Summary
Hepatitis E virus (HEV) is emerging as a global public health threat. Water-borne HEV outbreaks are common in developing countries and are associated with genotypes 1 and 2. In industrialised countries, sporadic cases of zoonotic transmission associated with genotypes 3 and 4 are increasingly being reported. Transfusion- and transplantation-transmitted HEV have been documented, although ingestion of contaminated food is thought to be the major transmission route. Severe disease is possible and chronic hepatitis infection occurs in solid-organ-transplant recipients and in patients with immunosuppressive disorders. In Australia, HEV cases are mainly travellers returning from disease endemic countries. Indeed, there are few reported cases of locally acquired HEV. Pigs in Australia have been shown to be infected with HEV, which indicates the possibility of zoonotic transmission. The extent of locally acquired infection is not known, however it may be greater than expected and may necessitate laboratory testing in patients reporting no overseas travel.
Keywords: Acute hepatitis, Australia, chronic hepatitis, hepatitis E virus, laboratory testing, seroprevalence, traveller, zoonotic
INTRODUCTION
Hepatitis E virus (HEV) is a causative agent of acute hepatitis,1 originally known as enterically transmitted non-A, non-B hepatitis virus, due to its faeco-oral transmission route.1–3 Such a mode of transmission is common today in developing countries, due to poor sanitation.1,2 Other routes of transmission, such as through ingestion of contaminated food and contact with infected animals, are increasingly being reported in many industrialised countries.2–4 Cases of transfusion-transmitted HEV have been reported from industrialised countries, highlighting transfusion as one of the possible routes of transmission.1–3 A recent study in the United Kingdom (UK) has detected HEV RNA at a rate of one in just under 3000 blood donations.1 The majority of HEV cases in developed countries are in travellers returning from countries endemic for HEV; however, autochthonous or locally acquired HEV is emerging as a public health concern.3,4 In the majority of individuals, infection with HEV is sub-clinical; however, more severe infection is possible, with chronic infection observed in solid-organ transplant recipients and in patients with immunosuppressive disorders.1,2
There are 4 genotypes of HEV (HEV 1–4), which differ in epidemiological distribution, modes of transmission and pathogenicity. HEV genotype 1 includes human strains reported from Asia and Africa.2,5 Genotype 2 is also solely a human virus, with strains occurring in Mexico and some African countries.2,3,5 HEV genotype 3 and 4, the former being globally ubiquitous and the latter restricted to south-east Asia, infect both humans and swine.2,3,5 Genotypes 1 and 2 are associated with the faeco-oral transmission route, predominantly in developing countries,4 whereas genotypes 3 and 4 are related to sporadic cases of zoonotic transmission in humans, particularly through the consumption of infected pork or contact with infected animals, and tend to occur in developed countries.2,5 HEV-3 RNA has been detected in pigs’ livers sold in grocery stores in the United States (US) the UK, France and Germany.4,6,7 A higher HEV seroprevalence among pig farm workers compared to the general population supports contact as a possible mode of transmission.5 Genotypes 1 and 2 result in self-limiting hepatitis in young adults, with increased mortality seen in pregnant women and immunocompromised patients.2,4 Genotypes 3 and 4 can cause clinically apparent hepatitis in middle-aged and older individuals.4 Such differences in clinical outcomes with genotypes are not well understood.4
CLINICAL FEATURES
Acute hepatitis caused by HEV resembles that caused by other hepatotropic viruses [hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), Epstein–Barr virus or cytomegalovirus], or due to drug induced liver injury (DILI).4 The average incubation period is 30–40 days (range 2–9 weeks),3 with the majority of HEV infections being asymptomatic (67–93%).2,8 Features of symptomatic infections include anorexia, epigastric pain, discoloured urine, nausea, vomiting, diarrhoea, fever, jaundice, elevation of serum transaminase and hepatomegaly.9,10 During outbreaks in developing countries, a case fatality rate of 0.5–4% has been reported.2,5 A higher mortality rate of 8–11% in non-endemic countries is possibly due to infection in individuals with pre-existing liver disease.3 Incidence of disease and fulminant hepatitis is even higher in pregnant women during the third trimester.2 Mortality in pregnancy also varies with geographical region, and reaches 10–25% in developing countries.2,4 Chronic HEV infection refers to a condition following the acute phase, in which HEV replication persists for more than 3 months.11 Cases of chronic HEV infection have been documented in solid-organ transplant (kidney, liver and pancreas) recipients,11 and in immunosuppressive conditions like infection with human immunodeficiency virus.12 Only genotype 3 has been shown to be involved in chronic infections.11 Non-hepatic manifestations of HEV can include pancreatitis, haematological manifestations (thrombocytopenia, haemolysis) or neurological syndromes (Guillain–Barre syndrome, meningoencephalitis, pseudotumour cerebri, nerve palsies).2,9
