Abstract
Mutations in the dhfr gene of Plasmodium vivax (pvdhfr) are associated with resistance to the antifolate antimalarial drugs. Polymorphisms in the pvdhfr gene were assessed by hybridization probe technology on the LightCycler instrument with 134 P. vivax-infected blood samples from Turkey (n = 24), Azerbaijan (n = 39), Thailand (n = 16), Indonesia (n = 53), and travelers (n = 19). Double mutations (S58R and S117N) or quadruple mutations (F57L/I, S58R, T61M, and S117N) in the pvdhfr genes were found in all Thai samples (100%). pvdhfr mutant-type alleles were significantly more common in samples from travelers (42%) than in those from patients from Indonesia (5%). Surprisingly, the pvdhfr single-mutation allele (S117N) was identified at a high frequency in parasites from Turkey and Azerbaijan (71 and 36%, respectively), where sulfadoxine-pyrimethamine is not recommended for the treatment of P. vivax malaria by the World Health Organization and the Malaria National Programs.
Malaria due to Plasmodium vivax is globally widespread not only in tropical and subtropical regions, with the exception of Central and West Africa, but also in many temperate zones, such as the southern states of the former USSR and neighboring regions, in which this disease was almost eradicated in the recent past (15, 17, 23, 29).
The recently reported resistance to antimalarials contributes to making the control of malaria more difficult. P. vivax resistance to chloroquine was first reported in Papua New Guinea in 1989 (22) and in Indonesia in 1991 (1, 24). Other reports of clinical resistance to chloroquine have been reported in India (4, 8), Myanmar (12, 27), and areas of Central and South America, including Guyana (20) and Brazil and Colombia (25). Chloroquine still constitutes the first-line therapy for uncomplicated malaria caused by P. vivax in most areas of endemicity. The combination therapeutic strategies under consideration include 4-amino-quinolines plus other standard antimalarials, including sulfadoxine-pyrimethamine (S-P). In countries where S-P was used extensively for the treatment of P. falciparum infection, such as Thailand, high-grade antifolate resistance has also emerged in P. vivax parasites (21, 30).
Molecular, clinical, and epidemiological studies have clearly shown that, as for P. falciparum, resistance to pyrimethamine results from specific point mutations in the P. vivax dhfr (dihydrofolate reductase [DHFR]) gene (pvdhfr), which lead to a reduced affinity of the drug for the DHFR enzyme (5, 6, 9-11, 13, 26, 28).
Detection of these mutations in isolates collected in the field has proved valuable in the mapping of resistance and the monitoring of malaria control measures (11).
For this purpose, through the use of a powerful method based on hybridization probe technology, we have analyzed point mutations in the pvdhfr gene using P. vivax-infected samples from Azerbaijan and Turkey, where no molecular investigation of the resistance markers of the P. vivax parasite have been conducted before; and from Thailand and Indonesia, which differ in their previous and present policies for the use of antimalarial drugs. The mutations targeted here were mapped on six P. vivax dhfr positions that have previously been suggested to be involved in S-P resistance.
MATERIALS AND METHODS
Human blood samples.
A total of 151 P. vivax-infected blood specimens, collected by either venous puncture or finger prick, were processed; but only 134 of these could be included in our study because they yielded a positive result by the fluorescence resonance energy transfer (FRET) assay.
During the summer of 2000, 24 blood samples were collected from symptomatic patients consulting at the Institute of Malariology of Adana (southeastern Turkey), after informed consent was obtained from each patient, and placed in EDTA anticoagulant.
During the summer of 2002, 39 blood samples were obtained during an active survey carried out in the framework of a malaria project supported by the European Commission and placed in EDTA anticoagulant. Samples were collected in seven districts of central Azerbaijan already operating as World Health Organization sentinel sites, namely, Sabirabad (n = 4), Saatli (n = 10), Beylagan (n = 1), Aghjabadi (n = 7), Imishly (n = 3), Barda (n = 8), and Mingaçevir (n = 6).
