenotype (17).
In summary, 2 g of either EPA or DHA supplementation for 12 weeks had no significant effect on the levels of TC, LDL-C, HDL-C, TC/HDL-C, TG/HDL-C, and LDL-C/HDL-C. However, it had a desirable effect on the level of TG in patients infected with H. pylori. The present study was the first to compare the effects of EPA and DHA on lipid profile in infected patients whose information regarding potential confounding variables including dietary intake and physical activity level were available. Nevertheless, this study had some limitations. First, several patients participating in the intervention were excluded from the study. The second limitation was lack of plasma levels measurement of N3FAs for better controlling the patients’ compliance. Moreover, frequent inclusion criteria, especially having no metabolic diseases, taking no supplements of N3FAs and antioxidant supplement, and no previous history of infection, led to further reduction in the number of eligible patients for the study and hampered the disease finding process. Finally, it is suggested to perform similar studies with larger number of patients and longer study period for a better comparison of the effects on serum lipids. Another research study should be conducted with different doses of N3FAs to investigate dose-related effects of N3FAs on lipid markers and plasma concentration measurement of N3FAs after the supplement intake to ensure the compliance rate of supplementation and effective dose of absorption, which would explain the mechanism of the compounds.
Acknowledgments
The authors express their gratitude to the honorable authorities of Minami-Nutrition Company, Belgium, that donated morEPA and morDHA supplements to this study. We are also grateful to all participants of the study who made this project possible by their cooperation and support.
Footnotes
Authors' Contributions:Nafiseh Khandouzi: study concept and design, acquisition of data, statistical analysis, analysis and interpretation of data, and drafting of the manuscript. Farzad Shidfar: assistance in the study design and critical revision of the manuscript for important intellectual content. Shahram Agah: assistance in acquisition of data and all experiments, especially in medical consultation, administrative, technical, and material support, and study supervision.
Funding/Support:This research was supported by Digestive Disease Research Institute (DDRC) of Tehran University of Medical Sciences.
References
- 1.Arabi MH, Alvani S, Ehteram H. Lipid profile in subjects with Helicobacter pylori Infection. Iran J Path. 2010;5(4):199–203. [Google Scholar]
- 2.Khadem Ansari MH, Omrani MD, Sayyah B, Khadem Ansari S. Effect of Helicobacter pylori infection on the lipid, lipoproteins, apolipoprotein A-1, lipoprotein (a) and apolipoprotein-B in patients with gastritis. Afr J Microbiol Res. 2010;4(1):84–7. [Google Scholar]
- 3.Fuccio L, Laterza L, Zagari RM, Cennamo V, Grilli D, Bazzoli F. Treatment of Helicobacter pylori infection. BMJ. 2008;337:a1454. doi: 10.1136/bmj.a1454. [DOI] [PubMed] [Google Scholar]
- 4.Nouraie M, Latifi-Navid S, Rezvan H, Radmard AR, Maghsudlu M, Zaer-Rezaii H, et al. Childhood hygienic practice and family education status determine the prevalence of Helicobacter pylori infection in Iran. Helicobacter. 2009;14(1):40–6. doi: 10.1111/j.1523-5378.2009.00657.x. [DOI] [PubMed] [Google Scholar]
- 5.Bergonzelli GE, Donnicola D, Porta N, Corthesy-Theulaz IE. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob Agents Chemother. 2003;47(10):3240–6. doi: 10.1128/AAC.47.10.3240-3246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurila A, Bloigu A, Nayha S, Hassi J, Leinonen M, Saikku P. Association of Helicobacter pylori infection with elevated serum lipids. Atherosclerosis. 1999;142(1):207–10. doi: 10.1016/s0021-9150(98)00194-4. [DOI] [PubMed] [Google Scholar]