PATHOGENESIS
HEV is difficult to culture in cell lines, therefore its pathogenesis and method of replication are not well understood.13,14 It is assumed that when the virus is faeco-orally transmitted, it replicates in the intestinal cells and reaches the liver through the portal vein.13 The virus attaches to hepatocytes through the binding of truncated peptide p239 to heparin sulphate proteoglycans on the cell wall, and enters the target cell by endocytosis.13,15 The virus has three open reading frames (ORFs): ORF1 encodes for non-structural proteins (such as RNA dependent RNA polymerase, methyltransferase, and RNA helicase);13,16 ORF2 encodes for the viral capsid;2,16 and ORF3 encodes phosphoproteins involved in viral morphogenesis and release.2,13,16 Both viral (such as the infecting genotype and infectious dose)4,13 and host factors (including age, pre-existing hepatic disease, pregnancy)2–4,13 influence the severity of disease.
TREATMENT AND PREVENTION
Acute cases of HEV that are self-limiting do not require treatment.4,9 Management of chronic cases of HEV includes treatment with ribavirin for 3 months, used alone or in combination with pegylated interferon-α.2 Ribavirin is contraindicated during pregnancy, and use of interferon increases risk of organ rejection in transplant recipients.2,4
Two HEV vaccines are in development, however neither is licensed worldwide.2 The first is a recombinant vaccine encoding ORF2 of a Pakistani HEV strain made using a baculovirus system in an insect (Spodoptera frugiperda, Sf9) cell line.17 A Phase I clinical trial of this vaccine (produced by DynCorp, USA) was conducted at the Walter Reed Army Institute of Research and was determined to be safe and immunogenic, but in need of further evaluation.17 A Phase II trial of the vaccine was performed with the Nepalese Army, and efficacy of three doses of vaccine was found to be 95.5% effective, with unclear information on durability of immunity.2,3 A second vaccine referred to as HEV 239, is based on a recombinant protein of ORF2 (HEV genotype 1) produced in an Escherichia coli expression system.2 A clinical Phase II trial conducted in China determined this prototype vaccine was safe and immunogenic.2 A Phase III trial of HEV 239 (Hecolin; Xiamen Innovax Biotech, China) determined an efficacy of 100%.18 This vaccine has recently been approved in China2 where its use is focussed on high-risk populations, such as women of child-bearing-age, chronic liver disease patients and food industry workers.19 In developed countries where genotypes 3 and 4 are present, this vaccine may be considered for certain patients, such as organ transplant recipients or those with chronic liver disease.20 However, this vaccine is not licensed worldwide.
Passive immunisation with immunoglobulin preparations in animal studies has been shown to be effective;10 however, immunoprophylaxis with low titre human serum immunoglobulin has not been successful in humans.10 Monoclonal antibodies against HEV for humans has not been tested.10
MARKERS OF INFECTION AND DIAGNOSIS
Acute HEV infection is characterised by an increase in bilirubin, alanine aminotransferase and aspartate aminotransferase.21 HEV RNA in blood and stool appears at the same time, 2–3 weeks after infection.2,3 Viraemia persists for up to 4 weeks, however can last longer in some individuals, whereas virus in stools is detectable up to 6 weeks.1–3 HEV IgM is detectable 3–4 weeks after exposure and persists for 6 months.2,3 HEV IgG is detectable 4–5 weeks post infection, and persists for 12 or more years.2,3
HEV cases are diagnosed based on the detection of the viral RNA and serological markers (HEV IgM, high or rising titre of HEV IgG).21 Liver biopsy for HEV diagnosis is not required, due to its course as a self-limiting illness.3 Histological examination of a liver with hepatitis E infection is similar to other causes of acute hepatitis, with lobular disarray with reticulin framework distortion.3 The portal tracts are infiltrated with lymphocytes, plasma cells and polymorphs including eosinophils.3,22 In HEV infected patients with underlying cirrhosis, liver biopsy resembles that of alcoholic hepatitis and therefore is non-specific.3
A recent study in southeastern Scotland in acute hepatitis patients has shown that HEV infection is more common than HAV, HBV and HCV.23 This study recommended that HEV screening be introduced in the initial testing panel for acute hepatitis.23 There is a possibility that patients with non-A, non-B, non-C hepatitis may be mis-diagnosed with non-viral related disease, such as DILI or alcohol consumption. This further necessitates testing for HEV infection markers in acute hepatitis patients.