Sixteen blood samples were collected by finger prick from adult patients visiting the Mae Sod malaria clinic, Tak Province Thailand. Whole blood was dotted on Whatman 3MM filter paper, air dried, and stored in individual plastic zip-lock bags with desiccant and away from direct light.
In June 2001, 53 blood samples were collected as blood spots from Indonesian children ages 6 to 10 years during a malariometric survey in Alor District, East Nusa Tenggara Province.
Between 2001 and 2003, 19 blood samples from patients presenting with imported vivax malaria and hospitalized in France were collected after biological confirmation by microscopic examination of thin smears and placed in EDTA anticoagulant. Those patients had contracted malaria during their travels in different areas of endemicity: French Guyana (n = 6), Comoros Islands (n = 4), Indonesia (n = 2), Madagascar (n = 2), Afghanistan (n = 1), Cameroon (n = 1), and unknown (n = 1).
Control blood samples that were noninfected or that were infected with P. falciparum, P. malariae, or P. ovale were collected from patients hospitalized in France and were used to check for the specificity of amplification.
DNA template preparation and PCR.
Parasite genomic DNA from all blood samples in EDTA anticoagulant was extracted by using a QIAamp DNA blood kit (Qiagen, Valencia, Calif.), according to the instructions manufacturer, with minor modifications (3). Extraction of P. vivax DNA from blood spot samples from Thailand was carried out with the QIAamp DNA blood kit and then by use of the Dried Blood Spots Protocol provided with the kit, with the same minor modifications mentioned above.
Parasite DNA from 17 Indonesian blood spots was previously isolated by using Instagene Matrix resin (Bio-Rad, Hercules, Calif.) by the protocol described previously (3). DNA was isolated from the other 36 Indonesian samples by using the Qiagen kit. Comparison of the efficiencies of the two different DNA isolation methods, which were evaluated by using blood spots infected with P. falciparum culture-adapted strain HB3 at different levels of parasitemia (0.01, 0.02, 0.05%), has shown no difference in the threshold cycle values (data not shown).
SYBR Green I PCR amplification.
The primers and probes used for the FRET assay were designed by Olfert Landt (DNA Synthesis Service, TibMolBiol, Berlin, Germany). Real-time PCR was performed by using SYBR Green I dye, which binds specifically to double-stranded DNA. The sequences of PCR primers dhfrS and dhfrA, which were used to amplify an approximately 450-bp fragment from the P. vivax dhfr gene (GenBank accession number X98123), are listed in Table 1.
TABLE 1.
Sequences of primers and oligonucleotides probes used for detection of pvdhfr mutations F57L/I, S58R, T61M, S117N/T, I172V, and I173L
| Oligonucleotide | Sequence (5′-3′)a | Position |
|---|---|---|
| dhfrS | TCTGGGCAATAAGGGGACT | 114-132 |
| dhfrA | AGTTTCTACTTAGGCATTCCCTAT | 559-536 |
| sensor 57/8 | GTAGGTCGTCACCGAGCTGAAGT-FL | 189-167 |
| anchor 57/8 | LC Red640-CTTCATATCGACGGAGTTGCATTTCCATG-PH | 165-137 |
| sensor [G] | GATGCTCTCCCAGCTGCTTC-FL | 363-344 |
| anchor 117 | LC Red640-CCCCATGACCACGACGTTTTGCAG-PH | 342-319 |
| sensor 172V | TGTGCTCCCCCAATGACGA-FL | 530-512 |
| anchor 172/3 | LC Red640-GCATTTGTAGTACTTCAGCTTCTTTAAGAGC-PH | 510-480 |
FL, fluorescein; LC Red640, LightCycler dye Red640; PH, phosphate; boldface, mutated nucleotides.