- 7.Gillum RF. Infection with Helicobacter pylori, coronary heart disease, cardiovascular risk factors, and systemic inflammation: the Third National Health and Nutrition Examination Survey. J Natl Med Assoc. 2004;96(11):1470–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Bone K. Helicobacter: a hidden factor in cardiovascular, digestive, autoimmune, and skin disorders. Townsend Let Doctors Patients. 2006 [Google Scholar]
- 9.Niemela S, Karttunen T, Korhonen T, Laara E, Karttunen R, Ikaheimo M, et al. Could Helicobacter pylori infection increase the risk of coronary heart disease by modifying serum lipid concentrations? Heart. 1996;75(6):573–5. doi: 10.1136/hrt.75.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanbay M, Gur G, Yucel M, Yilmaz U, Boyacioglu S. Does eradication of Helicobacter pylori infection help normalize serum lipid and CRP levels? Dig Dis Sci. 2005;50(7):1228–31. doi: 10.1007/s10620-005-2764-9. [DOI] [PubMed] [Google Scholar]
- 11.Turner D, Shah PS, Steinhart AH, Zlotkin S, Griffiths AM. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): a systematic review and meta-analyses. Inflamm Bowel Dis. 2011;17(1):336–45. doi: 10.1002/ibd.21374. [DOI] [PubMed] [Google Scholar]
- 12.Clarke SD. Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J Nutr. 2001;131(4):1129–32. doi: 10.1093/jn/131.4.1129. [DOI] [PubMed] [Google Scholar]
- 13.Borthwick L, U. K. Study Group The effects of an omega-3 ethyl ester concentrate on blood lipid concentrations in patients with hyperlipidaemia. Clin Drug Investig. 1998;15(5):397–404. doi: 10.2165/00044011-199815050-00004. [DOI] [PubMed] [Google Scholar]
- 14.Verlengia R, Gorjao R, Kanunfre CC, Bordin S, de Lima TM, Martins EF, et al. Effects of EPA and DHA on proliferation, cytokine production, and gene expression in Raji cells. Lipids. 2004;39(9):857–64. doi: 10.1007/s11745-004-1307-2. [DOI] [PubMed] [Google Scholar]
- 15.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9(2):95–104. doi: 10.1097/01.mco.0000214566.67439.58. [DOI] [PubMed] [Google Scholar]
- 16.Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34(2):253–60. doi: 10.1161/01.hyp.34.2.253. [DOI] [PubMed] [Google Scholar]
- 17.Mori TA, Burke V, Puddey IB, Watts GF, O'Neal DN, Best JD, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71(5):1085–94. doi: 10.1093/ajcn/71.5.1085. [DOI] [PubMed] [Google Scholar]
- 18.Buckley R, Shewring B, Turner R, Yaqoob P, Minihane AM. Circulating triacylglycerol and apoE levels in response to EPA and docosahexaenoic acid supplementation in adult human subjects. Br J Nutr. 2004;92(3):477–83. doi: 10.1079/bjn20041235. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2(10):787–95. doi: 10.1038/nri915. [DOI] [PubMed] [Google Scholar]
- 20.De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr. 2000;71(1 Suppl):213S–23S. doi: 10.1093/ajcn/71.1.213S. [DOI] [PubMed] [Google Scholar]
- 21.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68(7):1056–61. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 22.Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. 2007;6:21. doi: 10.1186/1476-511X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29(5):263–71. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci U S A. 2007;104(32):13152–7. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman DR, Birch DG. Docosahexaenoic acid in red blood cells of patients with X-linked retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1995;36(6):1009–18. [PubMed] [Google Scholar]
- 26.Hoffman DR, DeMar JC, Heird WC, Birch DG, Anderson RE. Impaired synthesis of DHA in patients with X-linked retinitis pigmentosa. J Lipid Res. 2001;42(9):1395–401. [PubMed] [Google Scholar]
- 27.Johnson EJ, Schaefer EJ. Potential role of dietary n-3 fatty acids in the prevention of dementia and macular degeneration. Am J Clin Nutr. 2006;83(6 Suppl):1494S–8S. doi: 10.1093/ajcn/83.6.1494S. [DOI] [PubMed] [Google Scholar]