In Australia, HEV infection is nationally notifiable and confirmed cases are notified based on laboratory definitive evidence or laboratory suggestive evidence with clinical and epidemiological evidence (travel to a HEV endemic country 15–64 days prior to onset or a link to confirmed case).24 Laboratory definitive evidence is referred to as: detection of HEV RNA by nucleic acid testing; HEV detection by electron microscopy in faeces; or IgG seroconversion (an increase in IgG antibody titre). Laboratory suggestive evidence refers to detection of HEV IgM or IgG.24 In Australia, the most common viral causes of acute hepatitis are HAV, HBV and HCV.25 Hence, the testing algorithm for laboratory diagnosis includes testing for infection markers for these viruses only. HEV testing is considered for acute hepatitis patients with recent overseas travel history. However, in the absence of HEV testing for patients with no overseas travel history, it is possible that HEV infection may be missed, or misdiagnosed (as non-A, non-B, non-C hepatitis, DILI or alcohol-induced hepatitis).3
GLOBAL EPIDEMIOLOGY
The global annual disease burden for HEV genotypes 1 and 2 in 2005 was estimated to be 20.1 million incident infections resulting in 3.4 million symptomatic cases, 70,000 deaths and 3000 still births.26 The study model represented 71% of the world's population with incidence rates greater for younger individuals (aged 15–20 years).26 The burden for HEV genotypes 3 and 4 is not known. HEV seroprevalence ranges from less than 5% to 52.5% in different countries.2
HEV EPIDEMIOLOGY IN AUSTRALIA
Data from the Commonwealth Department of Health indicates there were 378 HEV notified cases from 1999 to 2013, with an average of 25 cases per year (Fig. 1).27 Higher numbers of cases were reported from New South Wales and Victoria. Most cases are associated with travel to HEV endemic countries, mostly Asia,28 and few cases are reported to be locally acquired. The true incidence of HEV and exposure status of the Australian population, however, is unknown due to its subclinical nature.
Fig. 1.
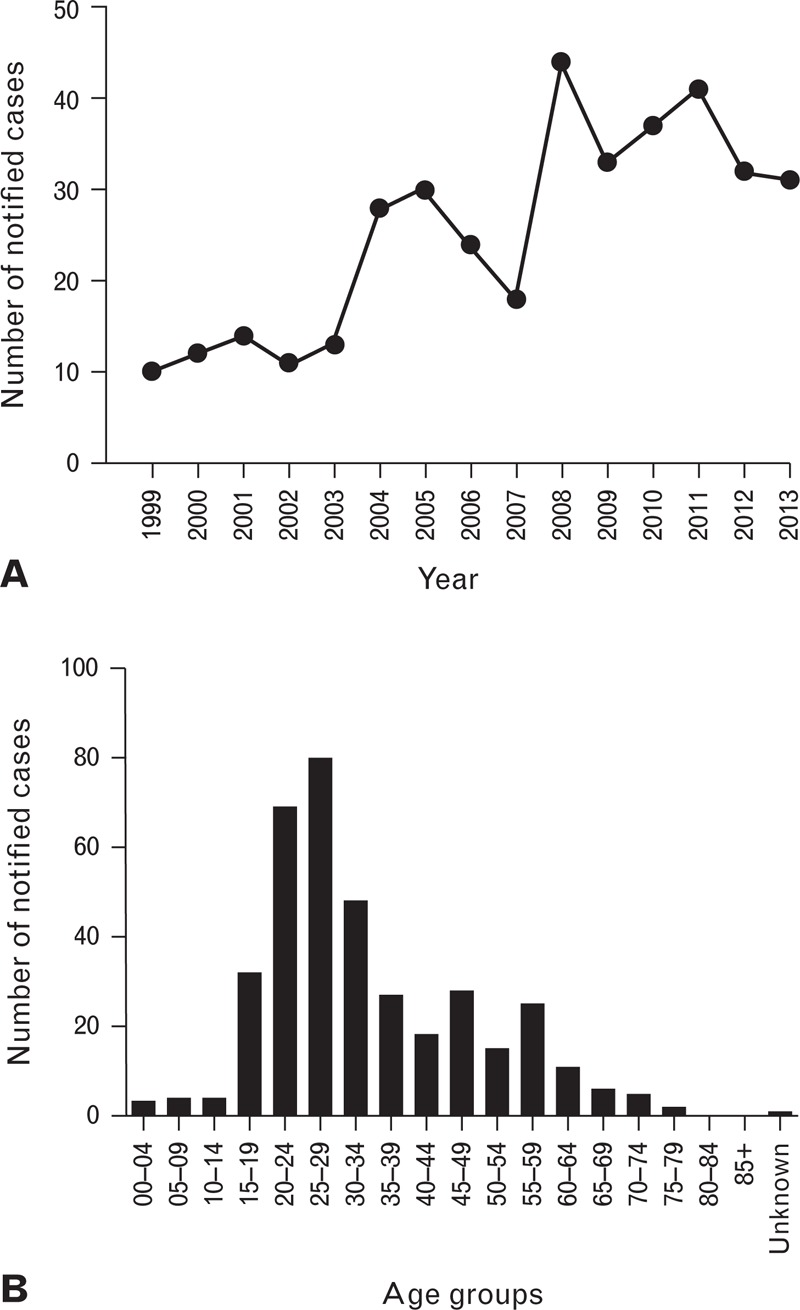
HEV notified cases in Australia from 1999–2013, by year (A) and age group (B).27