DNA template (5 μl) was added to the PCR mixture (20 μl) containing each of the primers at 0.5 μM, 3 mM MgCl2, and 2 μl of LightCycler FastStart DNA Master SYBR Green I buffer (Roche Molecular Biochemicals, Mannheim, Germany). The FastStart enzyme was activated at 95°C for 9 min prior to the start of cycling. The PCR program included 40 cycles of denaturation at 95°C for 10 s, annealing of the primers at 60°C for 10 s, and extension at 72°C for 20 s. The temperature change rates were 20°C/s for denaturation and annealing and 1°C/s for extension. Fluorescence was measured at channel F1 of the LightCycler instrument at the end of the elongation phase of each cycle. The melting curve program consisted of one cycle of 95°C for 2 s, 76°C for 20 s, and heating at 98°C. The temperature change rates were 20°C/s except for the final step, for which the temperature transition rate was 0.1°C/s. PCR product identity was assessed by the specific melting temperature obtained for each genotype.
FRET assay.
The oligonucleotide probes sequences used to detect six polymorphisms (F57L/I, S58R, T61M, S117N/T, I172V, and I173L) of the pvdhfr gene are listed in Table 1.
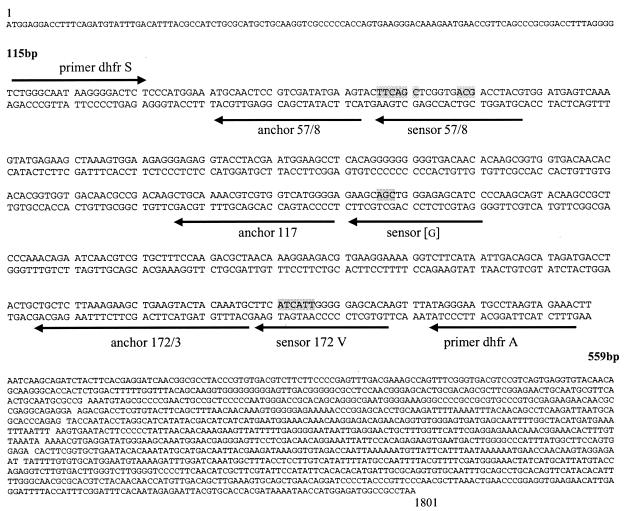
The sensor probe is designed to be perfectly complementary to the wild-type allele, and the anchor probe is designed to be perfectly complementary to conserved sequences adjacent to the mutation site (Fig. 1). The PCR mixture (20 μl) contained 5 μl of DNA template, probes (0.2 μM each), primers (0.5 μM each), MgCl2 (3 mM), and 2 μl of FastStart DNA Master Hybridization probes. The 40-cycle PCR program was performed as described above; however, a 45-cycle PCR program was used for the Indonesian isolates, due to the long-term storage of the blood samples, which were collected in 2001. Fluorescence was measured at channel F2 of the LightCycler instrument at the end of the annealing phase of each cycle.
FIG. 1.
Nucleotide sequence of the P. vivax DHFR-TS gene (GenBank accession no. X98123).
The melting curve program consisted of one cycle of 95°C for 2 s, 40°C for 20 s, and heating at 80°C.
RESULTS
Real-time PCR for pvdhfr gene detection in blood samples.
DNA from 134 of 151 P. vivax-infected samples yielded a specific melting temperature of 89.5 ± 0.3°C for the pvdhfr fragment (450 bp) amplified with SYBR Green I. The specificity of the assay was assessed by amplifying simultaneously, with primers dhfrS and dhfrA, DNA isolated from P. vivax, P. ovale, P. falciparum, and P. malariae and genomic DNA from human blood. None of the non-P. vivax species or the noninfected human blood samples gave a P. vivax-specific melting curve, confirming the absence of mixed infections with non-P. vivax Plasmodium species.
Distribution of mutations in the pvdhfr gene.
Wild-type and mutant pvdhfr alleles could rapidly be determined by comparison of the melting temperature for the alleles with the melting temperature of the reference allele obtained by the FRET assay (Table 2). The melting curves obtained for polymorphisms at positions 57/58, 117, 172/173 are shown in Fig. 2. In the same way that multiclonal P. vivax infections could be identified by the presence of two peaks simultaneously, each peak corresponded to the melting temperatures of the reference alleles. It was observed in two Thai samples and one Indonesian sample (Fig. 2D).