- 28.Barbosa VM, Miles EA, Calhau C, Lafuente E, Calder PC. Effects of a fish oil containing lipid emulsion on plasma phospholipid fatty acids, inflammatory markers, and clinical outcomes in septic patients: a randomized, controlled clinical trial. Crit Care. 2010;14(1):R5. doi: 10.1186/cc8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saifullah A, Watkins BA, Saha C, Li Y, Moe SM, Friedman AN. Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients--a pilot study. Nephrol Dial Transplant. 2007;22(12):3561–7. doi: 10.1093/ndt/gfm422. [DOI] [PubMed] [Google Scholar]
- 30.Ohno T, Kita M, Yamaoka Y, Imamura S, Yamamoto T, Mitsufuji S, et al. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter. 2003;8(3):207–15. doi: 10.1046/j.1523-5378.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 31.Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000;102(11):1264–9. doi: 10.1161/01.cir.102.11.1264. [DOI] [PubMed] [Google Scholar]
- 32.Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr. 2002;76(5):1007–15. doi: 10.1093/ajcn/76.5.1007. [DOI] [PubMed] [Google Scholar]
- 33.Medium chain triglycerides. Monograph. Altern Med Rev. 2002;7(5):418–20. [PubMed] [Google Scholar]
- 34.Clegg ME. Medium-chain triglycerides are advantageous in promoting weight loss although not beneficial to exercise performance. Int J Food Sci Nutr. 2010;61(7):653–79. doi: 10.3109/09637481003702114. [DOI] [PubMed] [Google Scholar]
- 35.Parinyasiri U, Ong-Ajyooth L, Parichatikanond P, Ong-Ajyooth S, Liammongkolkul S, Kanyog S. Effect of fish oil on oxidative stress, lipid profile and renal function in IgA nephropathy. J Med Assoc Thai. 2004;87(2):143–9. [PubMed] [Google Scholar]
- 36.Maki KC, Lawless AL, Kelley KM, Dicklin MR, Kaden VN, Schild AL, et al. Effects of prescription omega-3-acid ethyl esters on fasting lipid profile in subjects with primary hypercholesterolemia. J Cardiovasc Pharmacol. 2011;57(4):489–94. doi: 10.1097/FJC.0b013e318210fca5. [DOI] [PubMed] [Google Scholar]
- 37.Damsgaard CT, Schack-Nielsen L, Michaelsen KF, Fruekilde MB, Hels O, Lauritzen L. Fish oil affects blood pressure and the plasma lipid profile in healthy Danish infants. J Nutr. 2006;136(1):94–9. doi: 10.1093/jn/136.1.94. [DOI] [PubMed] [Google Scholar]
- 38.Illingworth DR, Connor WE, Hatcher LF, Harris WS. Hypolipidaemic effects of N3 fatty acids in primary hyperlipoproteinaemia. J Intern Med Suppl. 1989;731:91–7. doi: 10.1111/j.1365-2796.1989.tb01441.x. [DOI] [PubMed] [Google Scholar]
- 39.Nestel PJ, Connor WE, Reardon MF, Connor S, Wong S, Boston R. Suppression by diets rich in fish oil of very low density lipoprotein production in man. J Clin Invest. 1984;74(1):82–9. doi: 10.1172/JCI111422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris WS. Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. J Lipid Res. 1989;30(6):785–807. [PubMed] [Google Scholar]
- 41.McKenney JM, Sica D. Prescription omega-3 fatty acids for the treatment of hypertriglyceridemia. Am J Health Syst Pharm. 2007;64(6):595–605. doi: 10.2146/ajhp060164. [DOI] [PubMed] [Google Scholar]
- 42.Taziki O, Lessan-Pezeshki M, Akha O, Vasheghani F. The effect of low dose omega-3 on plasma lipids in hemodialysis patients. Saudi J Kidney Dis Transpl. 2007;18(4):571–6. [PubMed] [Google Scholar]
- 43.Bonaa KH, Bjerve KS, Nordoy A. Docosahexaenoic and eicosapentaenoic acids in plasma phospholipids are divergently associated with high density lipoprotein in humans. Arterioscler Thromb. 1992;12(6):675–81. doi: 10.1161/01.atv.12.6.675. [DOI] [PubMed] [Google Scholar]
- 44.Childs MT, King IB, Knopp RH. Divergent lipoprotein responses to fish oils with various ratios of eicosapentaenoic acid and docosahexaenoic acid. Am J Clin Nutr. 1990;52(4):632–9. doi: 10.1093/ajcn/52.4.632. [DOI] [PubMed] [Google Scholar]