A seroprevalence study of HEV in 1995 among selected groups determined HEV IgG prevalence of 0.4% in blood donors, 2.2% in travellers and 7.7% in non-A, non-B, non-C hepatitis patients as well as refugees.29 Retrospective study of stored sera from patients in the 1970s (non-A, non-B hepatitis) have shown serological evidence of HEV IgG.30 Our recent study among a cohort of Australian blood donors has demonstrated HEV seroprevalence of 5.9%.31
HEV in animals
Chandler et al. report HEV IgG seroprevalence to be 17% in wild caught pigs and 30–95% in commercially raised pigs.32 This study used an in-house assay with unknown sensitivity and specificity.32 Transmission from pigs to humans is possible through ingesting infected undercooked meat or contact with infected animals. An avian HEV strain associated with big liver and spleen disease in chickens was identified in Australia in 1980.33 However, avian strains are not known to be transmitted to humans.16
Locally acquired infection in humans
There are limited data on the extent of locally acquired HEV in Australia and therefore the burden of autochthonous HEV requires investigation. The first case of locally acquired HEV infection was reported in 1995 from the Northern Territory.34 The source of infection in this patient was not known.34 A case each in Victoria and Queensland were also notified as being locally acquired in 2005,35 however neither case has been published in peer reviewed journals. At least five other HEV cases have recently been reported in New South Wales, all with no recent overseas travel history and likely to be linked to the consumption of undercooked pork liver sausages.36,37 Our recent study in blood donors has reported an HEV seroprevalence of 3.37% in donors with no previous history of overseas travel (Table 1). Of these donors, 57% were males and 64% were >45 years of age. Demonstration of HEV serological markers in such donors who reported no international travel suggests the possibility of their infection being acquired within Australia. HEV seroprevalence in Australian pig herds shown in 199932 suggests that zoonotic transmission may be possible. Other modes of transmission such as congenital, transfusion and organ transplant have been documented overseas,1,2,5 which could contribute to the occurrence of locally acquired infection, and associated risks therefore need to be assessed.
Table 1.