TABLE 2.
Melting temperatures of the sensor probes for detection of pvdhfr mutations F57L/I, S58R, T61M, S117N/T, I172V, and I173L
| dhfr gene codon(s) | Amino acid (melting temp [°C]a) of sensor probes
|
|||
|---|---|---|---|---|
| Wild-type allele | Mutant-type allele 1 | Mutant-type allele 2 | Mutant-type allele 3 | |
| 57-58-61 | F-S-T (67.3 ± 0.4) | F-R-T (64.1 ± 0.1) | L-R-M (54.6 ± 0.3) | I-R-M (53.6 ± 0.3) |
| 117 | S (64.5 ± 0.2) | N (55.9 ± 0.6) | T (55 ± 0.3) | |
| 172-173 | I-I (54.5 ± 0.7) | I-L (49.3 ± 0.2) | ||
Melting temperatures are reported as means ± standard deviations for three to seven independent assays.
FIG. 2.
Genotyping of pvdhfr by melting curve analysis of 134 P. vivax-infected samples. (A) Melting temperature peaks of the alleles obtained for the F57I/L, S58R, and T61M mutations: a, wild-type alleles F57, S58, and T61; b, mutant type 1 alleles F57, S58R, and T61; c, mutant type 2 alleles F57L, S58R, and T61M; d, mutant type 3 alleles I57L, S58R, and T61M. (B) Melting peaks of the alleles for S117N/T. e, wild-type allele S117; f, mutant type 1 allele S117N; g, mutant type 2 allele S117T. (C) Melting peaks obtained for mutations I172V and I173L. h, wild-type alleles I172 and I173; i, mutant type alleles I172 and I173L. (D) Double peaks indicating the presence of both wild-type and mutant alleles simultaneously in the same sample.
Confirmation of FRET assay data by sequence analysis.
The sequences of 12 P. vivax dhfr genes obtained from isolates from four patients from Turkey, four patients from Thailand, two patients from Azerbaijan, one patient from French Guyana, and one patient of unknown origin were needed to confirm the uncertain results obtained by the FRET assay. Comparison of these sequences to that of a parasite isolated in Pakistan (ARI/Pakistan), which was considered to represent the wild-type sequence, revealed the presence of point mutations and size polymorphisms in the pvdhfr genes (Fig. 3). The geographical distributions of these mutations related to the areas of contamination are presented in Table 3. A point mutation (TGC→CGC) found only once at codon 49 (C→R) was excluded from the subsequent analysis since this unexpected and isolated mutation may have been generated as a result of artifacts during PCR amplification.
FIG. 3.
Comparison of the amino acid sequences of P. vivax DHFR enzymes deduced from the nucleotide sequences of the pvdhfr genes amplified from P. vivax isolates. Alignment of the sequences is based on the ARI-Pakistan sequence (GenBank accession no. X98123), considered to represent the wild-type sequence, from residues 39 to 185. Dashes represent deletions, and polymorphisms are in boldface with gray shading.
TABLE 3.
Distribution of pvdhfr mutations F57L/I, S58R, T61M, SI17N/T, I172V, and I173L among 134 human blood samples
| Sample source (no. of samples) and strain type | No. of strains of the indicated type at the following pvdhfr allele:
|
|||||
|---|---|---|---|---|---|---|
| Codon 57 | Codon 58 | Codon 61 | Codon 117 | Codon 172 | Codon 173 | |
| All samples (134) | ||||||
| Wild type | 125 | 109 | 125 | 78 | 134 | 131 |
| Mutant | 9 | 25 | 9 | 56 | 0 | 3 |
| Turkey, n = 24 | ||||||
| Wild type | 24 | 24 | 24 | 7 | 24 | 24 |
| Mutant | 0 | 0 | 0 | 17 | 0 | 0 |
| Azerbaijan, n = 39 | ||||||
| Wild type | 39 | 39 | 39 | 26 | 39 | 39 |
| Mutant | 0 | 0 | 0 | 13 | 0 | 0 |
| Thailand (16) | ||||||
| Wild type | 7 | 0 | 7 | 0 | 16 | 16 |
| Mutant | 9 | 16 | 9 | 16 | 0 | 0 |
| Indonesia (36) | ||||||
| Wild type | 36 | 34 | 36 | 34 | 36 | 36 |
| Mutant | 0 | 2 | 0 | 2 | 0 | 0 |
| Travelers (19) | ||||||
| Wild type | 19 | 12 | 19 | 11 | 19 | 16 |
| Mutant | 0 | 7 | 0 | 8 | 0 | 3 |
A mutation that resulted in a synonymous substitution (TAT→TAC) was observed at codon 69 in five samples. Mutations that resulted in nonsynonymous substitutions were found at five residues: 57, 58, 61, 117, and 173. The F→I change at codon 57 resulted from a TTC→ATA double mutation and was observed seven times; the second change found at this codon, F→L, due to a single mutation (TTC→TTA), was observed two times. The ACG→ATG mutation at codon 61 (which resulted in a T→M change) was observed nine times. The S→R change at position 58 resulted from a single mutation (AGC→ AGA or AGG) and was observed 25 times. Two different mutations at position 117 caused an S→N (AGC→AAC) change in 48 samples or an S→T (AGC→ACC) change in 9 samples.
The I→L change at codon 173 was found in only three of six samples from French Guyana. It should be noted that the samples from infected patients in French Guyana were sent to us following repeated chloroquine treatment failure, and the samples contained mutants with double mutations at positions 58 and 117. However, data collected during the monitoring of these patients were scarce, and clinical resistance could not be assumed in these cases. No polymorphism was detected at codon 172.
DISCUSSION
P. vivax is the most common human malaria species, causing an estimated 70 million to 80 millions cases of malaria annually (16). Although the drug resistance of P. vivax has not gained the same attention as that of P. falciparum, S-P resistance has been known in Thailand for more than two decades (21); and chloroquine resistance has been documented in Indonesia, Oceania, and South America (2, 7, 18). Because of the ability of treatment with S-P to produce relapses, the assessment of an adequate clinical response to treatment is difficult (31).
Moreover, the lack of efficient methods for in vitro and in vivo evaluations of P. vivax drug resistance increases the need for a database on the single-nucleotide polymorphisms known to be related to drug resistance.
We have conducted a retrospective study with partners in the field involved in malaria surveillance programs from western to eastern Asia. To our knowledge, no molecular investigation of the drug resistance marker for P. vivax parasites from a temperate zone of the Old World has previously been performed. In vivo tests were not available during these programs; thus, we did not evaluate the correlation between molecular markers and the resistance phenotype. The accuracy of the real-time PCR method for the detection of molecular markers in P. falciparum has been demonstrated previously (3), and the data presented here show that this method is applicable to P. vivax. This method could easily be applied to large numbers of samples (32 samples can be tested in a 1-h experiment) and is particularly adapted to large-scale studies of drug resistance.
All 134 samples analyzed could be classified into five different groups according to the mutation profiles of the isolates in the samples (Table 4). Approximately half (57.5%) of the samples tested here contained isolates that presented the wild-type genotype, and isolates with a single mutation (S117N) were observed in about a quarter (23.9%) of the samples tested.
TABLE 4.
Different pvdhfr single- and multiple-nucleotide polymorphisms found in the areas of endemicity analyzed
| Mutation(s) | No. of isolates from the following sourcea:
|
% Distribution | ||||
|---|---|---|---|---|---|---|
| Azerbaijan (n = 39) | Turkey (n = 24) | Indonesia (n = 36) | Thailand (n = 16) | Travelers (n = 19) | ||
| Wild type | 25 | 7 | 34 | 0 | 11 | 57.5 |
| S117N | 14 | 17 | 0 | 0 | 1 | 23.9 |
| S58R-S117N | 0 | 0 | 2 | 7 | 4 | 9.7 |
| S58R-S117N-I173L | 0 | 0 | 0 | 0 | 3 | 2.2 |
| F57L-S58R-T61M-S117T | 0 | 0 | 0 | 2 | 0 | 1.5 |
| F57I-S58R-T61M-S117T | 0 | 0 | 0 | 7 | 0 | 5.2 |
n, total number of isolates from the indicated source.