HEV IgG seroprevalence in a cohort of Australian blood donors reporting no overseas travel (based on re-analysis of data from our previous study31)
| Donors tested | Number reactive | Prevalence, % (95% CI) | |
| Total | 416 | 14 | 3.37 (1.63–5.10) |
| Gender | |||
| Female | 180 | 6 | 3.33 (0.71–5.96) |
| Male | 236 | 8 | 3.39 (1.08–5.70) |
| Age, years | |||
| <45 | 254 | 5 | 1.97 (0.26–3.68) |
| ≥45 | 162 | 9 | 5.56 (2.03–9.08) |
| State/Territory | |||
| Australian Capital Territory | 22 | – | – |
| New South Wales | 61 | 5 | – |
| Northern Territory | 30 | 2 | – |
| Queensland | 75 | 1 | – |
| South Australia | 66 | 4 | – |
| Tasmania | 104 | 2 | – |
| Victoria | 30 | – | – |
| Western Australia | 28 | – | – |
CONCLUSION
There is increasing public health concern in relation to HEV globally, and specifically among many industrialised countries traditionally considered non-endemic. Given this, there is a need for increased awareness in relation to this emerging virus in these countries, which include Australia. This awareness is required at the level of public health authorities, in the clinical settings and also with the general public. HEV is preventable through the control of the source of infection, which includes maintenance of a safe supply of drinking water, and prevention of human-to-human, and animal-to-human transmission by maintenance of personal hygiene and proper handling/cooking of food. While the occurrence of travel-associated HEV infection in Australia is well known, there is limited knowledge of the scale of locally acquired HEV in Australia and this needs further investigation. It is likely that autochthonous transmission will be greater than expected, and may necessitate laboratory testing in patients reporting no overseas travel.
Acknowledgements
The Australian government fully funds the Australian Red Cross Blood Service for the provision of blood products and services to the Australian community.
Conflicts of interest and sources of funding: The authors state that there are no conflicts of interest to disclose.
References
- 1.Hewitt PE, Ijaz S, Brailsford SR, et al. Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet 2014; 384:1766–1773. [DOI] [PubMed] [Google Scholar]
- 2.Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet 2012; 379:2477–2488. [DOI] [PubMed] [Google Scholar]
- 3.Dalton HR, Bendall R, Ijaz S, et al. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis 2008; 8:698–709. [DOI] [PubMed] [Google Scholar]
- 4.Scobie L, Dalton HR. Hepatitis E: source and route of infection, clinical manifestations and new developments. J Viral Hepat 2013; 20:1–11. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal R. The Global Prevalence of Hepatitis E Virus Infection and Susceptibility: A Systematic Review. Geneva: World Health Organization, 2010. http://whqlibdoc.who.int/hq/2010/WHO_IVB_10.14_eng.pdf. [Google Scholar]
- 6.Colson P, Borentain P, Queyriaux B, et al. Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis 2010; 202:825–834. [DOI] [PubMed] [Google Scholar]
- 7.Wenzel JJ, Preiss J, Schemmerer M, et al. Detection of hepatitis E virus (HEV) from porcine livers in Southeastern Germany and high sequence homology to human HEV isolates. J Clin Virol 2011; 52:50–54. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Hepatitis E Information for Health Professionals. Sep 2012; cited July 2014. http://www.cdc.gov/hepatitis/HEV/HEVfaq.htm. [Google Scholar]
- 9.Aggarwal R. Clinical presentation of hepatitis E. Virus Res 2011; 161:15–22. [DOI] [PubMed] [Google Scholar]
- 10.Mushahwar IK. Hepatitis E virus: Molecular virology, clinical features, diagnosis, transmission, epidemiology, and prevention. J Med Virol 2008; 80:646–658. [DOI] [PubMed] [Google Scholar]
- 11.Kamar N, Izopet J, Dalton HR. Chronic hepatitis E virus infection and treatment. J Clin Exp Hepatol 2013; 3:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalton HR, Bendall RP, Keane FE, et al. Persistent carriage of hepatitis E virus in patients with infection. N Engl J Med 2009; 361:1025–1027. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Gracia MT, Suay B, Mateos-Lindemann ML. Hepatitis E: an emerging disease. Infect Genet Evol 2014; 22:40–59. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad I, Holla RP, Jameel S. Molecular virology of hepatitis E virus. Virus Res 2011; 161:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandra V, Taneja S, Kalia M, et al. Molecular biology and pathogenesis of hepatitis E virus. J Biosci 2008; 33:451–464. [DOI] [PubMed] [Google Scholar]