Surprisingly, the single mutated allele (S117N) was identified at a high frequency in isolates in samples from Turkey and Azerbaijan (36 and 71%, respectively), areas where antifolate drug pressure or resistance is not obvious. The first-line treatment against P. vivax malaria in these areas is still the combination chloroquine-primaquine (16, 29), and a recent clinical study has demonstrated that treatment with chloroquine followed by treatment with primaquine is 100% effective in patients from Azerbaijan (29).
As is the case for the homologous position 108 in the dhfr gene of P. falciparum, we may hypothesize that the S117N mutation could represent the first step in the drug resistance selection process that has occurred in the parasite.
The isolates in all but two samples from Indonesia were of the wild type. Interestingly, an efficacy study with P. falciparum conducted in the same area (Alor Island) showed only a 10% rate of S-P treatment failure and did not demonstrate any dhps mutations.
The isolates in samples from travelers infected in French Guyana had double mutations (S58R and S117N) or triple mutations (S58R, S117N, and I173L). This is in agreement with the suspected frequency of treatment failure in this area, but the relationship of treatment failure to the use of antifolate drugs is speculative. The I173L mutation was observed only in isolates from samples from French Guyana and not in isolates from other samples. This could be related to data concerning the geographic subdivision of P. vivax between the Old World and the New World (14).
Analysis of field isolates from Thailand, where S-P has been extensively used in the past, revealed the predominance (100%) of parasites harboring three pvdhfr mutant alleles: S58R and S117N; F57L, S58R, T61M, and S117T; and F57I, S58R, T61M, and S117T.
This is in agreement with the findings of a recent study (19) in which high levels of P. vivax resistance in vivo were associated with the presence of double and triple mutations in pvdhfr (positions 57, 58, and 117) in 99% of the isolates tested.
If the spread of chloroquine resistance encourages health care policy makers to move toward antifolate drug regimens as first-line treatments, such data offer a snapshot of the prevalence of pvdhfr mutations in multiple geographical areas and can provide crucial information on the potential appearance of S-P resistance in these areas.
Acknowledgments
We are particularly indebted to Pascal Ringwald and Leonardo K. Basco for helpful discussions, Christelle Angei for skillful technical assistance, and Patrick Gerome and Lionel Crevon (French Army Medical Service) for providing samples from French Guyana. We are also grateful to Luigi Gradoni for continuous support and for providing samples from Turkey. Special thanks go to Daniel Schmitt (EA3732; Cellule de Langerhans, Peau Humaine et Immunité) for continuous support.
This study was partially supported by a grant from the European Commission (Copernicus-2 RTD project contract ICA2-CT-2000-10046) and a grant from the French Ministry of Research (Program PAL+).
REFERENCES
- 1.Baird, J. K., H. Basri, B. Subianto, L. C. Patchen, and S. L. Hoffman. 1991. Resistance to chloroquine by Plasmodium vivax in Irian Jaya, Indonesia. Am. J. Trop. Med. Hyg. 44:547-552. [DOI] [PubMed] [Google Scholar]
- 2.Baird, J. K., M. F. Sustriayu Nalim, H. Basri, S. Masbar, B. Leksana, E. Tjitra, R. M. Dewi, M. Khairani, and F. S. Wignall. 1996. Survey of resistance to chloroquine by Plasmodium vivax in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 90:409-411. [DOI] [PubMed] [Google Scholar]
- 3.de Monbrison, F., D. Raynaud, C. Latour-Foundanaiche, A. Staal, S. Favre, K. Kaiser, and S. Picot. 2003. Real-time PCR for chloroquine sensitivity assay and for pfmdr1-pfcrt single nucleotide polymorphisms in Plasmodium falciparum. J. Microbiol. Methods 54:391-401. [DOI] [PubMed] [Google Scholar]
- 4.Dua, V. K., P. K. Kar, and V. P. Sharma. 1996. Chloroquine resistant Plasmodium vivax malaria in India. Trop. Med. Int. Health 1:816-819. [DOI] [PubMed] [Google Scholar]
- 5.Eldin de Pecoulas, P., L. K. Basco, R. Tahar, T. Ouatas, and A. Mazabraud. 1998. Analysis of the Plasmodium vivax dihydrofolate reductase-thymidylate synthase gene sequences. Gene 211:177-185. [DOI] [PubMed] [Google Scholar]
- 6.Eldin de Pécoulas, P., R. Tahar, T. Ouatas, A. Mazabraud, and L. K. Basco. 1998. Sequences variations in the Plasmodium vivax dihydrofolate reductase-thymidylate synthase gene and their relationship with pyrimethamine resistance. Mol. Biochem. Parasitol. 92:265-273. [DOI] [PubMed] [Google Scholar]
- 7.Fryauff, D. F., S. Tuti, A. Mardi, S. Masbar, R. Patipelohi, B. Leksana, K. C. Kain, M. J. Bangs, T. L. Richie, and J. K. Baird. 1998. Chloroquine-resistant Plasmodium vivax in transmigration settlements of West Kalimantan, Indonesia. Am. J. Trop. Med. Hyg. 59:513-518. [DOI] [PubMed] [Google Scholar]
- 8.Garg, M., N. Gopinathan, P. Bodhe, and N. A. Khirsagar. 1995. Vivax malaria resistant to chloroquine: case reports from Bombay. Trans. R. Soc. Trop. Med. Hyg. 89:656-657. [DOI] [PubMed] [Google Scholar]
- 9.Hastings, M. D., and C. H. Sibley. 2002. Pyrimethamine and WR99210 exert opposing selection on dihydrofolate reductase from Plasmodium vivax. Proc. Natl. Acad. Sci. USA 99:13137-13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imwong, M., S. Pukrittakayamee, S. Looareesuwan, G. Pasvol, J. Poirriez, N. J. White, and G. Snounou. 2001. Association of genetic mutations in Plasmodium vivax dhfr with resistance to sulfadoxine-pyrimethamine: geographical and clinical correlates. Antimicrob. Agents Chemother. 45:3122-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imwong, M., S. Pukrittakayamee, L. Renia, F. Letourneur, J. P. Charlieu, U. Leartsakulpanich, S. Looareesuwan, N. J. White, and G. Snounou. 2003. Novel point mutations in the dihydrofolate reductase gene of Plasmodium vivax: evidence for sequential selection by drug pressure. Antimicrob. Agents Chemother. 47:1514-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyaw, M.-P., M.-P. Kyaw, O. Myint, L. Myint, Z. Thaw, K.-H. Aye, and N.-N. Yin. 1993. Emergence of chloroquine-resistant Plasmodium vivax in Myanmar (Burma). Trans. R. Soc. Trop. Med. Hyg. 87:687. [DOI] [PubMed] [Google Scholar]
- 13.Leartsakulpanich, U., M. Imwong, S. Pukrittakayamee, N. J. White, G. Snounou, S. Sirawaraporn, and Y. Yuthavong. 2002. Molecular characterization of dihydrofolate reductase in relation to antifolate resistance in Plasmodium vivax. Mol. Biochem. Parasitol. 119:63-73. [DOI] [PubMed] [Google Scholar]
- 14.Li, J., W. E. Collins, R. A. Wirtz, D. Rathore, A. Lal, and T. F. McCutchan. 2001. Geographic subdivision of the range of the malaria parasite Plasmodium vivax. Emerg. Infect. Dis. 7:35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majori, G., G. Sabatinelli, and A. V. Kondrachine. 1999. Re-emerging malaria in the WHO European region: control priorities and constraints. Parassitologia 41:327-328. [PubMed] [Google Scholar]
- 16.Mendis, K., B. J. Sina, P. Marchesini, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64(Suppl. 1-2):97-106. [DOI] [PubMed] [Google Scholar]
- 17.Mert, A., R. Ozaras, F. Tabak, M. Bilir, R. Ozturk, and Y. Aktuglu. 2003. Malaria in Turkey: a review of 33 cases. Eur. J. Epidemiol. 18:579-582. [DOI] [PubMed] [Google Scholar]
- 18.Noedl, H., C. Wongsrichanalai, and W. H. Wernsdorfer. 2003. Malaria drug-sensitivity testing: new assays, new perspectives. Trends Parasitol. 19:175-181. [DOI] [PubMed] [Google Scholar]
- 19.Oulette, M., and S. A. Ward. 2003. Drug resistance in parasites, p. 397-432. In J. J. Marr, T. M. Nilsen, and R. W. Komuniecki (ed.), Molecular medical parasitology. Academic Press, London, United Kingdom.