- 16.Kamar N, Dalton HR, Abravanel F, et al. Hepatitis E virus infection. Clin Microbiol Rev 2014; 27:116–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Safary A. Perspectives of vaccination against hepatitis E. Intervirol 2001; 44:162–166. [DOI] [PubMed] [Google Scholar]
- 18.Zhu F-C, Zhang J, Zhang X-F, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010; 376:895–902. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organisation. World's First Hepatitis E Vaccine Approved in China. Global Immunization News; January 2012; cited March 2014. http://www.who.int/immunization/GIN_January_2012.pdf [Google Scholar]
- 20.Zhang J, Zhang XF, Zhou C, et al. Protection against hepatitis E virus infection by naturally acquired and vaccine-induced immunity. Clin Microbiol Infect 2014; 20:O397–O405. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal R. Diagnosis of hepatitis E. Nat Rev Gastroenterol Hepatol 2013; 10:24–33. [DOI] [PubMed] [Google Scholar]
- 22.Drebber U, Odenthal M, Aberle SW, et al. Hepatitis E in liver biopsies from patients with acute hepatitis of clinically unexplained origin. Front Physiol 2013; 4:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harvala H, Wong V, Simmonds P, et al. Acute viral hepatitis – Should the current screening strategy be modified? J Clin Virol 2014; 59:184–187. [DOI] [PubMed] [Google Scholar]
- 24.Australian Government Department of Health. Commonwealth of Australia. Hepatitis E case definition. Cited July 2014. http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-surveil-nndss-casedefs-cd_hepe.htm [Google Scholar]
- 25.Post J, Marinos G, Beek IV, et al. HIV, viral hepatitis and STIs: a guide for primary care 2008. In: Australasian Society of HIV Medicine. HIV, Viral Hepatitis and STIs: A Guide for Primary Care. Darlinghurst: ASHM, 2008; 54–62 http://www.ashm.org.au/images/publications/monographs/HIV_viral_hepatitis_and_STIs_a_guide_for_primary_care/hiv_viral_hep_chapter_5.pdf. [Google Scholar]
- 26.Rein DB, Stevens GA, Theaker J, et al. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012; 55:988–997. [DOI] [PubMed] [Google Scholar]
- 27.Australian Government Department of Health and Ageing. Commonwealth of Australia. Number of notifications of Hepatitis E∗, Australia, in the period of 1991 to 2013 and year-to-date notifications for 2014: National Notifiable Diseases Surveillance System; 2013. Cited Aug 2014. http://www9.health.gov.au/cda/source/cda-index.cfm. [Google Scholar]
- 28.Cowie BC, Adamopoulos J, Carter K, et al. Hepatitis E infections, Victoria, Australia. Emerg Infect Dis 2005; 11:482–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moaven L, Van Asten M, Crofts N, et al. Seroepidemiology of hepatitis E in selected Australian populations. J Med Virol 1995; 45:326–330. [DOI] [PubMed] [Google Scholar]
- 30.Binotto E, Boughton CR, Vollmer-Conna U, et al. A serological re-evaluation of acute nonA nonB hepatitis from the early 1970s. Aust NZ J Med 2000; 30:668–674. [DOI] [PubMed] [Google Scholar]
- 31.Shrestha AC, Seed CR, Flower RL, et al. Hepatitis E virus and implications for blood supply safety, Australia. Emerg Infect Dis 2014; 20:1940–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandler JD, Riddell MA, Li F, et al. Serological evidence for swine hepatitis E virus infection in Australian pig herds. Vet Microbiol 1999; 68:95–105. [DOI] [PubMed] [Google Scholar]
- 33.Bilic I, Jaskulska B, Basic A, et al. Sequence analysis and comparison of avian hepatitis E viruses from Australia and Europe indicate the existence of different genotypes. J Gen Virol 2009; 90:863–873. [DOI] [PubMed] [Google Scholar]
- 34.Heath TC, Burrow JN, Currie BJ, et al. Locally acquired hepatitis E in the Northern Territory of Australia. Med J Aust 1995; 162:318–319. [DOI] [PubMed] [Google Scholar]
- 35.Owen R, Roche PW, Hope K, et al. Australia's Notifiable Diseases Status, 2005: Annual Report of the National Notifiable Diseases Surveillance System. National Notifiable Diseases Surveillance System. Canberra: Commonwealth of Australia, 2005; 27–28 http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-cdi3101-pdf-cnt.htm/FILE/cdi3101a.pdf. [Google Scholar]
- 36.New South Wales Health. Communicable Diseases Weekly Reports – 2014. Cited Nov 2014. http://www.health.nsw.gov.au/Infectious/reports/Pages/Communicable-Diseases-Weekly-Report.aspx. [Google Scholar]
- 37.NSW Health. Warning about Hepatitis E cases linked with pork liver. 11 Sep 2014; cited Nov 2014. http://www.foodauthority.nsw.gov.au/news/media-releases/mr-11-Sep-14-warning-Hep-E-pork-liver. [Google Scholar]