- 20.Phillips, E. J., J. S. Keystone, and K. C. Kain. 1996. Failure of combined chloroquine and high-dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana. South Am. Clin. Infect. Dis. 23:1171-1173. [DOI] [PubMed] [Google Scholar]
- 21.Pukrittayakamee, S., A. Chantra, J. Simpson, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob. Agents Chemother. 44:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieckmann, K. H., D. R. Davis, and D. C. Hutton. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183-1184. [DOI] [PubMed] [Google Scholar]
- 23.Sabatinelli, G. 1999. Determinants in malaria resurgence in the former USSR. G. Ital. Med. Trop. 4:53-62. [Google Scholar]
- 24.Schwartz, I. K., E. M. Lackrtiz, and L. C. Patchen. 1991. Chloroquine-resistant Plasmodium vivax from Indonesia. N. Engl. J. Med. 324:927. [DOI] [PubMed] [Google Scholar]
- 25.Soto, J., J. Toledo, P. Gutierrez, M. Luzz, M. Llinas, N. Cedeño, M. Dunne, and J. Berman. 2001. Plasmodium vivax clinically resistant to chloroquine in Colombia. Am. J. Trop. Med. Hyg. 65:90-93. [DOI] [PubMed] [Google Scholar]
- 26.Tahar, R., P. Eldin de Pecoulas, L. K. Basco, M. Chiadmi, and A. Mazabraud. 2001. Kinetic properties of dihydrofolate reductase from wild-type and mutant Plasmodium vivax expressed in Escherichia coli. Mol. Biochem. Parasitol. 113:241-249. [DOI] [PubMed] [Google Scholar]
- 27.Than, M., M. P. Kyaw, A. Y. Soe, K. K. Gyi, and M. S. Myint-Oo. 1995. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans. R. Soc. Trop. Med. Hyg. 89:307-308. [DOI] [PubMed] [Google Scholar]
- 28.Tjitra, E., J. Baker, S. Suprianto, Q. Cheng, and N. M. Anstey. 2002. Therapeutic efficacies of artesunate-sulfadoxine-pyrimethamine and chloroquine-sulfadoxine-pyrimethamine in vivax malaria pilot studies: relationship to Plasmodium vivax dhfr mutations. Antimicrob. Agents Chemother. 46:3947-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valibayov, A., F. Abdullayev, S. Mammadov, E. Gasimov, G. Sabatinelli, A. V. Kondrachine, and P. Ringwald. 2003. Clinical efficacy of chloroquine followed by primaquine for Plasmodium vivax treatment in Azerbaijan. Acta Trop. 88:99-103. [DOI] [PubMed] [Google Scholar]
- 30.White, N. J. 1997. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob. Agents Chemother. 41:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White, N. J. 2002. The assessment of antimalarial drug efficacy. Trends Parasitol. 18:458-464. [DOI] [PubMed] [Google Scholar]